Abstract
Pigeon protozoal encephalitis (PPE) is an emerging central-nervous disease of domestic pigeons (Columba livia f. domestica) reported in Germany and the United States. It is caused by the apicomplexan parasite Sarcocystis calchasi which is transmitted by Accipter hawks. In contrast to other members of the Apicomplexa such as Toxoplasma and Plasmodium, the knowledge about the pathophysiology and host manipulation of Sarcocystis is scarce and almost nothing is known about PPE. Here we show by mRNA expression profiling a significant down-modulation of the interleukin (IL)-12/IL-18/interferon (IFN)-γ axis in the brains of experimentally infected pigeons during the schizogonic phase of disease. Concomitantly, no cellular immune response was observed in histopathology while immunohistochemistry and nested PCR detected S. calchasi. In contrast, in the late central-nervous phase, IFN-γ and tumor necrosis factor (TNF) α-related cytokines were significantly up-modulated, which correlated with a prominent MHC-II protein expression in areas of mononuclear cell infiltration and necrosis. The mononuclear cell fraction was mainly composed of T-lymphocytes, fewer macrophages and B-lymphocytes. Surprisingly, the severity and composition of the immune cell response appears unrelated to the infectious dose, although the severity and onset of the central nervous signs clearly was dose-dependent. We identified no or only very few tissue cysts by immunohistochemistry in pigeons with severe encephalitis of which one pigeon repeatedly remained negative by PCR despite severe lesions. Taken together, these observations may suggest an immune evasion strategy of S. calchasi during the early phase and a delayed-type hypersensitivity reaction as cause of the extensive cerebral lesions during the late neurological phase of disease.
Introduction
Sarcocystis calchasi is an apicomplexan parasite and the causative agent of pigeon protozoal encephalitis (PPE), an emerging neurological disease of the domestic pigeon (Columba livia f. domestica) [1,2]. The definitive hosts of S. calchasi are Accipiter hawks of which the European subspecies of the Northern goshawk (Accipiter g. gentilis) has been experimentally identified to shed large quantities of infectious sporocysts [3,4]. So far the domestic pigeon is the only identified intermediate host of the parasite. Pigeons show a biphasic disease with polyuria, diarrhea and apathy during the schizogonic first phase and severe central nervous signs such as torticollis and opisthotonus associated with severe brain lesions about eight weeks post infection. At the same time mature tissue cysts were present in skeletal muscles. The severity and onset of central nervous signs clearly were dose dependent. In contrast, the intensities of histopathologic lesions and immune cell infiltration in the brains appear to be independent of the amount of administered sporocysts (102-105 per oral dose) [5]. Moreover, no intralesional parasitic stages were observed in H&E histopathology. It therefore appears that the immune system of the pigeon is incapable of preventing infection and an immunopathological basis of the central-nervous lesions has been hypothesized [3]. In this context it can be speculated that S. calchasi may benefit from the induction of central-nervous malfunctioning and immobilization as they may influence the rate of parasite’s transmission to its definitive host similar as it is proposed for related apicomplexan parasites [6].
Several avian Sarcocystis spp. have been reported to induce central nervous signs (see [1] for overview). Encephalitis is most often reported to be associated with the schizont stage of the parasite’s development. One notable example is Sarcocystis neurona. This parasite which is closely related to S. calchasi and most probably of avian origin is capable of inducing a central nervous disease in a broad range of avian and mammalian species such as horses, cats, and dogs [7-10]. In many cases and even in extensive lesions the number of intralesional S. neurona merozoites and schizonts can be very low. It has been proposed that an immune response triggered by cytokines and metabolites of the parasite may cause the extensive lesions [11]. Recently the presence of S. neurona tissue cysts together with schizonts and merozoites has been confirmed for the first time in southern sea otters (Enhydra lutris nereis) with encephalitis [12]. More significantly, ovine Sarcocystis spp. such as Sarcocystis tenella has been found capable of inducing a widespread encephalomyelitis associated with degenerating tissue cysts and prominent central nervous signs [13,14].
Until now the biology of the hosts’ immune response against Sarcocystis spp. in general has only scarcely been addressed and whether this genus of parasites may manipulate the immune response similarly to other Apicomplexa is unknown. However, in vitro results suggest that S. neurona might be capable of down-modulating the IFN-γ signaling pathway [15,16]. It has therefore been proposed that Sarcocystis spp. may use similar evasion strategies than Toxoplasma gondii, a well-studied apicomplexan parasite that interferes with the IFN-γ signaling pathway [17,18]. Here, we aimed at investigating the immune response and pathophysiology of PPE due to S. calchasi during the schizogonic and late chronic phase of disease associated with central-nervous signs. In most cases we confirmed the presence of parasitic stages in the brains of the pigeons by immunohistochemistry and nested PCR. The cytokine expression profile together with the morphological results of this study may suggest an immune evasion strategy of the parasite that interferes with the Th1 response in the first phase of the disease, while an overstimulated T-cell mediated immune response appears to be characteristic for the second phase of the disease.
Material and methods
Samples of pigeons
The samples used for the present study originate from an experimental infection study of S. calchasi in domestic pigeons [3]. All experiments were performed under governmental approval (No. Reg 0111/08). The pigeons were orally inoculated with a range of 102 to 3 × 106 sporocysts shed by an experimentally infected Northern goshawk (A. g. gentilis) as described previously [5]. The pigeons depicted a biphasic disease. Two animal groups were established for the purpose of this study. The eight pigeons of group A inoculated with 8 × 104 to 3 × 106 sporocysts deceased 7–12 dpi during the schizogonic early phase of disease. The five pigeons of group B inoculated with 102 to 104 sporocysts were euthanized 51–65 dpi in the central nervous late phase of disease. Two additional pigeons (L60, L74) were inoculated with 103 sporocysts, euthanized in the second phase at day 53 and 59 dpi and integrated into group B (Table 1). Five uninfected pigeons were used as reference animals. The brains of all pigeons were removed immediately after death [5]. One half was snap frozen and stored at −80°C until further use. The other half was fixed in 4% neutral-buffered formaldehyde and embedded in paraffin 24 h later. In addition, tissue samples from lung, heart, liver, spleen, kidneys, gizzard and skeletal muscles were taken and processed equally.
Table 1.
Numbers of immunohistochemically detected schizonts and tissue cysts in five consecutive transversal section of brains of pigeons during first (group A) and second, central nervous phase of disease (group B)
|
Group |
Pigeon ID no. |
Dose of administered sporocysts |
Post mortem (dpi) |
Occurrence of central nervous signs (dpi) |
Encephalitis |
No. of |
|
|---|---|---|---|---|---|---|---|
| schizonts | tissue cysts | ||||||
| A |
2 |
3 × 106 |
7 |
/ |
/ |
1 |
0 |
| 3 |
3 × 106 |
7 |
/ |
/ |
1 |
0 |
|
| 4 |
3 × 105 |
9 |
/ |
/ |
0 |
0 |
|
| 5 |
3 × 105 |
9 |
/ |
/ |
0 |
0 |
|
| 6 |
105 |
8 |
/ |
/ |
0 |
0 |
|
| 7 |
105 |
12 |
/ |
/ |
3 |
0 |
|
| 8 |
8 × 104 |
9 |
/ |
/ |
1 |
0 |
|
| 9 |
8 × 104 |
10 |
/ |
/ |
0 |
0 |
|
| B |
10 |
104 |
51 |
51 |
+++ |
0 |
8 |
| 11 |
104 |
53 |
52 |
+++ |
0 |
2 |
|
| 12 |
103 |
58 |
57 |
+++ |
0 |
0 |
|
| 14 |
102 |
65 |
64 |
+++ |
0 |
0 |
|
| 15 |
102 |
58 |
57 |
+++ |
0 |
0 |
|
| L60 |
103 |
53 |
52 |
+++ |
0 |
4 |
|
| L74 | 103 | 59 | 58 | +++ | 0 | 0 | |
+++, severe.
For details on histopathology of further organs see [5].
Antibodies against S. calchasi
S. calchasi sporocysts derived from a Northern goshawk euthanized 14 days after oral infection were used for generation of S. calchasi-specific polyclonal antibodies [3]. Washed sporocysts were pretreated with 5% sodium hypochlorite for 30 min, washed and resuspended in 15 mL Roswell Park Memorial Institute medium (RMPI) medium supplemented with 10% fetal bovine serum (FBS) and 15% bovine bile and were incubated for 1 h at 37°C. Vero cells grown in RMPI medium were inoculated with 108 excysted sporozoites and supplemented with FBS, 10 000 IU/mL penicillin and 10 000 μg/mL dihydrostreptomycin. Merozoites were harvested after 12 days by rinsing the monolayer with 10 mL 4°C Hank’s buffered salt solution, washed and resuspended in 2.5 mL PBS and passed through a PD-10 desalting column (GE Healthcare, Freiburg, Germany) as previously described [19]. Purified merozoites were incubated in 4% formalin for 30 min and washed three times in PBS. Standard immunization of two rabbits was conducted with 1 × 107 merozoites each. Histological sections of skeletal muscle infested with tissue cysts and livers infested with merozoites and schizonts of S. calchasi from experimentally infected domestic pigeons were used to assess the specificity of the serum.
Histopathology and immunohistochemistry
Formalin-fixed paraffin-embedded tissue was sectioned at 4 μm, mounted on glass slides and stained with haematoxylin and eosin (H&E).
Immunohistochemistry was used to analyze the prevalence of parasitic stages of S. calchasi and expression of MHC-II, CD3 for T-cells and Pax-5 for B-cells in pigeon brains. Serial sections of frozen brain samples were cut at 4 μm, mounted on adhesive glass slides and were fixed in acetone for 10 min and dried for 20 min. Avidin-biotin blocking of the cryostat sections was performed according to the manufacturer’s protocol (Dako North America, Inc., Carpinteria, CA, USA). The slides were washed in PBS containing 0.05% Triton X-100 and blocked with PBS containing 2% BSA and 20% normal goat serum for 30 min. Finally the sections were incubated with mouse-anti-chicken MHC-II specific antibody 2G11 (1:50) for 1 h. The antibody 2G11 has been shown to cross-react with MHC-II of multiple avian and non-avian species [20]. A goat anti-mouse IgG (1:200, Vector Laboratories, Burlingame, CA, USA) was used as secondary antibody. MHC-II immunoreaction was visualized by incubating in ABC solution, followed by HistoGreen-staining (Linaris, Wertheim-Bettingen, Germany) for 4 min at room temperature.
For detection of CD3 and Pax-5, sections of formalin-fixed paraffin-embedded tissue samples were cut at about 2 μm and mounted on glass slides. Consecutive sections were dewaxed in xylene, followed by rehydration in descending graded ethanol. Endogenous peroxidase was blocked by incubating the slides with 0.5% H2O2 in methanol for 30 min at room temperature. Antigen retrieval was performed using 15 min microwave heating (600 W) in 10 mM citric acid, pH 6.0, containing 0.05% Triton X-100. A polyclonal rabbit antibody specific for the highly conserved ε-chain of human CD3 (1:3000, DAKO, Glostrup, Denmark) and a monoclonal mouse anti-human Pax-5 specific antibody (clone 24, 1:1000, BD Biosciences, San Jose, CA, USA) were diluted in Tris-buffered saline (TBS, 50 mM, pH 7.6) and incubated at 4°C overnight after a blocking step with 50% goat serum in TBS (30 min at room temperature). Both antibodies have been tested on spleen cell populations for cross-reactivity with pigeon T- and B-cells, respectively. Goat anti-rabbit IgG (1:200) and anti-mouse IgG (1:200, Vector Laboratories), respectively, was used as secondary antibody. Color was developed by incubating the slides in avidin-biotin-peroxidase complex (ABC) solution (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA, USA), followed by exposure to diaminobenzidine tetrahydrochloride (DAB, Merck, Darmstadt, Germany). All slides were counterstained with Mayer’s haematoxylin. In each run, negative controls were incubated with irrelevant commercial mouse or rabbit immunoglobulins (BioGenex, Fremont, CA, USA) instead of primary antibodies listed above. No unspecific labelling was detected in any tissue examined.
RNA isolation, cDNA preparation and DNA isolation
About 40 mg cerebrum of each pigeon was minced into small pieces. RNA was extracted and purified using the NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany). Concentrations were measured by OD 260 and OD 280 (NanoDrop 1000 Spectrophotometer, Thermo Fisher Scientific, Wilmington, DE, USA). RNA integrity numbers (RIN) above 8.0 were measured in all samples using an Agilent 2100 Bioanalyzer (RNA 6000 Nano Kit, Agilent, Santa Clara, CA, USA) and regarded as good quality [21]. For cDNA synthesis, 50 ng RNA was reverse transcribed using the iScript kit (Bio-Rad, Hercules, CA, USA). DNA was extracted using the NucleoSpin Tissue kit (Macherey-Nagel).
Nested and quantitative real time (RT) PCR
S. calchasi DNA was detected by nested PCR targeting the ITS1 region as described previously [4].
For candidate reference genes, three primer pairs for beta-actin, beta glucuronidase (GUSB) and hydroxymethylbilane synthetase (HMBS) were used (A. Meyer, unpublished observations). In addition, two new primer pairs for ribosomal protein L13 (RPL13) and transferrin receptor protein (TFRC) were designed using Netprimer software (PREMIER Biosoft, Palo Alto, CA, USA) based on a comparative sequence analysis of published mRNA sequences of the domestic chicken (G. gallus f. dom.) and the zebra finch (Taeniopygia guttata) using MEGA4 (Table 2) [22]. In the same way a novel set of primers was designed for 10 cytokines of the domestic pigeon including interleukin (IL) 1, IL-6, IL-7, IL-12, IL-15, IL-18, interferon gamma (IFN-γ), transforming growth factor beta 2 (TGF-β2), LPS-induced TNF-α factor (LITAF), TNF-like ligand 1A (TL1A) and the chemokine IL-8 (Tab. 2). For RT-PCR, the 15 μL reaction mix included 10 μL Brilliant SYBR Green QPCR Master Mix (Applied Biosystems, Carlsbad, CA) with 300 nM of each primer and 5 μL sample cDNA. Cycling conditions were 10 min at 95°C followed by 40 cycles at 30 s at 95°C, 1 min at 58°C and 30 s at 72°C. Reference genes were evaluated to quantify relative cytokine mRNA expression levels. RT-PCR and data were analyzed using the MX 3000P Quantitative PCR System and MX Pro software (Agilent). Each sample was analyzed in triplicate. Nuclease-free water was used as negative controls in each run. Initially, primer efficiencies were determined and primers with an efficiency below 90.0 excluded from further analysis (Table 1). Specificity of amplicons was evaluated against chicken sequences derived from GenBank using MEGA5 (Table 1) and by melting curve analysis. Relative cytokine mRNA expression levels were normalized by the efficiency-corrected ΔΔCt method against the expression of the three most stable reference genes determined by the GeNorm algorithm [23-25]. Data are presented as fold change (FC) in cytokine expression levels in pigeons of group A and B, respectively, normalized to the reference genes and relative to the group of reference animals. Cut off values were set at > 2.0 for increased and < 0.5 for reduced gene expression.
Table 2.
Sequences of primers used for RT-qPCR and uncorrected (p) sequence distance of obtained pigeon amplicons to chicken mRNA
| RNA target | 5′-3′ primer sequences | Amplicon size (bp) | Primer efficiency (%) | p-distance* | Accession number** |
|---|---|---|---|---|---|
| beta-actin |
F: AAGGACCTGTACGCCAACAC |
211 |
91.8 |
0.024 |
NM_205518 |
| |
R: CCTGCTTGCTGATCCACATC |
|
|
|
|
| GUSB |
F: GGGGCAAACTCCTTCCG |
223 |
92.2 |
0.127 |
NM_001039316 |
| |
R: ATCCACCAGCTTGATGTCACTAAC |
|
|
|
|
| HMBS |
F: CTGGCCCGGATTCAGAC |
154 |
96.3 |
0.166 |
XM_417846 |
| |
R: GCTCTTTGGTGAAGAGGCTC |
|
|
|
|
| RPL13 |
F: CCACAAGGACTGGCAGCG |
135 |
92.0 |
0.095 |
NM_204999 |
| |
R: ACGATGGGCCGGATGG |
|
|
|
|
| TFRC |
F: GCCCTGAATGACAGGATGATG |
206 |
87.3 |
0.17 |
NM_205256 |
| |
R: GTCCACGTCGCTAGGGCC |
|
|
|
|
| IL-1 |
F: CGAGAGCAGCTACGCCG |
271 |
99.6 |
0.176 |
DQ393270 |
| |
R: GCCGCTCAGCACACACG |
|
|
|
|
| IL-6 |
F: CTGCCCAAGGTGACGGAG |
178 |
97.9 |
0.3 |
HM179640 |
| |
R: CCAGGTGCTTTGTGCTGTAGC |
|
|
|
|
| IL-7 |
F: CAGAGTATCGTGACAGATGCTGC |
174 |
101.3 |
0.111 |
NM_001037833 |
| |
R: ATGAGACTAATGCTGCTTTCCTTC |
|
|
|
|
| IL-8 |
F: CAAGACGTGAAGCTGACACAGAG |
161 |
99.5 |
0.117 |
DQ393275 |
| |
R: GGTGCATCAGAATTGAGTTGAG |
|
|
|
|
| IL-12 |
F: AGTGAAGGAGTTCCCAGATGC |
188 |
90.0 |
0.194 |
DQ202328 |
| |
R: TTCCAGAGTAGTTCTTTGCCTCAC |
|
|
|
|
| IL-15 |
F: GAATGCCAGGAACCTGTAATG |
246 |
102.5 |
0.142 |
HQ005358 |
| |
R: GCATTCCCTCTGTATAACCTTTAC |
|
|
|
|
| IL-18 |
F: GCCAGTTGCTTGTGGTTCG |
160 |
100.8 |
0.141 |
AY775782 |
| |
R: TCTACCTGGACGCTGAATGC |
|
|
|
|
| IFN-γ |
F: CAGATGTAGCTGACGGTGGAC |
276 |
93.2 |
0.178 |
DQ479967 |
| |
R: GCTCATGCACAGCTTTGCG |
|
|
|
|
| TGF-β2 |
F: GAAGAAGCGTGCTCTAGATGC |
105 |
100.1 |
0.019 |
ENSGALT00000015664 |
| |
R: CATTTCCAGCCAAGATCCC |
|
|
|
|
| LITAF |
F: CCCATCTGCACCACCTTCAT |
157 |
100.8 |
0.252 |
NM_204267 |
| |
R: TGCTGCACATACACAGTCTGAAC |
|
|
|
|
| TL1A |
F: CCTGAGTTATTCCAGCAACGCA |
285 |
95.3 |
0.111 |
NM_001024578 |
| R: ATCCACCAGCTTGATGTCACTAAC |
*compared to chicken by MEGA5 software. **of chicken.
Statistical analysis
Gene expression data were analyzed with the Mann–Whitney-U test using SPSS software, version 20.0 (SPSS, X). Results were considered statistically significant at p < 0.05 between two groups of animals.
Results
Histopathology and immunohistochemistry
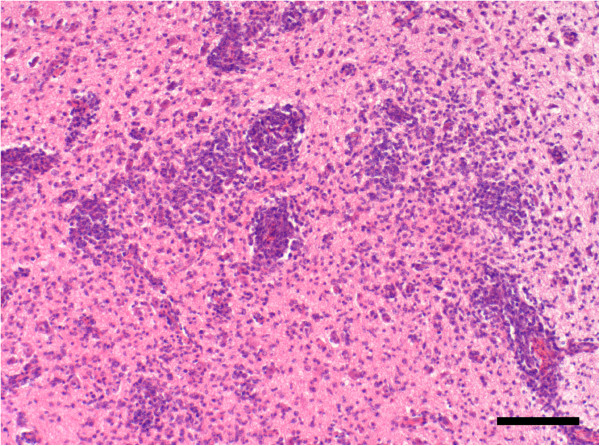
Pigeons of group A that died during the schizogonic first phase of disease had no histopathological lesions in the brain. No inflammatory cell response was discernible in any area. Pigeons of group B were euthanized in the neurological second phase of disease. All pigeons showing central nervous signs uniformly had a severe multifocal lymphohistocytic and necrotizing encephalitis with prominent perivascular cuffing (Figure 1) [5]. No pathological lesions were found in the reference animals.
Figure 1.
Encephalitis of pigeon no. 14 infected with 102 sporocysts and negative for S. calchasi by immunohistochemistry and PCR. Note the extensive mononuclear cell infiltration and perivascular cuffing in the cerebrum. H&E stain. Bar, 100 μm.
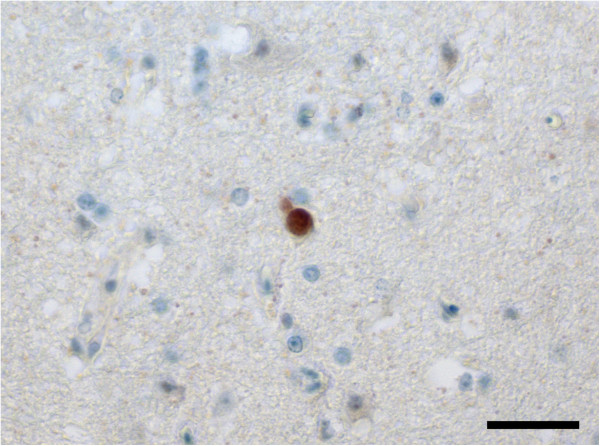
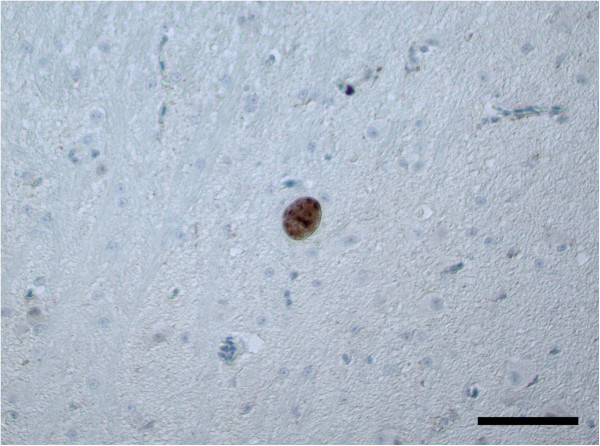
Immunohistochemical testing revealed that the anti-merozoite antibody is capable of labelling S. calchasi merozoites, schizonts and tissue cysts containing bradyzoites (data not shown). A positive labelling for S. calchasi was observed in the cerebrum of pigeons of group A and B. Four of eight pigeons of group A had rare schizonts in the neuropil (Figure 2 and Table 1). Mean schizont dimensions were 11.6 × 9.8 μm (n = 6, range = 7.3-13.4 × 6.5-13.2 μm). Three of seven pigeons of group B had few, randomly distributed tissue cysts located in areas of the neuropil not associated with pathological lesions or inflammatory cell reactions (Figure 3 and Table 1). The size of tissue cysts was in mean 19.0 × 15.1 μm (n = 14, range = 12.1-26.2 × 10.6-24.8 μm). All reference animals were negative for S. calchasi by immunohistochemistry.
Figure 2.
Immunohistochemical labelling of a schizont with polyclonal antiserum to S. calchasi in the brain of pigeon no. 7 during the first phase of disease. No associated cellular immune reaction or necrosis is discernible. In total, three schizonts were identified in consecutive section of the brain of this pigeon. Bar, 30 μm.
Figure 3.
Immunohistochemical labelling of a tissue cyst with polyclonal antiserum to S. calchasi in the brain of pigeon no. L60 during the second phase of disease. No associated pathological lesions can be seen. In total, four tissue cysts were identified in the neuropil of this pigeon. Bar, 50 μm.
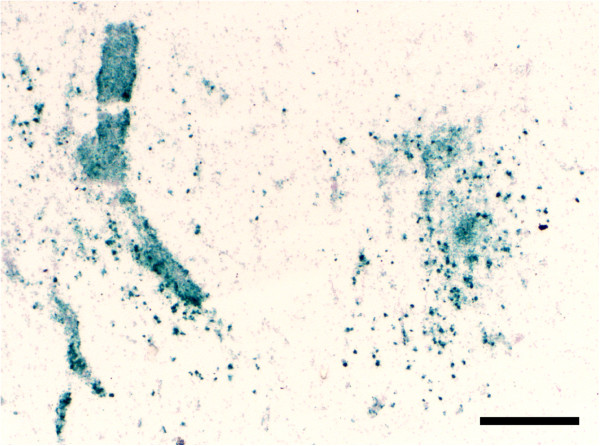
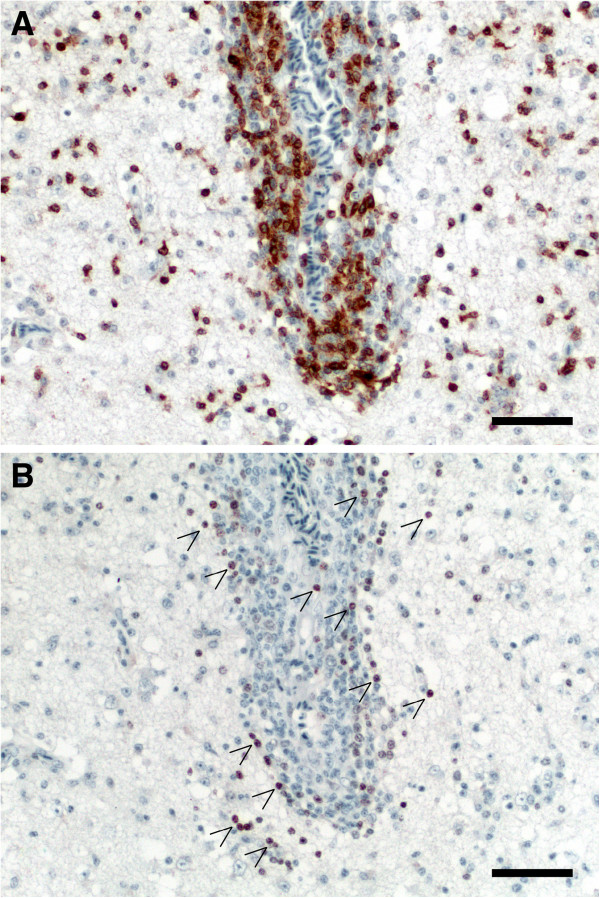
Areas with cerebral lesions including necrosis and gliosis as well as areas with mononuclear cell infiltration had strong MHC-II labelling in all pigeons of group B (Figure 4). Most mononuclear cells were strongly CD3 positive (T-lymphocytes) while only few cells were positive for Pax-5 (B-lymphocytes; Figure 5).
Figure 4.
Immunohistochemical detection of MHC-II of a pigeon with cerebral lesions in the second phase of disease. Most prominent signaling is discernable in areas of perivasular cuffing of mononuclear cells (left) and areas of necrosis and gliosis (right). Bar, 300 μm.
Figure 5.
Immunohistochemical demonstration of CD3+ T-cells (A), and Pax-5+ B cells (B) surrounding the same cerebral blood vessel of pigeon no. 12. Note the dominance of T lymphocytes in perivascular cuffs and the neuropil. Arrows, positive stained B-cells. Bars 50 μm.
Molecular detection of S. calchasi
The nested PCR amplified DNA specific for S. calchasi in the brains of all pigeons of group A and all but one (pigeon no. 14) of group B (Figure 6). Reference animals were all negative. Processing controls and no template control also gave negative results.
Figure 6.
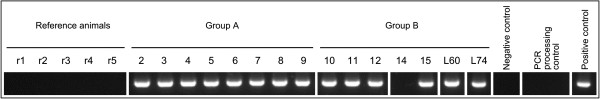
PCR detection of S. calchasi DNA in brains of pigeons with specific nested primer pairs targeting the ITS1 region. All brains of pigeons of group A (no. 2–9) and pigeons of group B (no. 10–12, 15, L60 and L74) except for no. 14 were positive. Five reference animals (r1-r5) used for RT-qPCR as well as negative controls and two processing controls were negative.
Cytokine expression profile
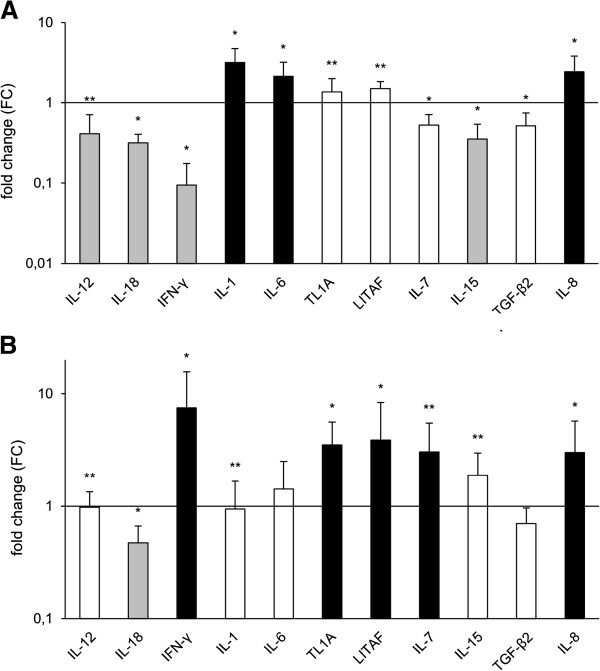
Most stable reference genes for the cerebrum of pigeons of this study calculated by the GeNorm algorithm were RPL13, HMBS and beta-actin. For group A, mRNA expression levels of the Th-1 cytokines IL-12, IL-18 and IFN-γ were significantly down-modulated while the proinflammatory cytokines IL-1, IL-6, IL-15 and the chemokine IL-8 were significantly up-modulated when compared to the reference animals (Figure 7A). No regulation in mRNA expression levels was measured for TL1A, LITAF, IL-7 and TGF-β2. In contrast, for group B IFN-γ expression was significantly up-modulated while IL-18 was down-modulated and IL-12 was not regulated (Figure 7B). Furthermore, TL1A, LITAF and IL-7 were significantly up-modulated. IL-8 remained up-modulated, while IL-1 and IL-15 were unregulated when compared with the reference pigeons. IL-6 and TGF-β2 were also unregulated, but without statistical significance.
Figure 7.
Cytokine and chemokine expression profiles in the cerebrum from pigeons of group A (A) and group B (B) compared with non-infected pigeons (n = 5). gray bar = down-modulated (FC < 0.5), white bar = unregulated (0.5 < FC <2), “black square” = up-modulated (FC ≥ 2). * = statistically significant at p ≤ 0.05 by Mann–Whitney-U-test between group of infected and uninfected pigeons. ** = significant differences between group A and B.
Discussion
As demonstrated previously, S. calchasi is capable of causing a severe biphasic central nervous disease in the domestic pigeon [3]. Histologically, a severe lymphohistiocytic and necrotizing encephalitis was found in the late neurological phase of disease. So far, intralesional stages of the parasite had not been confirmed in the pigeon’s brains [5]. Because in tissue sections of cerebral sarcosporidiosis the parasitic load can be low or difficult to detect despite extensive pathological lesions [3,11], we generated and established an anti-merozoite antiserum against S. calchasi. The antibody reliably detected merozoites, schizonts and tissue cysts including bradyzoites by immunohistochemical analysis. Hereby we demonstrate that S. calchasi stages - although only very few - are present in about half of the brains in both clinical phases of PPE. The presence of S. calchasi DNA could be confirmed by PCR results for the ITS1 region from the cerebrum in all but one pigeon. Together the results indicate that S. calchasi is present in the brains of pigeons with PPE in both disease phases and may suggest a direct involvement of the parasite in the development of the cerebral lesions. However, since no direct association of parasitic stages with histopathological lesions was detected, an unknown immunopathological mechanism may trigger the extensive inflammatory lesions. This notion is underlined by one pigeon infected with 102 sporocysts with a strong cellular immune response but negative results for parasite protein and DNA in the brain. To verify this, further experimental studies are needed since it cannot be ruled out that at the onset of central nervous signs all parasites are effectively eliminated by the immune system.
To further clarify the effect of S. calchasi on the cerebral immune response we established a novel panel of primers to measure the expression level of 11 key immune effector genes and 5 reference genes by RT-qPCR. We characterized an anti-parasite response profile of the host immune system. Most notably, during the schizogonic, first phase of disease the important Th1 cytokines IL-12, IL-18 and IFN-γ as well as IL-15 were significantly down-modulated. During this phase, schizonts were present in various organs, most prominently in the liver, spleen and endothelial cells [5]. In the brains, only very few schizonts were detected in the neuropil without discernible lesions or immune cell infiltrations, although the expression of the major pro-inflammatory cytokines IL-1 and IL-6 and the chemokine IL-8 were significantly up-modulated. This may suggest that similar to other members of the Apicomplexa, S. calchasi is capable of manipulating the IL-12/IL-18/IFN-γ axis to evade the cellular immune response [17].
Compared to closely related members of the Apicomplexa such as Toxoplasma gondii and Neospora caninum very little is known about the host immune response against Sarcocystis infection. IFN-γ has been shown to be essential for the protection against S. neurona neurological disease in mice [26]. IFN-γ is produced by T-cells, natural killer (NK) cells, monocytes and microglia in the brain [27]. While IFN-γ KO mice show severe neurological disease after experimental infection with S. neurona, SCID mice, which still have functional IFN-γ producing NK cells, only develop disease after treatment with neutralizing anti-IFN-γ antibodies [26]. Furthermore, it has been shown that CD8+ T-cells are critical for the protection from meningoencephalitis in C57BL/6 mice [28], while a humoral immune response seems to play no major role [29]. There is also first evidence that S. neurona may be capable of interfering with the cytokine signaling of the Th1 immune response. IFN-γ production was reduced in lymphocytes extracted from Equine protozoal meningoencephalitis (EPM) positive horses [16]. Isolated peripheral blood lymphocytes from horses with EPM that were co-cultured with SnSAG1 produced significantly less IFN-γ after 48 h [15] and the cell-mediated immune responses to SnSAG1 were significantly reduced in horses with EPM [16]. Together with the results of this study it is plausible to assume that Sarcocystis spp. in general may exhibit an immune evasion strategy that disrupts IFN-γ signaling. Since the Th1-biased immune response is of major importance in clearing infection with T. gondii and N. caninum [30,31], a balanced immune-modulation by the parasite is crucial for survival in its host. Impairment of the IL-12 and IFN-γ expression is therefore one central immune evasion strategy of T. gondii[17,18]. Furthermore, an impaired synthesis of IL-15 reduces the expression of IFN-γ which enhances the survival of T. gondii[32]. IL-15 also activates CD4+ and CD8+ T-cells, of which CD8+ T cells are stimulated to produce IFN-γ [33]. Interestingly, besides IL-12, IL-18 and IFN-γ, IL-15 was significantly down-modulated in the first phase of S. calchasi infection. This temporary immune suppression may, similar to T. gondii[30], facilitate S. calchasi to infect host cells and to replicate in the absence of a protective immune response.
In contrast to the first phase, the second neurological phase of disease is associated with a massive mononuclear cell infiltration and characterized by a markedly up-modulated IFN-γ expression. Notably, IL-12 and IL-18 as important inducers of IFN-γ were not up-modulated. IFN-γ is the key cytokine for the activation of mononuclear cells. This correlates well with the prominence of T-cells, the granulomatous character of the lesions and prominent MHC-II signaling in the pigeon brains. Chicken IFN-γ increases the expression of class II MHC on antigen presenting cells and directly activates macrophages and natural killer cells which can excrete further inflammatory effector molecules such as TNF-α that may destroy tissue. [34-36]. Since TNF-α has not been identified in the avian genome, we tested TL1A and LITAF which both were significantly up-modulated. Taken together this suggests an extensive Th1-biased T-cell driven immune response, which appears inappropriate in the light of a very low or absent parasite load.
Pigeons infected with S. calchasi depict neurological signs such as incoordination about eight weeks after infection when tissue cysts mainly located in skeletal muscles contain infectious bradyzoites [5]. This alteration in intermediate host behavior may lead to an increased predation rate by the final host, the Northern goshawk. In contrast to pigeons, several natural and aberrant hosts of avian Sarcocystis spp. (i.e. S. neurona) depict neurological signs associated with encephalitis only during the schizogonic phase which does not allow transmission to a final host [7,9,37,38]. Changes in intermediate host behavior as reported for common voles (Microtus arvalis) and lemmings (Dicrostonyx richardsoni) infected by birds of prey-transmitted Sarcocystis cernae and Sarcocystis rauschorum, respectively, have been suggested to enhance parasite transmission due to increased predation rates [39,40]. However, whether the change in behavior of pigeons induced by S. calchasi may be regarded as a parasite’s adaptation to enhance its fitness or simply as side effect due to a delayed-type hypersensitivity reaction requires further investigation to meet the manipulation hypothesis [41].
In conclusion, the observations of this study suggest that the Th-1 immune response during the schizogonic phase of the S. calchasi development is down-modulated in the intermediate host. The absence of a strong host’s cellular immune response in the pigeon may facilitate parasite evasion during acute disease and subsequent formation of tissue cysts. The results of this study further suggest that during the late central nervous phase of PPE a T-cell mediated delayed-type hypersensitivity reaction may cause the cerebral lesions.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
Conceived and designed the experiments: PO, AM, ML, AG. Performed the experiments: PO, AM. Analyzed the data: PO, AM, RK, BK. Contributed reagents/ materials/ analysis tools: PO, ML, BK, AG. Wrote the paper: PO. All authors have read and approved the final manuscript.
Contributor Information
Philipp Olias, Email: philipp.olias@fu-berlin.de.
Anne Meyer, Email: anne.meyer@fu-berlin.de.
Robert Klopfleisch, Email: robert.klopfleisch@fu-berlin.de.
Michael Lierz, Email: michael.lierz@vetmed.uni-giessen.de.
Bernd Kaspers, Email: kaspers@tiph.vetmed.uni-muenchen.de.
Achim D Gruber, Email: achim.gruber@fu-berlin.de.
Acknowledgements
We thank M. Schaerig, J. Enders, M. Dauer and A. Linke for excellent technical assistance.
References
- Olias P, Gruber AD, Heydorn AO, Kohls A, Mehlhorn H, Hafez HM, Lierz M. A novel Sarcocystis-associated encephalitis and myositis in racing pigeons. Avian Pathol. 2009;38:121–128. doi: 10.1080/03079450902737847. [DOI] [PubMed] [Google Scholar]
- Wünschmann A, Armien AG, Reed L, Gruber AD, Olias P. Sarcocystis calchasi-associated neurologic disease in a domestic pigeon in North America. Transbound Emerg Dis. 2011;58:526–530. doi: 10.1111/j.1865-1682.2011.01254.x. [DOI] [PubMed] [Google Scholar]
- Olias P, Gruber AD, Kohls A, Hafez HM, Heydorn AO, Mehlhorn H, Lierz M. Sarcocystis species lethal for domestic pigeons. Emerg Infect Dis. 2010;16:497–499. doi: 10.3201/eid1603.090860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olias P, Olias L, Krücken J, Lierz M, Gruber AD. High prevalence of Sarcocystis calchasi sporocysts in European Accipiter hawks. Vet Parasitol. 2011;175:230–236. doi: 10.1016/j.vetpar.2010.10.025. [DOI] [PubMed] [Google Scholar]
- Olias P, Gruber AD, Heydorn AO, Kohls A, Hafez HM, Lierz M. Unusual biphasic disease in domestic pigeons (Columba livia f. domestica) following experimental infection with Sarcocystis calchasi. Avian Dis. 2010;54:1032–1037. doi: 10.1637/9303-031110-Reg.1. [DOI] [PubMed] [Google Scholar]
- Berdoy M, Webster JP, MacDonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc R Soc Lond B Biol Sci. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield LS, Mehler S, Nelson K, Elsheikha HM, Murphy AJ, Knust B, Tanhauser SM, Gearhart PM, Rossano MG, Bowman DD, Schott HC, Patterson JS. Brown-headed cowbirds (Molothrus ater) harbor Sarcocystis neurona and act as intermediate hosts. Vet Parasitol. 2008;153:24–43. doi: 10.1016/j.vetpar.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Chapman JL, Rosenthal BM, Mense M, Schueler RL. Clinical Sarcocystis neurona, Sarcocystis canis, Toxoplasma gondii, and Neospora caninum infections in dogs. Vet Parasitol. 2006;137:36–49. doi: 10.1016/j.vetpar.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Davis SW, Speer CA, Bowman DD, de Lahunta A, Granstrom DE, Topper MJ, Hamir AN, Cummings JF, Suter MM. Sarcocystis neurona n. sp. (Protozoa: Apicomplexa), the etiologic agent of equine protozoal myeloencephalitis. J Parasitol. 1991;77:212–218. [PubMed] [Google Scholar]
- Dubey JP, Hamir AN. Immunohistochemical confirmation of Sarcocystis neurona infections in raccoons, mink, cat, skunk, and pony. J Parasitol. 2000;86:1150–1152. doi: 10.1645/0022-3395(2000)086[1150:ICOSNI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS, Saville WJ, Reed SM, Granstrom DE, Speer CA. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM) Vet Parasitol. 2001;95:89–131. doi: 10.1016/S0304-4017(00)00384-8. [DOI] [PubMed] [Google Scholar]
- Miller MA, Barr BC, Nordhausen R, James ER, Magargal SL, Murray M, Conrad PA, Toy-Choutka S, Jessup DA, Grigg ME. Ultrastructural and molecular confirmation of the development of Sarcocystis neurona tissue cysts in the central nervous system of southern sea otters (Enhydra lutris nereis) Int J Parasitol. 2009;39:1363–1372. doi: 10.1016/j.ijpara.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP. Lesions in sheep inoculated with Sarcocystis tenella sporocysts from canine feces. Vet Parasitol. 1988;26:237–252. doi: 10.1016/0304-4017(88)90092-1. [DOI] [PubMed] [Google Scholar]
- Henderson JM, Dies KH, Haines DM, Higgs GW, Ayroud M. Neurological symptoms associated with sarcocystosis in adult sheep. Can Vet J. 1997;38:168–168. [PMC free article] [PubMed] [Google Scholar]
- Spencer JA, Deinnocentes P, Moyana EM, Guarino AJ, Ellison SE, Bird RC, Blagburn BL. Cytokine gene expression in response to SnSAG1 in horses with equine protozoal myeloencephalitis. Clin Diagn Lab Immunol. 2005;12:644–646. doi: 10.1128/CDLI.12.5.644-646.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JA, Ellison SE, Guarino AJ, Blagburn BL. Cell-mediated immune responses in horses with equine protozoal myeloencephalitis. J Parasitol. 2004;90:428–430. doi: 10.1645/GE-3289RN. [DOI] [PubMed] [Google Scholar]
- Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- Shapira S, Speirs K, Gerstein A, Caamano J, Hunter CA. Suppression of NF‐κB activation by infection with Toxoplasma gondii. J Infect Dis. 2002;185:S66–S72. doi: 10.1086/338000. [DOI] [PubMed] [Google Scholar]
- Elsheikha HM, Rosenthal BM, Murphy AJ, Dunams DB, Neelis DA, Mansfield LS. Generally applicable methods to purify intracellular coccidia from cell cultures and to quantify purification efficacy using quantitative PCR. Vet Parasitol. 2006;135:223–234. doi: 10.1016/j.vetpar.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Skjoedt K, Salomonsen J, Simonsen M, Du Pasquier L, Parisot R, Riegert P. MHC-like molecules in some nonmammalian vertebrates can be detected by some cross-reactive xenoantisera. J Immunol. 1990;144:2258–2272. [PubMed] [Google Scholar]
- Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: an RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etschmann B, Wilcken B, Stoevesand K, Von Der Schulenburg A, Sterner-Kock A. Selection of reference genes for quantitative real-time PCR analysis in canine mammary tumors using the GeNorm algorithm. Vet Pathol. 2006;43:934–942. doi: 10.1354/vp.43-6-934. [DOI] [PubMed] [Google Scholar]
- Sellon DC, Knowles DP, Greiner EC, Long MT, Hines MT, Hochstatter T, Hasel KM, Ueti M, Gillis K, Dame JB. Depletion of natural killer cells does not result in neurologic disease due to Sarcocystis neurona in mice with severe combined immunodeficiency. J Parasitol. 2004;90:782–788. doi: 10.1645/GE-205R. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Host resistance in the brain against Toxoplasma gondii. J Infect Dis. 2002;185(Suppl 1):S58–65. doi: 10.1086/337999. [DOI] [PubMed] [Google Scholar]
- Witonsky SG, Gogal RM, Duncan RB, Norton H, Ward D, Lindsay DS. Prevention of meningo/encephalomyelitis due to Sarcocystis neurona infection in mice is mediated by CD8 cells. Int J Parasitol. 2005;35:113–123. doi: 10.1016/j.ijpara.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Witonsky SG, Gogal RM, Duncan RB, Norton H, Ward D, Yang J, Lindsay DS. Humoral immunity is not critical for protection against experimental infection with Sarcocystis neurona in B-cell-deficient mice. J Parasitol. 2005;91:830–837. doi: 10.1645/GE-488R.1. [DOI] [PubMed] [Google Scholar]
- Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol. 2009;39:23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Innes EA, Wright S, Bartley P, Maley S, Macaldowie C, Esteban-Redondo I, Buxton D. The host-parasite relationship in bovine neosporosis. Vet Immun Immunopathol. 2005;108:29–36. doi: 10.1016/j.vetimm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Combe CL, Moretto MM, Schwartzman JD, Gigley JP, Bzik DJ, Khan IA. Lack of IL-15 results in the suboptimal priming of CD4+ T cell response against an intracellular parasite. Proc Natl Acad Sci U S A. 2006;103:6635–6640. doi: 10.1073/pnas.0506180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IA, Kasper LH. IL-15 augments CD8+ T cell-mediated immunity against Toxoplasma gondii infection in mice. J Immunol. 1996;157:2103–2108. [PubMed] [Google Scholar]
- Staeheli P, Puehler F, Schneider K, Göbel TW, Kaspers B. Cytokines of birds: conserved functions—a largely different look. J Interferon Cytokine Res. 2001;21:993–1010. doi: 10.1089/107999001317205123. [DOI] [PubMed] [Google Scholar]
- Weining KC, Schultz U, Münster U, Kaspers B, Staeheli P. Biological properties of recombinant chicken interferon-gamma. Europ J Immunol. 1996;26:2440–2447. doi: 10.1002/eji.1830261026. [DOI] [PubMed] [Google Scholar]
- Merlino PG, Marsh JA. The enhancement of avian NK cell cytotoxicity by thymulin is not mediated by the regulation of IFN-γ production. Dev Comp Immunol. 2002;26:103–110. doi: 10.1016/S0145-305X(01)00042-8. [DOI] [PubMed] [Google Scholar]
- Wünschmann A, Rejmanek D, Conrad PA, Hall N, Cruz-Martinez L, Vaughn SB, Barr BC. Natural fatal Sarcocystis falcatula infections in free-ranging eagles in North America. J Vet Diag Invest. 2010;22:282–289. doi: 10.1177/104063871002200222. [DOI] [PubMed] [Google Scholar]
- Mutalib A, Keirs R, Maslin W, Topper M, Dubey JP. Sarcocystis-associated encephalitis in chickens. Avian Dis. 1995;39:436–440. doi: 10.2307/1591891. [DOI] [PubMed] [Google Scholar]
- Hoogenboom I, Dijkstra C. Sarcocystis cernae - a parasite increasing the risk of predation of its intermediate host, Microtus arvalis. Oecologia. 1987;74:86–92. doi: 10.1007/BF00377350. [DOI] [PubMed] [Google Scholar]
- Quinn SC, Brooks RJ, Cawthorn RJ. Effects of the protozoan parasite Sarcocystis rauschorum on open-field behaviour of its intermediate vertebrate host, Dicrostonyx richardsoni. J Parasitol. 1987;73:265–271. [PubMed] [Google Scholar]
- Poulin R. “Adaptive” changes in the behaviour of parasitized animals: A critical review. Int J Parasitol. 1995;25:1371–1383. doi: 10.1016/0020-7519(95)00100-X. [DOI] [PubMed] [Google Scholar]