Abstract
Functional neuroimaging studies have largely established the prominence of amygdala during emotion processing and prefrontal areas such as anterior cingulate cortex (ACC) during attentional modulation. In general, emotion processing paradigms known to probe amygdala have not been adapted to recruit prefrontal areas. In this study we used a well-known perceptual face matching paradigm, designed to elicit amygdala response, and asked volunteers to shift their focus in order to recruit regions responsible for attentional control. Stimuli comprised a trio of geometric shapes (circles, rectangles, triangles) presented alongside a trio of emotional faces (angry, fear, or happy) within the same field of view, and subjects were instructed to Match Faces or Match Shapes, as a means of attending to and away from the emotional content, respectively. We observed greater amygdala reactivity to Match Faces (>Match Shapes), and greater rostral ACC response to Match Shapes (>Match Faces). Results indicate that simply and volitionally directing attention towards or away from emotional content correspondingly modulates amygdala and ACC activity.
Introduction
Facial expressions convey salient information and their motivational influence naturally captures attention [13]. Though among types of expressions, threat signals are thought to be most readily captured given their significance in responding to danger [17]. Much of the work delineating neural mechanisms of face processing can be traced to: 1) studies regarding the emotional influence of expressions and, 2) those concerning the effects of emotion on attentional control.
The former includes the examination of task-relevant face effects—that is, basic perceptual matching paradigms serve to isolate the influence of facial expressions by contrasting a matching face task with a sensorimotor control task (i.e., matching shapes) [11, 12]. In support of amygdala as a key emotion processing region [15], perceptual assessment paradigms have, for nearly a decade, consistently demonstrated robust amygdala responses (for review see [22]).
In contrast, attentional control paradigms are based on a biased competition model, in which top-down control is needed to supersede task-irrelevant distractors (e.g. emotional faces) to carry out cognitive goals [19]. Frequently used spatial tasks such as modified dot probe detection [3, 20] and “faces/houses” [2, 24] have in common a very brief temporal window of information processing. Namely, relevant and irrelevant stimuli (e.g., neutral versus threat faces) are rapidly presented (e.g., 250 ms or less) in the same field of view. Data showing enhanced anterior cingulate cortex (ACC) to task-irrelevant threat faces [2, 24] is consistent with findings of prefrontal recruitment when higher-order control is required (e.g., ACC, dorso- and ventrolateral prefrontal cortex areas [2–5, 16, 19, 24, 25].
In addition to prefrontal engagement, some of these paradigms also show amygdala response to threat faces [3, 24], which supports the function of amygdala in mediating attention to crude threat cues [15]. However, these paradigms are not well-validated probes of amygdala due to inconsistencies in amygdala results [2, 20].
In summary, simple perceptual matching paradigms reliably elicit amygdala response whereas more challenging attentional control paradigms are known to recruit prefrontal areas. Not well understood is prefrontal response over task-irrelevant emotional faces when the information processing window extends beyond very brief stimuli presentation. Hypothetically, prefrontal areas associated with sustained goal-directed attention should engage given neurophysiological evidence demonstrating emotional cues not only capture but sustain visual attention [10]. Yet, few paradigms exist that permit the evaluation of continued attentional control in the context of stimuli that robustly elicit emotion processing circuitry.
Accordingly, we modified the well-known perceptual face processing paradigm by configuring the traditional faces-only and shapes-only images to be in the same field of view. Here, subjects were instructed to “Match Faces” to engage emotion processing or “Match Shapes” to alter the focus of attention by shifting it away from faces. Over each 4 second trial, the emotional faces are still in full view and should regain attentional focus once the simple shapes matching task is successfully completed (Fig. 1). Based on the literature, we predicted: 1) amygdala reactivity when attending to emotional faces, 2) prefrontal (e.g., ACC, dorsolateral and ventrolateral prefrontal cortex) response when attending to shapes, and 3) attention-emotion interactions, specifically, threat versus happy expressions would enhance amygdala response during “Match Faces”, however, threat would enhance prefrontal areas for “Match Shapes”.
Figure 1.
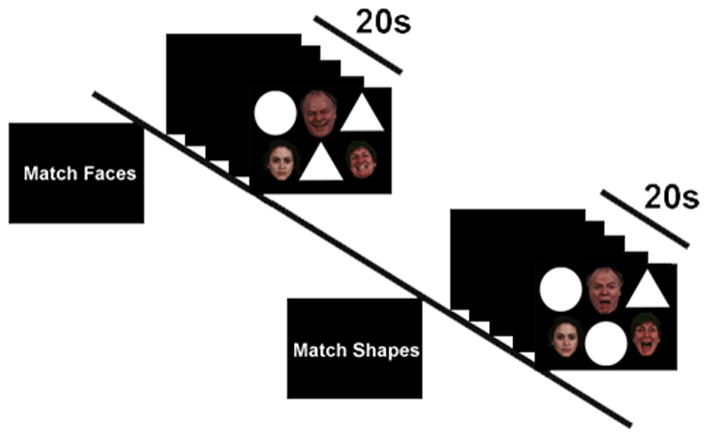
Schematic of an exemplar Match Faces and Match Shapes blocks in the functional magnetic resonance imaging (fMRI) paradigm.
Methods
Participants
There were 21 right-handed healthy adults (38% male; χ2 test for gender p=0.14) with a mean age 24.5 ± 5.3 years who were physically, neurologically, and psychiatrically healthy, as confirmed by a physician-conducted medical exam and psychiatric evaluation that included the Structured Clinical Interview for DSM-IV [6]. All participants provided written informed consent, as approved by the local Institutional Review Board.
Experimental Task
During fMRI participants performed our “Emotional Faces Shifting Attention Task” (EFSAT) comprising a trio of geometric shapes (circles, rectangles, triangles) alongside a trio of faces within the same field of view. For “Match Faces”, participants selected one of two bottom faces (neutral vs. emotional) that matched the emotion of the top target face, and similar instructions were used for “Match Shapes”. Consequently, “Match Shapes” was a baseline to “Match Faces” as opposed to a less cognitive, more ambiguous baseline (e.g., fixation) [23]. Face stimuli were from a validated stimulus set [9], the identities were always different, and an equal number of male and female faces were presented.
The paradigm comprised 36 blocks: 18 blocks of matching shapes interleaved with 18 blocks of matching emotional faces, counterbalanced across 2 runs. Each target face condition (angry, fear, happy) was presented for an entire block 6 times without repetition. Each 20 second ‘task’ block contained four sequential matching trials, 4 sec each, preceded by a 4-sec instruction image to either “Match Faces” (attend to faces) or “Match Shapes” (attend away from faces). Participants responded by pressing response buttons.
Functional imaging: acquisition and analysis
Functional imaging was performed with blood-oxygen-level-dependent (BOLD) sensitive whole-brain fMRI on a 3.0 Tesla GE Signa System (General Electric; Milwaukee, WI) using a standard radio frequency coil. Images were acquired from 30 axial, 5-mm-thick sli0ces using a standard T2*-sensitive gradient echo reverse spiral acquisition sequence (repetition time, 2000 ms; echo time, 25 ms; 64 × 64 matrix; 24 cm field of view; flip angle, 77). A high-resolution, T1-weighted volumetric anatomical scan was also acquired for anatomical localization. High quality and scan stability with minimum motion corrections was set at < 3 mm displacement in any one direction. Conventional preprocessing steps were used in Statistical Parametric Mapping (SPM5) software package (Wellcome Trust Centre for Neuroimaging, London; www.fil.ion.ucl.ac.uk/spm)[7]. Briefly, images were temporally corrected to account for differences in slice time collection, spatially realigned to the first image of the first run, normalized to a Montreal Neurological Institute (MNI) template, and smoothed with an 8 mm isotropic Gaussian kernel.
A general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128 s high-pass filter. Task effects of Match Faces (shapes in ‘background’) and Match Shapes (faces in ‘background’) and emotion effects of angry, fear, and happy faces were modeled with box-car regressors representing the occurrence of each block type, and effects were estimated at each voxel for each participant and taken to the second level for random effects analysis. In addition, six movement parameters obtained during realignment were included in the model as regressors to account for motion-related effects in BOLD signal.
Whole-brain voxel-wise Analysis of Variance (ANOVA) was conducted to evaluate main effects of Task (Match Faces vs. Match Shapes), Emotion (angry, fear, happy), and Task by Emotion interactions. A stringent threshold for significance was set at p<0.05, corrected for multiple comparisons across the entire brain using a False Discovery Rate with a cluster size of at least 10 contiguous voxels. Significant main effects and interactions were followed by post hoc t-tests to clarify the direction of effects.
Results
Whole-brain ANOVA revealed a robust main effect for Task in the right amygdala and right rostral anterior cingulate cortex (ACC). As expected, the post hoc t-test showed amygdala activity was greater for Match Faces than for Match Shapes (Fig. 2A), whereas rostral ACC activity was greater for Match Shapes than for Match Faces (Fig. 2B). The Match Faces>Match Shapes contrast also revealed activation of the primary visual (fusiform gyrus) and paralimbic (medial prefrontal gyrus, orbital frontal gyrus) areas whereas Match Shapes>Match Faces showed activation of visual association cortices (middle occipital, middle temporal gyrus, supramarginal gyrus) and prefrontal areas (middle and superior frontal gyrus). See Table 1 for all results. However, the main effect of Emotion or interaction between Task and Emotion were both non-significant.
Figure 2.
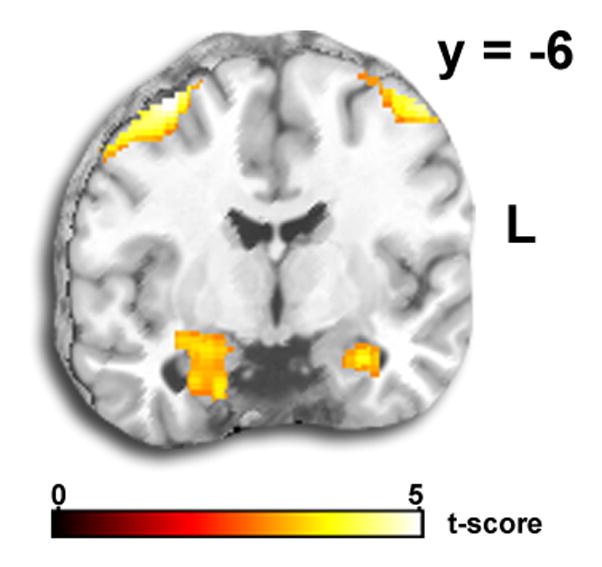
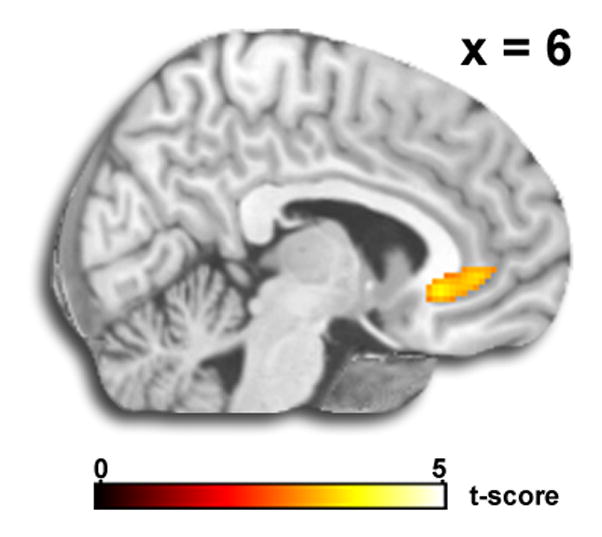
A) Voxel-wise statistical t-map displayed on a canonical brain showing amygdala activation to Match Faces (>Match Shapes). B) Voxel-wise statistical t-map displayed on a canonical brain showing rostral anterior cingulate cortex activation to Match Shapes (>Match Faces).
Table 1.
Whole-brain voxel-wise Analysis of Variance: Main effect of task; activation results presented at p<0.05 (false-discovery rate corrected for multiple comparisons across whole brain); cluster size >10 contiguous voxels
| Region | MNI Coordinates | Volume | F statistic | ||
|---|---|---|---|---|---|
| Lingual gyrusa | 28 | −98 | −2 | 147,216 | 209.16 |
| Fusiform gyrus | −42 | −46 | −16 | 51.36 | |
| Amygdala | 24 | −10 | −18 | 15.85 | |
| Inferior frontal gyrusa | −54 | 28 | 28 | 15,440 | 70.60 |
| Precentral gyrusa | 36 | −10 | 70 | 17,504 | 47.59 |
| Frontal superior medial gyrusa | 18 | 30 | 62 | 576 | 27.79 |
| Frontal inferior orbital gyrusa | 50 | 48 | 4 | 2,752 | 25.46 |
| Frontal middle orbital gyrusa | −26 | 36 | −16 | 496 | 24.16 |
| Middle occipital gyrusb | 42 | −72 | 8 | 1,144 | 23.83 |
| 44 | −80 | 38 | 120 | 12.71 | |
| Precentral gyrusa | −28 | 2 | 70 | 1,392 | 20.01 |
| Temporal middle gyrusb | 62 | −46 | −6 | 248 | 18.38 |
| Cerebellar tonsila | 40 | −52 | −50 | 144 | 16.80 |
| Frontal middle gyrusb | 24 | 24 | 24 | 1,152 | 16.66 |
| Anterior cingulate cortexb | 6 | 30 | −6 | 440 | 13.83 |
| Frontal superior gyrusb | −18 | 12 | 44 | 304 | 13.81 |
| Parietal inferior gyrusa | −30 | −56 | 36 | 160 | 13.18 |
| Dorsal medial frontal gyrusa | −2 | 18 | 50 | 280 | 12.87 |
| Supramarginal gyrusb | 58 | −26 | 32 | 168 | 12.43 |
| −60 | −44 | 34 | 544 | 12.21 | |
| Parietal superior gryusb | 16 | −60 | 52 | 88 | 11.43 |
| Temporal middle gyrusb | −60 | −28 | 0 | 80 | 10.93 |
A priori areas are shown in bold.
MNI, Montreal Neurological Institute
Match Faces>Match Shapes based on post hoc t-test
Match Shapes>Match Faces based on post hoc t-test
Discussion
To date, the delineation of emotional face processing networks primarily correspond to basic perceptual paradigms or cognitively demanding attentional modulation paradigms, which may tap into relatively distinct networks. Therefore, it is unclear to what extent attentional control mechanisms engage when the only cognitive goal is to pay attention to neutral stimuli amid a background of task-irrelevant emotional faces.
Our contribution to this gap in the literature is the development of an Emotional Faces Shifting Attention Task (EFSAT) to examine regions associated with attentional control in a widely used emotional faces paradigm well-known to elicit robust amygdala response (for review see [22]). By spatially combining the traditionally separate faces-only and shapes-only image trials into one trial within one field of view, attention was modulated by having it directed towards or away from emotional faces in order to complete the matching task.
Prior evidence led us to hypothesize that the Match Faces instruction would selectively engage emotion processing regions (e.g., amygdala; [11, 12]), whereas, Match Shapes would selectively engage prefrontal regions associated with top-down control (e.g., anterior cingulate cortex, dorsolateral and ventrolateral prefrontal cortex; [2–5, 16, 19, 24, 25]). As predicted, Match Faces (attend to angry, fear, or happy) versus Match Shapes (ignore angry, fear, or happy by attending to shapes) elicited an amygdala response.
In addition to amygdala, there was evidence of significant activation in other crucial emotion processing and visual areas such as fusiform, medial prefrontal, and orbitofrontal areas. Though these areas are commonly found in emotional face processing networks, we did not find evidence of activation in other areas previously implicated in emotion processing (e.g., parahippocampal gyrus, insula; [8, 21]). Our conversative analytic approach may have reduced detection of certain emotion processing areas, nevertheless, results indicate attention to faces effectively recruited key regions implicated in socio-emotional circuitry.
Our hypothesis regarding Match Shapes (ignore angry, fear, or happy by attending to shapes) versus Match Faces (attend to angry, fear, or happy) was also supported. Specifically, there was an anterior cingulate cortex (ACC) response. In this study, simply attending to shapes that were alongside emotional faces was sufficient to recruit rostral ACC. Along with data showing rostral ACC responds to task-irrelevant faces [2, 24], findings are in keeping with rostral ACC recruitment when ‘resolving’ emotional conflict [5]. Our results suggest rostral ACC engagement even when demands on attentional resources are relatively low. In light of the simplicity of the task and long stimuli presentation, attention directed to shapes likely shifted covertly or overtly, to task-irrelevant faces present in the same field of view. We speculate that rostral ACC activity, in its role to effectively resolve conflict [5] helped initiate and maintain control by attenuating salient face signals.
Our prediction of greater amygdala response to threat versus happy expressions was not supported. Lack of differential emotion effects has also been noted in other basic perceptual matching tasks in that amygdala activated regardless of emotion type (i.e., happy, threating faces) [1, 18]. Similar to amygdala response, emotion type did not modulate ACC activation. Together with evidence that positive and threat signals are motivationally relevant compared to neutral events (for review see [14]), failure of differential effects suggests more complex cognitive processes may modulate emotional signals when the temporal window of processing is prolonged [25].
Additionally, our hypothesis of other prefrontal recruitment (e.g., dorso- and ventrolateral prefrontal cortex) to Match Shapes versus Match Faces, and an effect of attentional task demands when processing certain emotional expressions (fear versus happy) were not supported. Potentially, focusing on shapes next to faces in the absence of other demands on attention did not exert the type of cognitive demand on higher order resources shown to elicit a more robust network of prefrontal regions [2–5, 16, 19, 24, 25].
Futhermore, the study has limitations and findings should be interpreted with caution. There was no non-cognitive baseline (e.g., fixation) condition; hence, findings cannot be interpreted in relation to a change from rest. Also, the lack of neutral target expressions does not permit dissociation between face- or emotion-processing influences. Lastly, the task failed to elicit differential activation to expression type.
Despite limitations, results indicate our modification of a basic perceptual task well-known to elicit amygdala response is adequately sensitive to recruit top-down control. It appears that a simple, volitional shift in attention away from emotional faces effectively engages anterior cingulate cortex, whereas, attention to faces elicits an amygdala response.
Highlights.
Perceptual face matching tasks have been shown to robustly elicit amygdala activity
We modified a well-known face matching task to examine attentional control
When attention was directed to emotional faces next to shapes, amygdala responded
When attention was focused on shapes alongside faces, anterior cingulate responded
Our modified perceptual matching task probes an area involved in attentional control
Acknowledgments
This work was supported by a grant from the National Institutes of Health, National Institute of Mental Health Patient-Oriented Career Development Award K23MH076198 (KLP) and by a grant from the National Center for Research Resources (NCRR) UL1RR024 986 (HK). The content is solely the responsibility of the authors and does not necessarily represent the official views of NCRR or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arce E, Simmons AN, Lovero KL, Stein MB, Paulus MP. Escitalopram effects on insula and amygdala BOLD activation during emotional processing. Psychopharmacology (Berl) 2008;196:661–672. doi: 10.1007/s00213-007-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- 3.Carlson JM, Reinke KS, Habib R. A left amygdala mediated network for rapid orienting to masked fearful faces. Neuropsychologia. 2009;47:1386–1389. doi: 10.1016/j.neuropsychologia.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 4.Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- 5.Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 6.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV-Patient Edition (SCID-P) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 7.Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995:189–210. [Google Scholar]
- 8.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34:418–432. [PMC free article] [PubMed] [Google Scholar]
- 9.Gur RC, Schroeder L, Turner T, McGrath C, Chan RM, Turetsky BI, Alsop D, Maldjian J, Gur RE. Brain activation during facial emotion processing. Neuroimage. 2002;16:651–662. doi: 10.1006/nimg.2002.1097. [DOI] [PubMed] [Google Scholar]
- 10.Hajcak G, Olvet DM. The persistence of attention to emotion: brain potentials during and after picture presentation. Emotion. 2008;8:250–255. doi: 10.1037/1528-3542.8.2.250. [DOI] [PubMed] [Google Scholar]
- 11.Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Arch Gen Psychiatry. 2005;62:146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- 12.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 13.Lang P, Simons RF, Balaban M, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Hillsdale, NJ: 1997. [Google Scholar]
- 14.Lang PJ, Bradley MM. Emotion and the motivational brain. Biological Psychology. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeDoux JE. The Emotional Brain. Touchstone; New York: 1996. [Google Scholar]
- 16.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 17.Ohman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 18.Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62:282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- 19.Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neutral and emotional stimuli. Brain Res Cogn Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 20.Pourtois G, Schwartz S, Seghier ML, Lazeyras F, Vuilleumier P. Neural systems for orienting attention to the location of threat signals: an event-related fMRI study. NeuroImage. 2006;31:920–933. doi: 10.1016/j.neuroimage.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 21.Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. NeuroImage. 2011;54:2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Sergerie K, Chochol C, Armony JL. The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2008;32:811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: an event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 25.Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci. 2007;362:837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]


