INTRODUCTION
Obesity is a global health concern. One of the complications associated with morbid obesity is obesity hypoventilation syndrome (OHS). OHS is defined by the triad of obesity (body mass index [BMI] ≥30 kg/m2), daytime hypoventilation with hypercapnia (partial pressure of arterial carbon dioxide [PacO2] ≥45 mm Hg at sea level), and hypoxemia (partial pressure of arterial oxygen [Pao2] less than 70 mm Hg at sea level), and sleep-disordered breathing.1 OHS is diagnosed after excluding other known causes of hypoventilation, such as severe obstructive or restrictive parenchymal lung disease, kyphoscoliosis, severe hypothyroidism, neuromuscular disease, and congenital central hypoventilation syndrome. In 90% of cases of OHS, the sleep-disordered breathing present is obstructive sleep apnea (OSA).2 The prevalence of OHS in the general adult population is estimated to be 0.15% to 0.3%.2 In patients undergoing bariatric surgery, approximately 8% present with OHS.3
Patients with OHS have a higher burden of comorbidities and increased risk for perioperative morbidity and mortality.4-6 Therefore, a thorough plan of evaluation and management is essential for patients with OHS who undergo surgery. Currently, information on the perioperative management of OHS is extremely limited in the literature. As the prevalence of OHS is likely to increase as a result of the current global obesity epidemic, it is crucial for physicians to recognize and manage patients with this syndrome. This review examines the current data on OHS and discusses its optimal perioperative management.
PATHOPHYSIOLOGY
Daytime hypercapnia is a distinguishing feature in OHS and is entirely due to hypoventilation; a short course of noninvasive positive airway pressure (PAP) therapy (<2 weeks) improves hypercapnia without any significant changes in body weight, carbon dioxide (CO2) production, or dead space volume.7 There are currently 3 main hypotheses regarding the development of OHS: obesity-induced impairment in respiratory mechanics, leptin resistance, and impaired compensation for acute hypercapnia in OSA.2,8
Obesity-Induced Impairment in Respiratory Mechanics
Obesity induces hypoventilation by increasing the mechanical load on the respiratory system, resulting in fatigue and weakness of the respiratory muscles.9-11 In several studies, patients with OHS were shown to have higher BMI than eucapnic obese individuals.12-15 However, because less than one third of morbidly obese individuals develop hypercapnia, other mechanisms may contribute to hypoventilation.14-16
Leptin Resistance
Leptin is a protein secreted specifically by adipocytes to regulate appetite and energy expenditure.17-19 Leptin crosses the blood-brain barrier and exerts its effect by binding to leptin receptors in various areas of the brain.18 In obese individuals, a higher level of leptin is found to be associated with an increase in ventilation to compensate for the increased CO2 production by excess body mass.17,20,21 Patients with OHS have an even higher serum leptin level than eucapnic individuals matched for BMI.22,23 Although the precise relationship between leptin and OHS remains to be determined, it is speculated that leptin resistance may lead to central hypoventilation in OHS.
Impaired Compensation of Acute Hypercapnia in OSA
Hypoventilation during sleep secondary to obstructive apneas and hypopneas results in transient episodes of acute hypercapnia and serum bicarbonate (HCO3−) retention. Eucapnic patients with OSA present several compensatory mechanisms to maintain acid-base homeostasis. During sleep, they hyperventilate between periods of apnea.24 In addition, during wakefulness in daytime, acute hypercapnia is corrected and the excess HCO3− is excreted. On the other hand, patients with OHS have a reduced duration of ventilation between periods of apnea while sleeping.25 The resulting acute hypercapnia persists during wakefulness and HCO3− retention occurs, causing gradual adaptation by chemoreceptors and further blunting of ventilatory CO2 responsiveness. In a computer model, when both CO2 response and the rate of renal HCO3− excretion was abnormally low, an increase in awake PacO2 and HCO3− developed over multiple days.26
DISTINGUISHING CLINICAL FEATURES
Compared with eucapnic obese individuals with and without OSA, patients with OHS demonstrate more severe upper airway obstruction, restrictive pulmonary physiology, blunted central respiratory drive, and increased incidence of pulmonary hypertension. Patients with OHS display increased upper airway resistance in both the sitting and supine position in comparison with obese individuals with eucapnia.27 In perioperative settings, patients with OHS are at increased risk of life-threatening apneic events because sedatives and narcotics increase the collapsibility of the upper airway and attenuate respiratory drive.28,29
Spirometric values from morbidly obese patients typically reveal a restrictive pattern with a reduction in forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) but normal FEV1/FVC ratio. This restrictive pulmonary physiology is further impaired in OHS.3 Chest wall compliance is reduced and respiratory resistance is increased, likely secondary to the reduction in functional residual capacity and expiratory reserve volume. As a result, the work of breathing in OHS patients is twice that of obese eucapnic individuals30,31 and increases further when these patients are positioned supine from sitting as a result of the cephaled shift of abdominal contents.30,32
In contrast to obese eucapnic individuals who possess a substantially increased central respiratory drive,32 patients with OHS have a blunted central respiratory drive to both hypercapnia and hypoxia. They do not hyperventilate to the same extent as obese eucapnic individuals when forced to rebreathe CO233-35 or breathe a hypoxic gas mixture.35
The prevalence of pulmonary hypertension in patients with OHS is high, ranging from 30% to 88%.4,36-38 Seventy-seven percent of patients with OHS with respiratory failure in the intensive care unit have moderate to severe pulmonary hypertension (pulmonary systolic pressure >45 mm Hg).37 The cause of pulmonary hypertension is likely secondary to chronic alveolar hypoxia and hypercapnia. In morbidly obese patients, left-heart failure is not uncommon and may increase pulmonary arterial pressure.39
MORBIDITY AND MORTALITY
Obesity and OSA are associated with a spectrum of comorbidities such as coronary artery disease, heart failure, stroke, and metabolic syndrome, which result in increased morbidity and mortality.40-44 Furthermore, patients with OSA are at increased risk of developing postoperative complications including arrhythmias and hypoxemia.45-47 An increased risk of transfer to the intensive care unit and increased length of hospital stay were also observed among patients with OSA who underwent noncardiac surgery.46
Several studies have shown that patients with OHS may experience higher morbidity and mortality than patients who are similarly obese and have OSA. Compared with obese individuals with eucapnia, patients with OHS were more likely to develop heart failure (odds ratio [OR] 9, 95% confidence interval [CI] 2.3–35), angina pectoris (OR 9, 95% CI 1.4–57.1), and cor pulmonale (OR 9, 95% CI 1.4–57.1).4 They also received higher rates of long-term care at discharge (19% vs 2%, P = .01), and invasive mechanical ventilation (6% vs 0%, P 5 .01).48
Hospitalized patients with untreated OHS had a high mortality rate of 46% during a 50-month follow-up period after discharge.49 In addition, their mortality rate is higher compared with obese eucapnic patients after hospital discharge at 18 months (23% vs 9%).48 In patients undergoing open bariatric surgery, those with either OHS or OSA suffered a surgical mortality rate of 4%, significantly higher than those without the disease (0.2%, P<.01).50 In patients with OHS with additional risk factors (previous history of venous thromboembolism, BMI ≥50 kg/m2, male sex, hypertension and age ≥45 years) undergoing bariatric surgery, mortality ranges between 2% and 8%.5,6,51
In summary, patients with OHS experience higher morbidity and mortality than those with eucapnia who are obese. Previous history of venous thromboembolism, morbid obesity, male sex, hypertension, increasing age, and noncompliance with PAP treatment may further increase mortality risk. The surgical mortality rate in high-risk patients with OHS undergoing bariatric surgery is between 2% and 8%.
TREATMENT
Therapeutic interventions for OHS include 4 main components: PAP therapy, supplemental oxygen, bariatric surgery, and pharmacologic respiratory stimulants.
PAP Therapy
The 2 main forms of PAP therapy currently being used are continuous positive airway pressure (CPAP) and bilevel PAP. The overall short-term and long-term benefits were summarized in a recent systematic review.3
Short-term benefits of PAP include an improvement in gas exchange and sleep-disordered breathing. A short course (≤3 weeks) of PAP results in a significant decrease in PacO2 and an increase in Pao2.49,52-54 Furthermore, sleep study parameters, including the apnea-hypopnea index (AHI) and oxygen saturation during sleep, were reported to be significantly improved.53-56
Long-term benefits of PAP include an improvement in pulmonary function and central respiratory drive to CO2. A course of PAP therapy for 24 to 48 weeks significantly increased FEV1 and FVC.57-59 The effect of PAP on central respiratory drive, measured as the change in minute ventilation per unit change in end-tidal CO2, was reported in several studies to be favorable.52,58,60
PAP may also reduce mortality in OHS. Two retrospective studies have reported a mortality rate of 13% to 19% in patients with OHS on PAP throughout a mean period of 4 years.57,61 Through indirect comparison, this mortality rate is lower than the 23% mortality rate reported in patients with untreated OHS at 18 months of follow-up.48
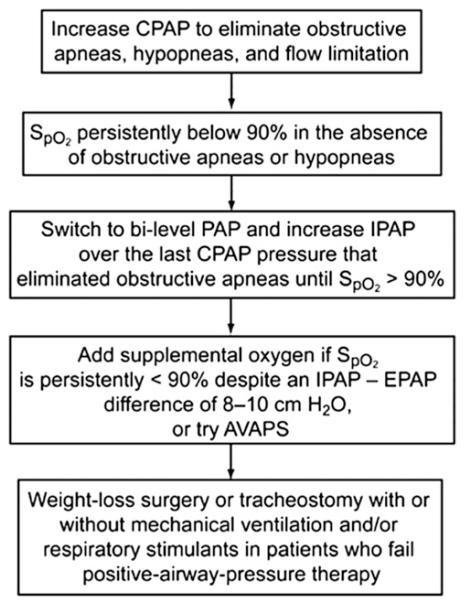
CPAP failure, defined by a residual AHI of 5 or more or a mean nocturnal pulse oximeter oxygen saturation (SpO2) less than 90%, has been reported.62 A recent prospective randomized study compared the long-term efficacy of bilevel PAP versus CPAP.63 Two groups of 18 patients with OHS who underwent successful CPAP titration were randomized to either bilevel PAP or CPAP for 3 months. Both groups experienced a similar degree of improvement in PacO2 and daytime sleepiness. Overall, bilevel PAP was not considerably superior to CPAP if CPAP titration was successful. However, if CPAP titration is unsuccessful, bilevel PAP should be strongly considered and treatment should be individualized to each patient.2 Bilevel PAP should be instituted if the patient is intolerant of higher CPAP pressure (>15 cm H2O) or if hypoxemia persists despite adequate resolution of obstructive respiratory events.64 A therapeutic algorithm for CPAP titration in OHS patients is shown in Fig. 1.
Fig. 1.
Algorithm for continuous PAP titration in patients with OHS. AVAPS, average volume-assured pressure-support ventilation; CPAP, continuous positive airway pressure; EPAP, expiratory positive airway pressure; IPAP, inspiratory positive airway pressure.
A new treatment modality in patients with OHS is average volume-assured pressure-support (AVAPS) ventilation. This mode of PAP therapy ensures the delivery of a preset tidal volume during bilevel PAP mode. The expiratory tidal volume and leak are estimated based on pneumotacographic inspiratory and expiratory flows. Target tidal volume is typically set at 8 to 10 mL/kg of ideal body weight. The expiratory PAP is set to resolve upper airway obstruction and the inspiratory PAP is automatically adjusted to achieve the targeted tidal volume. This mode of PAP also provides a backup rate to alleviate central apneas that may emerge during PAP therapy.65 In 1 clinical trial, AVAPS was more effective in improving nocturnal hypoventilation compared with CPAP and bilevel PAP in ST mode (activated backup rate). However, the degree of improvement in daytime PacO2 did not reach statistical significance.66 In a recent randomized controlled trial of patients with OHS, there was no significant difference between AVAPS and bilevel PAP ST mode in the degree of improvement in daytime and nocturnal gas exchange.67 However, in this study, those randomized to bilevel PAP ST mode underwent aggressive bilevel PAP titration focusing on achieving adequate tidal volumes (mean inspiratory PAP of 23 cm H2O and mean expiratory PAP of 10 cm H2O), as recommended by the American Academy of Sleep Medicine guidelines on noninvasive ventilation.68
In summary, PAP therapy improves gas exchange, sleep-disordered breathing, lung function, and central respiratory drive to CO2. Long-term PAP therapy also lowers the mortality rate in patients with OHS. Because of its noninvasiveness and effectiveness, PAP is the first-line therapy for OHS. CPAP is usually the initials modality of choice because of its relative simplicity and low cost. However, if CPAP titration fails, bilevel PAP or AVAPS should be applied. A need for a backup rate should be strongly considered because central apneas occur commonly in patients with OHS undergoing PAP therapy.
Supplemental Oxygen
Approximately 20% to 30% of patients with OHS continue to desaturate to SpO2 less than 90% during sleep while on adequate CPAP or bilevel PAP settings, thereby requiring supplemental oxygen.55 Administration of high-concentration supplemental oxygen without PAP therapy may induce hypoventilation and worsen hyercapnia.69,70 In a recent study, a significant decrease in minute ventilation, resulting in an increase in transcutaneous CO2 tension by 5 mm Hg, was found in patients with OHS while breathing 100% oxygen compared with those breathing room air.71 Therefore, clinicians should administer the lowest concentration of oxygen to patients with OHS to avoid worsening of hypercapnia while maintaining optimized oxygenation, particularly in patients with OHS experiencing an exacerbation or recovering from sedatives/narcotics or general anesthesia.72
Bariatric Surgery
Bariatric surgery is a mainstay treatment of obesity, especially for morbidly obese patients in whom more conservative approaches have failed or who have developed comorbidities. Bariatric surgery improves gas exchange and lung function in OHS. At 1 year after surgery, Pao2, PacO2, FEV1, and FVC all improved significantly.50,73 To better understand the effect of surgical weight loss on OSA, Greenburg and colleagues74 performed a meta-analysis of 12 studies including a total of 342 patients in whom polysomnography before and after maximum weight loss were available. They found that bariatric surgery led to significant weight loss with a mean reduction in BMI from 55.3 kg/m2 to 37.7 kg/m2. This robust weight loss was accompanied by a reduction in the AHI from baseline values of 55 events/h (95% CI 49–60) to 16 events/h (95% CI 13–19). However, many of these patients (62%) had persistent OSA of moderate severity (AHI ≥15 events/h). Thus, although improvements should be anticipated, OSA does not resolve in all patients after surgically achieved weight loss. CPAP therapy could still benefit patients with residual OSA after maximum weight loss. Similarly, 14% of patients with OHS still require PAP therapy after weight loss.73 Therefore, patients with OHS should undergo reevaluation after bariatric surgery before discontinuing PAP therapy. As patients age and/or regain some weight over the years, the severity of OSA can increase.
Bariatric surgery is associated with significant risk. The overall perioperative mortality ranges between 0.5% and 1.5%.75,76 The presence of OSA and extreme preoperative weight are independent risk factors associated with perioperative death and adverse events including venous thromboembolism, surgical reintervention, and prolonged hospital stay.76,77 Ideally, PAP should be initiated in all patients with OHS and bariatric surgery should be considered as a second-line intervention.
Pharmacotherapy
Medications that increase respiratory drive have been investigated for the treatment of OHS. Limited evidence is available for 2 respiratory stimulants: medroxyprogesterone acetate and acetazolamide.
Medroxyprogesterone acetate stimulates respiration at the hypothalamic level.78 Its role in OHS is uncertain. An early study reported an increase in Pao2 and a decrease in PacO2 in patients with OHS treated with medroxyprogesterone acetate.79 However, a later study did not demonstrate the same benefits.7 Because medroxyprogesterone acetate increases the risk of venous thromboembolism,80 administration to patients with OHS whose mobility is limited may be unsafe.
Acetazolamide is a carbonic anhydrase inhibitor that increases minute ventilation by inducing metabolic acidosis through increased excretion of bicarbonate by the kidneys. Acetazolamide has been shown to improve AHI, increase Pao2, and reduce PacO2 in patients with OSA.81,82 More recently, in mechanically ventilated patients with OHS, acetazolamide reduced plasma HCO3− and increased hypercapnic drive response.83 Given the limited data on pharmacotherapy and because it is not widely used, the authors do not recommend it as a mainstay therapy but rather an adjunctive therapy in patients with OHS who remain hypercapnic despite adequate adherence to optimally titrated PAP therapy. Specifically, OHS patients requiring high doses of loop diuretics, which can lead to further HCO3− retention, may be ideal candidates for acetazolamide. Caution should be exercised in prescribing a respiratory stimulant in patients with ventilatory limitation because it can lead to exacerbation of acidosis and worsening of dyspnea. Acetazolamide should not be prescribed as a respiratory stimulant if a patient cannot normalize or near normalize their PacO2 (or endtidal CO2) levels with 1 to 2 minutes of voluntary hyperventilation.
PREOPERATIVE ASSESSMENT OF PATIENTS WITH OHS
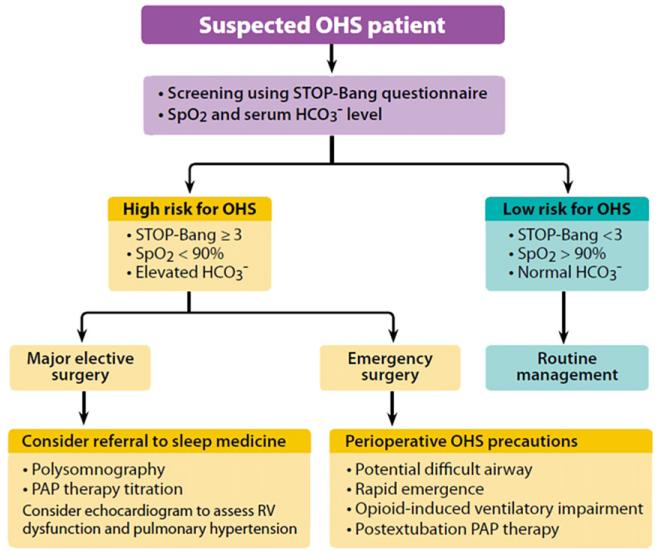
The 3 main challenges in OHS are OSA, obesity, and hypoventilation (hypercapnia and hypoxemia). For a patient with suspected OHS presenting for elective surgery, the preoperative assessment should begin with the history and physical examination directed to identify comorbidities in OHS, including coronary artery disease, congestive heart failure, pulmonary hypertension, and diabetes mellitus. A detailed examination of the airway and sites for venous access should also be performed. Further laboratory and imaging investigations should be focused on screening for sleep-disordered breathing and stratification of surgical risk. An algorithm for the perioperative evaluation and management of OHS is given in Fig. 2.
Fig. 2.
Perioperative management of the patient suspected to have OHS. HCO3−, serum bicarbonate; RV, right ventricular. (Adapted from Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care 2010;55:1347–62; with permission.)
Preoperative Screening for OHS
OHS is often undiagnosed and may increase perioperative risk. Appropriate screening facilitates the identification of patients at risk for OHS, referral to sleep medicine for PAP therapy, and modifications in the surgical approach, anesthetic technique, and postoperative monitoring.
The definitive test for alveolar hypoventilation is an arterial blood gas performed on room air during wakefulness. As this is a relatively invasive procedure, several screening tools have been proposed. Mokhlesi and colleagues15 suggested 3 clinical predictors of OHS: serum HCO3−, AHI, and lowest oxygen saturation during sleep. Increased serum HCO3− level caused by metabolic compensation of chronic respiratory acidosis is common in patients with OHS. In a cohort of obese patients with OSA referred to the sleep laboratory for suspicion of OSA, a serum HCO3− threshold of 27 mEq/L demonstrated a 92% sensitivity in predicting hypercapnia on arterial blood gas.15 To complement the highly sensitive serum HCO3−, a highly specific (95%) AHI threshold of 100 was identified. A 2-step screening process was proposed, with serum HCO3− as the initial test to exclude patients without OHS and then AHI asthe second test to improve specificity. In addition, hypoxemia (Spo2 <90%, corresponding to Pao2 <60 mm Hg)84 during wakefulness should lead clinicians to suspect OHS in patients with OSA. In a recent meta-analysis, patients with OSA and higher BMI, higher AHI, and more restrictive chest wall mechanics were more likely to develop OHS.85 In these patients with OHS, the mean BMI, AHI, percent predicted FEV1, and percent predicted FVC were 39 kg/m2, 64 events/h, 71%, and 85%, respectively.
In summary, patients presenting with a high BMI and AHI should alert the physician to screen for OHS. The serum HCO3− is an easy initial screening test. If it is increased or hypoxemia by room air Spo2 during wakefulness is present, a measurement of arterial blood gases, or end-tidal CO2, is recommended. Once hypercapnia is confirmed, referral to sleep medicine and further testing, such as pulmonary function testing, chest imaging, measurement of thyroid-stimulating hormone level, and clinical assessment of neuromuscular strength, should be considered to rule out other important causes of hypoventilation.
Preoperative Screening for OSA
OSA screening in patients suspected to have OHS provides valuable information that may modify perioperative management. Approximately 90% of patients with OHS present with OSA, therefore a positive screen increases the index of suspicion for OHS. Multiple screening tools have been developed to evaluate patients at risk for OSA. The STOP-Bang questionnaire was used in preoperative patients.86,87 It is a scoring model combining the STOP (snoring, tiredness, observed apneas, and increased blood pressure) questionnaire and Bang (BMI ≥35 kg/m2, age >50 years, neck circumference >40 cm, and male gender). A systematic review has suggested using the STOP-Bang questionnaire in the surgical population due to its high methodological quality and easy-to-use features.88 A positive screen (≥3 questions answered yes) is highly sensitive for moderate to severe OSA and is useful to exclude patients with the disease. On the other hand, patients with an STOP-Bang score of 5 to 8 have been shown to be at higher risk for moderate to severe OSA.89 If these patients present with concurrent morbid obesity, they are at high risk for OHS and should be referred to sleep medicine for further evaluation.
In summary, the STOP-Bang questionnaire is a validated and easy tool to screen for OSA as part of the preoperative evaluation for patients with suspected OHS. A high STOP-Bang score with coexisting morbid obesity indicates an increased risk for OHS.
Preoperative Risk Stratification and Cardiopulmonary Testing
The Lee revised cardiac risk index represents a valuable tool to predict cardiac risk for elective major noncardiac surgery in the general population.90 However, other risk factors specifically related to OHS, such as pulmonary hypertension and a history of venous thromboembolism, should be considered when evaluating perioperative risk. The Obesity Surgery Mortality Risk Score was developed for patients undergoing gastric bypass, and includes 5 risk factors: hypertension, BMI of 50 kg/m2 or greater, male sex, age 45 years or more, and known risk factors for pulmonary embolism (OHS, previous thromboembolism, preoperative vena cava filter, pulmonary hypertension).5,6,51 This risk score stratifies mortality risk into low (0 or 1 comorbidity), intermediate (2 to 3 comorbidities) and high (4 to 5 comorbidities). Mortality rates were 0.2%, 1.2%, and 2.4% for low-risk, intermediate-risk, and high-risk classes, respectively.6 The most common causes of death were pulmonary embolism (30%), cardiac causes (27%), and gastrointestinal leak (21%).
A 12-lead electrocardiogram and chest radiograph should be obtained in patients suspected to have OHS to evaluate for coronary artery disease, congestive heart failure, and pulmonary hypertension. Indications for further cardiovascular testing should be based on the patient’s cardiovascular risk factors and the invasiveness of surgery according to current American Heart Association guidelines.90,91 The assessment of functional capacity is of particular importance in obese individuals because cardiorespiratory fitness levels and the postoperative complication rate are inversely related to BMI.92,93 If these patients are undergoing major surgery and present with multiple cardiac risk factors, stress testing and transthoracic echocardiogram may be considered if management may be changed.91
Studies evaluating postoperative pulmonary complications have generally found no increased risk attributable to obesity.94 However, patients with OSA were found to have a higher risk of pulmonary complications than patients without OSA in a recent retrospective study.95 Routine pulmonary function tests may not translate into an effective risk prediction for postoperative pulmonary complications in noncardiothoracic surgery.96 However, if coexisting chronic obstructive pulmonary disease is suspected in the patient with OHS, spirometry may be considered for diagnosis and subsequent optimization.
INTRAOPERATIVE MANAGEMENT OF OHS
Key considerations specific to the intraoperative management of OHS include airway management and emergence from anesthesia.
Airway Management
OSA is a risk factor for both difficult mask ventilation and tracheal intubation.97 In addition, patients with severe OSA (AHI ≥40 events/h) showed a significantly higher prevalence of difficult intubation than patients with lower AHI.98
Obesity results in a threefold increase in difficulty with mask ventilation.99 Whether obesity increases the difficulty of tracheal intubation is more controversial. A retrospective study of 18,500 surgical patients reported that obesity is a risk factor for difficult intubation.100 However, other studies have not found an association between BMI and intubation difficulties.101,102 More recently, Kheterpal and colleagues103 identified 5 risk factors (limited mandibular protrusion, thick/obese neck anatomy, OSA, snoring, and BMI more than 30 kg/m2) as independent predictors of difficult mask ventilation and intubation during anesthesia induction, which suggests that patients with OHS with limited mandibular protrusion are in the highest risk group for airway complications.
During induction of anesthesia, patients with OHS should be placed in the ramp position with tilting of the torso and head by 25°. This position has been shown to improve the glottic view during intubation and reduce atelectasis.104 Preoxygenation for more than 3 minutes with a tightly fitted mask can increase apnea tolerance time. A variety of airway adjuncts and skilled anesthesiology assistance should be made available in advance. Awake fiber optic intubation should be considered in patients with OHS with markers for difficult mask ventilation and intubation. In situations during which a patient with OHS is hypoxemic, concomitant use of PAP during fiber optic intubation prevents further deterioration of oxygen saturation.105,106 In addition, PAP splints the airway open and thus facilitates the visualization of anatomic landmarks.107
Emergence from Anesthesia
Patients with OHS are sensitive to the respiratory depressant effects of anesthetic agents due to the propensity for airway collapse, sleep deprivation, and blunting of physiologic response to hypercapnia and hypoxemia. A semi-upright or lateral position is recommended at the end of surgery for better oxygenation and airway maintenance.108 Rapid emergence from anesthesia is preferred because tracheal extubation should be performed only after the patient is fully conscious. A systematic analysis of the literature comparing postoperative recovery after propofol, isoflurane, desflurane, and sevoflurane-based anesthesia in adults demonstrated that early recovery was faster in the desflurane and sevoflurane groups.109 Another strategy to accelerate emergence is to decrease volatile anesthetic requirement and minimize washout time from fat/muscle by using other short-acting anesthetic adjuvants, such as remifentanil, or a combined general regional anesthetic.29
POSTOPERATIVE MANAGEMENT OF OHS
Key considerations specific to the postoperative management of OHS include monitoring for opioid-induced ventilatory impairment (OIVI) and prompt use of PAP therapy. The dual roles of postoperative PAP therapy are to prevent and treat respiratory failure secondary to sleep-disordered breathing.
OIVI
OIVI induces central respiratory depression, decreased level of consciousness, and upper airway obstruction, ultimately resulting in alveolar ventilation. The incidence of OIVI after major surgery varies with the different routes of opioid administration. The incidence of decreased respiratory rate was 0.8%, 1.2%, and 1.1% for intramuscular, intravenous patient-controlled analgesia, and epidural analgesia, respectively.110 The incidence of oxygen desaturation was 37%, 11.5%, and 15.1% for intramuscular, intravenous patient-controlled analgesia, and epidural analgesia, respectively.110 Patients with OHS could be at significant risk for OIVI because of their susceptibility to upper airway obstruction, depressed central respiratory drive, and impaired pulmonary mechanics. An opioid-sparing analgesic regimen, including local anesthetic–infused nerve block catheters and nonopioid adjuncts (acetaminophen, nonsteroidal antiinflammatory drugs), should be considered in these patients.
Improved postoperative monitoring is key in reducing the risk of OIVI. Patient-specific, anesthetic, and surgical factors determine the requirements for postoperative monitoring. Patients with OHS undergoing major surgery who require high doses of postoperative opioid should be monitored with continuous oximetry. Recurrent respiratory events in the postanesthesia care unit, including apnea for 10 seconds or more, bradypnea of less than 8 breaths/min, pain-sedation mismatch, or desaturations to less than 90%, can be used to identify patients at high risk of postoperative respiratory complications.111 Recently, Macintyre and colleagues112 proposed that sedation level is a more reliable sign of OIVI than respiratory rate because multiple reports suggest that OIVI is not always accompanied by a decrease in respiratory rate.112-114 Thus, sedation scoring systems should be used postoperatively to recognize OIVI so that appropriate interventions are triggered. In patients with OHS requiring high doses of postoperative opioids, sedation monitoring should be considered every 1 to 2 hours for the first 24 hours.115
Postoperative PAP Therapy: Prevention of Respiratory Failure
There is limited evidence demonstrating a reduction in postoperative complications with PAP in patients with OHS. However, a case series of 14 patients with OSA suggested that the use of CPAP continuously for 24 to 48 hours after extubation may reduce the risk of postoperative complications.116 In addition, PAP was found to decrease respiratory failure after extubation in severely obese patients admitted to the intensive care unit (absolute risk reduction of 16%).117 Subgroup analysis of patients with hypercapnia showed reduced hospital mortality in the PAP group compared with the control group. Other potential benefits of postoperative PAP include reduced hemodynamic fluctuations and arrhythmia related to hypoxemia.
In summary, patients with OHS who were previously on PAP should resume therapy as soon as possible postoperatively. In patients suspected to have OHS experiencing postoperative ventilatory impairment, PAP should be considered. Based on the available literature, patients with OHS typically require an inspiratory PAP and the expiratory PAP of 16 to 22 cm H2O and 9 to 10 cm H2O, respectively, to achieve adequate resolution of upper airway obstruction and to improve ventilation. Bilevel PAP can be empirically set at these pressures in patients suspected of having OHS.
Postoperative PAP Therapy: Treatment of Respiratory Failure
Although the incidence of postoperative respiratory failure in patients with OHS is unknown, these patients are particularly susceptible to cardiopulmonary complications secondary to increased respiratory load, blunted central drive, pulmonary hypertension, and impaired ventricular function. In the postoperative period, these patients may decompensate acutely due to multiple factors, including sedation, sleep deprivation, and deconditioning.118 Of concern, misdiagnosis is common if the physician is not aware of the potential for sleep-disordered breathing causing acute cardiopulmonary failure.37,118 It was reported that 77% of patients with OHS admitted to the intensive care unit for hypercapnic respiratory failure were erroneously diagnosed and treated for chronic obstructive pulmonary disease/asthma.37
Four presentations of acute cardiopulmonary failure may be encountered postoperatively: hypercapnic respiratory failure, acute congestive heart failure, acute cor pulmonale, and sudden death, an extreme manifestation. The mechanisms leading to the development of such complications were described by Carr and colleagues118 in detail. A high index of suspicion and early initiation of PAP therapy are key in managing patients with suspected OHS who develop respiratory failure postoperatively. Adjunctive interventions include judicious sedation/analgesia, minimal sleep disruption at night, and close follow-up with a sleep specialist.
SUMMARY
OHS is an important disease entity that requires the anesthesiologist’s thorough understanding. The prevalence of OHS is estimated to be 0.15% to 0.3% in the general population and 8% in patients undergoing bariatric surgery.2,3 Patients with OHS present with severe upper airway obstruction, restrictive pulmonary physiology, blunted central respiratory drive, and pulmonary hypertension. The primary therapy for OHS is PAP.
Perioperative management begins with a high index of suspicion for OHS in the morbidly obese patient. Screening questionnaires such as the validated STOP-Bang questionnaire can identify patients at high risk of OSA. This screening tool can be further complemented by the presence of low SpO2, increased end-tidal CO2 or PacO2, and serum HCO3− level to identify patients at high risk of OHS. Before major elective surgery, these patients should be referred to sleep medicine for polysomnography and PAP titration. An echocardiogram should be considered to assess right ventricular function and pulmonary hypertension. Perioperative precautions for OHS include prudent airway management, rapid emergence, monitoring for ventilatory impairment, and early resumption of PAP therapy. Future research should focus on the perioperative strategies of screening, monitoring, and treatment of OHS and associated complications.
KEY POINTS.
Obesity hypoventilation syndrome (OHS) is an important disease entity that requires the anesthesiologist’s thorough understanding.
Patients with OHS present with severe upper airway obstruction, restrictive pulmonary physiology, blunted central respiratory drive, and pulmonary hypertension. The primary therapy for OHS is positive airway pressure (PAP) therapy.
Screening questionnaires such as the validated STOP-Bang questionnaire can identify patients at high risk of obstructive sleep apnea.
Before major elective surgery, these patients should be referred to sleep medicine for polysomnography and PAP titration.
Future research should focus on the perioperative strategies of screening, monitoring, and treatment of OHS and associated complications.
REFERENCES
- 1.Olson A, Zwillich C. The obesity hypoventilation syndrome. Am J Med. 2005;118:948–56. doi: 10.1016/j.amjmed.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 2.Mokhlesi B. Obesity hypoventilation syndrome: a state-of-the-art review. Respir Care. 2010;55:1347–62. [PubMed] [Google Scholar]
- 3.Chau E, Lam D, Wong J, et al. Obesity hypoventilation syndrome: a review of epidemiology, pathophysiology, and perioperative considerations. Anesthesiology. 2012;117:188–205. doi: 10.1097/ALN.0b013e31825add60. [DOI] [PubMed] [Google Scholar]
- 4.Berg G, Delaive K, Manfreda J, et al. The use of health-care resources in obesity-hypoventilation syndrome. Chest. 2001;120:377–83. doi: 10.1378/chest.120.2.377. [DOI] [PubMed] [Google Scholar]
- 5.DeMaria E, Portenier D, Wolfe L. Obesity surgery mortality risk score: proposal for a clinically useful score to predict mortality risk in patients undergoing gastric bypass. Surg Obes Relat Dis. 2007;3:134–40. doi: 10.1016/j.soard.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 6.DeMaria E, Murr M, Byrne TK, et al. Validation of the obesity surgery mortality risk score in a multi-center study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg. 2007;246:578–82. doi: 10.1097/SLA.0b013e318157206e. [DOI] [PubMed] [Google Scholar]
- 7.Rapoport DM, Garay SM, Epstein H, et al. Hypercapnia in the obstructive sleep apnea syndrome. A reevaluation of the “Pickwickian syndrome”. Chest. 1986;89:627–35. doi: 10.1378/chest.89.5.627. [DOI] [PubMed] [Google Scholar]
- 8.Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med. 2011;183:292–8. doi: 10.1164/rccm.201008-1280CI. [DOI] [PubMed] [Google Scholar]
- 9.Aldrich T, Arora NS, Rochester DF. The influence of airway obstruction and respiratory muscle strength on maximal voluntary ventilation. Am Rev Respir Dis. 1982;126:195–9. doi: 10.1164/arrd.1982.126.2.195. [DOI] [PubMed] [Google Scholar]
- 10.Ladosky W, Botelho MA, Albuquerque JP., Jr. Chest mechanics in morbidly obese non-hypoventilated patients. Respir Med. 2001;95:281–6. doi: 10.1053/rmed.2001.1035. [DOI] [PubMed] [Google Scholar]
- 11.Lavietes M, Clifford E, Silverstein D, et al. Relationship of static respiratory muscle pressure and maximum ventilatory ventilation. Respiration. 1979;38:121–6. doi: 10.1159/000194068. [DOI] [PubMed] [Google Scholar]
- 12.Akashiba T, Akahoshi T, Kawahara S, et al. Clinical characteristics of obesity-hypoventilation syndrome in Japan: a multi-center study. Intern Med. 2006;45:1121–5. doi: 10.2169/internalmedicine.45.1747. [DOI] [PubMed] [Google Scholar]
- 13.Kawata N, Tatsumi K, Terada J, et al. Daytime hypercapnia in obstructive sleep apnea syndrome. Chest. 2007;132:1832–8. doi: 10.1378/chest.07-0673. [DOI] [PubMed] [Google Scholar]
- 14.Laaban J, Chailleux E. Daytime hypercapnia in adult patients with obstructive sleep apnea syndrome in France, before initiating nocturnal nasal continuous positive airway pressure therapy. Chest. 2005;127:710–5. doi: 10.1378/chest.127.3.710. [DOI] [PubMed] [Google Scholar]
- 15.Mokhlesi B, Tulaimat A, Faibussowitsch I, et al. Obesity hypoventilation syndrome: prevalence and predictors in patients with obstructive sleep apnea. Sleep Breath. 2007;11:117–24. doi: 10.1007/s11325-006-0092-8. [DOI] [PubMed] [Google Scholar]
- 16.Javaheri S, Colangelo G, Lacey W, et al. Chronic hypercapnia in obstructive sleep apneahypopnea syndrome. Sleep. 1994;17:416–23. doi: 10.1093/sleep/17.5.416. [DOI] [PubMed] [Google Scholar]
- 17.Considine R, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 18.Kalra S. Central leptin insufficiency syndrome: an interactive etiology for obesity, metabolic and neural diseases and for designing new therapeutic interventions. Peptides. 2008;29:127–38. doi: 10.1016/j.peptides.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tankersley C, O’Donnell C, Daood MJ, et al. Leptin attenuates respiratory complications associated with the obese phenotype. J Appl Physiol. 1998;85:2261–9. doi: 10.1152/jappl.1998.85.6.2261. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert R, Sipple JH, Auchincloss JH., Jr. Respiratory control and work of breathing in obese subjects. J Appl Physiol. 1961;16:21–6. doi: 10.1152/jappl.1961.16.1.21. [DOI] [PubMed] [Google Scholar]
- 21.Kress J, Pohlman AS, Alverdy J, et al. The impact of morbid obesity on oxygen cost of breathing (VO2(RESP)) at rest. Am J Respir Crit Care Med. 1999;160:883–6. doi: 10.1164/ajrccm.160.3.9902058. [DOI] [PubMed] [Google Scholar]
- 22.Phipps P, Starritt E, Caterson I, et al. Association of serum leptin with hypoventilation in human obesity. Thorax. 2002;57:75–6. doi: 10.1136/thorax.57.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimura R, Tatsumi K, Nakamura A, et al. Fat accumulation, leptin, and hypercapnia in obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127:543–9. doi: 10.1378/chest.127.2.543. [DOI] [PubMed] [Google Scholar]
- 24.Berger KI, Goldring RM, Rapoport DM. Obesity hypoventilation syndrome. Semin Respir Crit Care Med. 2009;30:253–61. doi: 10.1055/s-0029-1222439. [DOI] [PubMed] [Google Scholar]
- 25.Ayappa I, Berger KI, Norman RG, et al. Hypercapnia and ventilatory periodicity in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:1112–5. doi: 10.1164/rccm.200203-212OC. [DOI] [PubMed] [Google Scholar]
- 26.Norman R, Goldring RM, Clain JM, et al. Transition from acute to chronic hypercapnia in patients with periodic breathing: predictions from a computer model. J Appl Physiol. 2006;100:1733–41. doi: 10.1152/japplphysiol.00502.2005. [DOI] [PubMed] [Google Scholar]
- 27.Lin C, Wu KM, Chou CS, et al. Oral airway resistance during wakefulness in eucapnic and hypercapnic sleep apnea syndrome. Respir Physiol Neurobiol. 2004;139:215–24. doi: 10.1016/j.resp.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Adesanya A, Lee W, Greilich NB, et al. Perioperative management of obstructive sleep apnea. Chest. 2010;138:1489–98. doi: 10.1378/chest.10-1108. [DOI] [PubMed] [Google Scholar]
- 29.Seet E, Chung F. Management of sleep apnea in adults - functional algorithms for the perioperative period. Can J Anaesth. 2010;57:849–64. doi: 10.1007/s12630-010-9344-y. [DOI] [PubMed] [Google Scholar]
- 30.Lee M, Lin CC, Shen SY, et al. Work of breathing in eucapnic and hypercapnic sleep apnea syndrome. Respiration. 2009;77:146–53. doi: 10.1159/000140491. [DOI] [PubMed] [Google Scholar]
- 31.Sharp J, Henry JP, Sweany SK, et al. The total work of breathing in normal and obese men. J Clin Invest. 1964;43:728–39. doi: 10.1172/JCI104957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steier J, Jolley CJ, Seymour J, et al. Neural respiratory drive in obesity. Thorax. 2009;64:719–25. doi: 10.1136/thx.2008.109728. [DOI] [PubMed] [Google Scholar]
- 33.Lopata M, Onal E. Mass loading, sleep apnea, and the pathogenesis of obesity hypoventilation. Am Rev Respir Dis. 1982;126:640–5. doi: 10.1164/arrd.1982.126.4.640. [DOI] [PubMed] [Google Scholar]
- 34.Sampson M, Grassino K. Neuromechanical properties in obese patients during carbon dioxide re-breathing. Am J Med. 1983;75:81–90. doi: 10.1016/0002-9343(83)91171-3. [DOI] [PubMed] [Google Scholar]
- 35.Zwillich CW, Sutton FD, Pierson DJ, et al. Decreased hypoxic ventilatory drive in the obesity-hypoventilation syndrome. Am J Med. 1975;59:343–8. doi: 10.1016/0002-9343(75)90392-7. [DOI] [PubMed] [Google Scholar]
- 36.Kessler R, Chaouat A, Schinkewitch P, et al. The obesity-hypoventilation syndrome revisited: a prospective study of 34 consecutive cases. Chest. 2001;120:369–76. doi: 10.1378/chest.120.2.369. [DOI] [PubMed] [Google Scholar]
- 37.Marik P, Desai H. Characteristics of patients with the “malignant obesity hypoventilation syndrome” admitted to an ICU. J Intensive Care Med. 2012 doi: 10.1177/0885066612444261. http://dx.doi.org/10.1177/0885066612444261. [DOI] [PubMed] [Google Scholar]
- 38.Sugerman HJ, Baron PL, Fairman RP, et al. Hemodynamic dysfunction in obesity hypoventilation syndrome and the effects of treatment with surgically induced weight loss. Ann Surg. 1988;207:604–13. doi: 10.1097/00000658-198805000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Divitiis O, Fazio S, Petitto M, et al. Obesity and cardiac function. Circulation. 1981;64:477–82. doi: 10.1161/01.cir.64.3.477. [DOI] [PubMed] [Google Scholar]
- 40.Arzt M, Young T, Finn L, et al. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172:1447–51. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coughlin S, Mawdsley L, Mugarza JA, et al. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur Heart J. 2004;25:735–41. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Peker Y, Kraiczi H, Hedner J, et al. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. 1999;14:179–84. doi: 10.1034/j.1399-3003.1999.14a30.x. [DOI] [PubMed] [Google Scholar]
- 43.Sin D, Fitzgerald F, Parker JD, et al. Relationship of systolic BP to obstructive sleep apnea in patients with heart failure. Chest. 2003;123:1536–43. doi: 10.1378/chest.123.5.1536. [DOI] [PubMed] [Google Scholar]
- 44.Tung A. Anaesthetic considerations with the metabolic syndrome. Br J Anaesth. 2010;105:24–33. doi: 10.1093/bja/aeq293. [DOI] [PubMed] [Google Scholar]
- 45.Chung S, Yuan H, Chung F. A systemic review of obstructive sleep apnea and its implications for anesthesiologists. Anesth Analg. 2008;107:1543–63. doi: 10.1213/ane.0b013e318187c83a. [DOI] [PubMed] [Google Scholar]
- 46.Kaw R, Pasupuleti V, Walker E, et al. Postoperative complications in patients with obstructive sleep apnea. Chest. 2011;141:436–41. doi: 10.1378/chest.11-0283. [DOI] [PubMed] [Google Scholar]
- 47.Liao P, Yegneswaran B, Vairavanathan S, et al. Postoperative complications in patients with obstructive sleep apnea: a retrospective matched cohort study. Can J Anaesth. 2009;56:819–28. doi: 10.1007/s12630-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 48.Nowbar S, Burkart KM, Gonzales R, et al. Obesity-associated hypoventilation in hospitalized patients: prevalence, effects, and outcome. Am J Med. 2004;116:1–7. doi: 10.1016/j.amjmed.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 49.Perez de Llano LA, Golpe R, Ortiz Piquer M, et al. Short-term and long-term effects of nasal intermittent positive pressure ventilation in patients with obesity-hypoventilation syndrome. Chest. 2005;128:587–94. doi: 10.1378/chest.128.2.587. [DOI] [PubMed] [Google Scholar]
- 50.Sugerman HJ, Fairman RP, Sood RK, et al. Long-term effects of gastric surgery for treating respiratory insufficiency of obesity. Am J Clin Nutr. 1992;55:597S–601S. doi: 10.1093/ajcn/55.2.597s. [DOI] [PubMed] [Google Scholar]
- 51.Efthimiou E, Sampalis J, Christou N. Validation of obesity surgery mortality risk score in patients undergoing gastric bypass in a Canadian center. Surg Obes Relat Dis. 2009;5:643–7. doi: 10.1016/j.soard.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Lin C. Effect of nasal CPAP on ventilatory drive in normocapnic and hypercapnic patients with obstructive sleep apnoea syndrome. Eur Respir J. 1994;7:2005–10. [PubMed] [Google Scholar]
- 53.Perez de Llano LA, Golpe R, Piquer MO, et al. Clinical heterogeneity among patients with obesity hypoventilation syndrome: therapeutic implications. Respiration. 2008;75:34–9. doi: 10.1159/000105460. [DOI] [PubMed] [Google Scholar]
- 54.Piper AJ, Sullivan CE. Effects of short-term NIPPV in the treatment of patients with severe obstructive sleep apnea and hypercapnia. Chest. 1994;105:434–40. doi: 10.1378/chest.105.2.434. [DOI] [PubMed] [Google Scholar]
- 55.Banerjee D, Yee BJ, Piper AJ, et al. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest. 2007;131:1678–84. doi: 10.1378/chest.06-2447. [DOI] [PubMed] [Google Scholar]
- 56.Chouri-Pontarollo N, Borel JC, Tamisier R, et al. Impaired objective daytime vigilance in obesity-hypoventilation syndrome: impact of noninvasive ventilation. Chest. 2007;131:148–55. doi: 10.1378/chest.06-1159. [DOI] [PubMed] [Google Scholar]
- 57.Budweiser S, Riedl SG, Jörres RA, et al. Mortality and prognostic factors in patients with obesity-hypoventilation syndrome undergoing noninvasive ventilation. J Intern Med. 2007;261:375–83. doi: 10.1111/j.1365-2796.2007.01765.x. [DOI] [PubMed] [Google Scholar]
- 58.de Lucas-Ramos P, de Miguel-Díez J, Santacruz-Siminiani A, et al. Benefits at 1 year of nocturnal intermittent positive pressure ventilation in patients with obesity-hypoventilation syndrome. Respir Med. 2004;98:961–7. doi: 10.1016/j.rmed.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 59.Heinemann F, Budweiser S, Dobroschke J, et al. Non-invasive positive pressure ventilation improves lung volumes in the obesity hypoventilation syndrome. Respir Med. 2007;101:1229–35. doi: 10.1016/j.rmed.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 60.Han F, Chen E, Wei H, et al. Treatment effects on carbon dioxide retention in patients with obstructive sleep apnea-hypopnea syndrome. Chest. 2001;119:1814–9. doi: 10.1378/chest.119.6.1814. [DOI] [PubMed] [Google Scholar]
- 61.Priou P, Hamel JF, Person C, et al. Long-term outcome of noninvasive positive pressure ventilation for obesity hypoventilation syndrome. Chest. 2010;138:84–90. doi: 10.1378/chest.09-2472. [DOI] [PubMed] [Google Scholar]
- 62.Schafer H, Ewig S, Hasper E, et al. Failure of CPAP therapy in obstructive sleep apnoea syndrome: predictive factors and treatment with bilevel-positive airway pressure. Respir Med. 1998;92:208–15. doi: 10.1016/s0954-6111(98)90097-x. [DOI] [PubMed] [Google Scholar]
- 63.Piper A, Wang D, Yee BJ, et al. Randomised trial of CPAP versus bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008;63:395–401. doi: 10.1136/thx.2007.081315. [DOI] [PubMed] [Google Scholar]
- 64.American Academy of Sleep Medicine Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–71. [PMC free article] [PubMed] [Google Scholar]
- 65.Contal O, Adler D, Borel JC, et al. Impact of different back-up respiratory rates on the efficacy of non-invasive positive pressure ventilation in obesity hypoventilation syndrome: a randomized trial. Chest. 2012 doi: 10.1378/chest.11-2848. http://dx.doi.org/10.1378/chest.11-2848. [DOI] [PubMed] [Google Scholar]
- 66.Storre JH, Seuthe B, Fiechter R, et al. Average volume-assured pressure support in obesity hypoventilation: a randomized crossover trial. Chest. 2006;130:815–21. doi: 10.1378/chest.130.3.815. [DOI] [PubMed] [Google Scholar]
- 67.Murphy P, Davidson C, Hind MD, et al. Volume targeted versus pressure support non-invasive ventilation in patients with super obesity and chronic respiratory failure: a randomised controlled trial. Thorax. 2012;67:727–34. doi: 10.1136/thoraxjnl-2011-201081. [DOI] [PubMed] [Google Scholar]
- 68.Berry RB, Chediak A, Brown LK, et al. Best clinical practices for the sleep center adjustment of noninvasive positive pressure ventilation (NPPV) in stable chronic alveolar hypoventilation syndromes. J Clin Sleep Med. 2010;6:491–509. [PMC free article] [PubMed] [Google Scholar]
- 69.Aubier M, Murciano D, Milic-Emili J, et al. Effects of the administration of O2 on ventilation and blood gases in patients with chronic obstructive pulmonary disease during acute respiratory failure. Am Rev Respir Dis. 1980;122:747–54. doi: 10.1164/arrd.1980.122.5.747. [DOI] [PubMed] [Google Scholar]
- 70.Robinson T, Freiberg DB, Regnis JA, et al. The role of hypoventilation and ventilation-perfusion redistribution in oxygen-induced hypercapnia during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1524–9. doi: 10.1164/ajrccm.161.5.9904119. [DOI] [PubMed] [Google Scholar]
- 71.Wijesinghe M, Williams M, Perrin K, et al. The effect of supplemental oxygen on hypercapnia in subjects with obesity-associated hypoventilation: a randomized, crossover, clinical study. Chest. 2011;139:1018–24. doi: 10.1378/chest.10-1280. [DOI] [PubMed] [Google Scholar]
- 72.Mokhlesi B, Tulaimat A, Parthasarathy S. Oxygen for obesity hypoventilation syndrome: a double-edged sword? Chest. 2011;139:975–7. doi: 10.1378/chest.10-2858. [DOI] [PubMed] [Google Scholar]
- 73.Marti-Valeri C, Sabate A, Masdevall C, et al. Improvement of associated respiratory problems in morbidly obese patients after open Roux-en-Y gastric bypass. Obes Surg. 2007;17:1102–10. doi: 10.1007/s11695-007-9186-z. [DOI] [PubMed] [Google Scholar]
- 74.Greenburg D, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–42. doi: 10.1016/j.amjmed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 75.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 76.Fernandez AJ, Demaria EJ, Tichansky DS, et al. Multivariate analysis of risk factors for death following gastric bypass for treatment of morbid obesity. Ann Surg. 2004;239:698–702. doi: 10.1097/01.sla.0000124295.41578.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flum D, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–54. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bayliss D, Millhorn DE. Central neural mechanisms of progesterone action: application to the respiratory system. J Appl Physiol. 1992;73:393–404. doi: 10.1152/jappl.1992.73.2.393. [DOI] [PubMed] [Google Scholar]
- 79.Sutton FJ, Zwillich CW, Creagh CE, et al. Progesterone for outpatient treatment of Pickwickian syndrome. Ann Intern Med. 1975;83:476–9. doi: 10.7326/0003-4819-83-4-476. [DOI] [PubMed] [Google Scholar]
- 80.Poulter N, Chang CL, Farley TM, et al. Risk of cardiovascular diseases associated with oral progestagen preparations with therapeutic indications [letter] Lancet. 1999;354:1610. doi: 10.1016/s0140-6736(99)03132-3. [DOI] [PubMed] [Google Scholar]
- 81.Tojima H, Kunitomo F, Kimura H, et al. Effects of acetazolamide in patients with the sleep apnoea syndrome. Thorax. 1988;43:113–9. doi: 10.1136/thx.43.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Whyte K, Gould GA, Airlie MA, et al. Role of protrip-tyline and acetazolamide in the sleep apnea/hypopnea syndrome. Sleep. 1988;11:463–72. doi: 10.1093/sleep/11.5.463. [DOI] [PubMed] [Google Scholar]
- 83.Raurich J, Rialp G, Ibáñez J, et al. Hypercapnic respiratory failure in obesity-hypoventilation syndrome: CO2 response and acetazolamide treatment effects. Respir Care. 2010;55:1442–8. [PubMed] [Google Scholar]
- 84.Pedersen T, Møller AM, Pedersen BD. Pulse oximetry for perioperative monitoring: systematic review of randomized, controlled trials. Anesth Analg. 2003;96:426–31. doi: 10.1097/00000539-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 85.Kaw R, Hernandez AV, Walker E, et al. Determinants of hypercapnia in obese patients with obstructive sleep apnea: a systematic review and meta-analysis of cohort studies. Chest. 2009;136:787–96. doi: 10.1378/chest.09-0615. [DOI] [PubMed] [Google Scholar]
- 86.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812–21. doi: 10.1097/ALN.0b013e31816d83e4. [DOI] [PubMed] [Google Scholar]
- 87.Chung F, Yegneswaran B, Liao P, et al. Validation of the Berlin questionnaire and American Society of Anesthesiologists checklist as screening tools for obstructive sleep apnea in surgical patients. Anesthesiology. 2008;108:822–30. doi: 10.1097/ALN.0b013e31816d91b5. [DOI] [PubMed] [Google Scholar]
- 88.Abrishami A, Khajehdehi A, Chung F. A systematic review of screening questionnaires for obstructive sleep apnea. Can J Anaesth. 2010;57:423–38. doi: 10.1007/s12630-010-9280-x. [DOI] [PubMed] [Google Scholar]
- 89.Chung F, Subramanyam R, Liao P, et al. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. 2012;108:768–75. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee T, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–9. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 91.Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116:418–99. [Google Scholar]
- 92.Gallagher M, Franklin BA, Ehrman JK, et al. Comparative impact of morbid obesity vs heart failure on cardiorespiratory fitness. Chest. 2005;127:2197–203. doi: 10.1378/chest.127.6.2197. [DOI] [PubMed] [Google Scholar]
- 93.McCullough P, Gallagher MJ, Dejong AT, et al. Cardiorespiratory fitness and short-term complications after bariatric surgery. Chest. 2006;130:517–25. doi: 10.1378/chest.130.2.517. [DOI] [PubMed] [Google Scholar]
- 94.Smetana G. Preoperative pulmonary evaluation. N Engl J Med. 1999;340:937–44. doi: 10.1056/NEJM199903253401207. [DOI] [PubMed] [Google Scholar]
- 95.Memtsoudis S, Liu SS, Ma Y, et al. Perioperative pulmonary outcomes in patients with sleep apnea after noncardiac surgery. Anesth Analg. 2011;112:113–21. doi: 10.1213/ANE.0b013e3182009abf. [DOI] [PubMed] [Google Scholar]
- 96.Qaseem A, Snow V, Fitterman N, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Ann Intern Med. 2006;144:575–80. doi: 10.7326/0003-4819-144-8-200604180-00008. [DOI] [PubMed] [Google Scholar]
- 97.Siyam M, Benhamou D. Difficult endotracheal intubation in patients with sleep apnea syndrome. Anesth Analg. 2002;95:1098–102. doi: 10.1097/00000539-200210000-00058. [DOI] [PubMed] [Google Scholar]
- 98.Kim J, Lee JJ. Preoperative predictors of difficult intubation in patients with obstructive sleep apnea syndrome. Can J Anaesth. 2006;53:393–7. doi: 10.1007/BF03022506. [DOI] [PubMed] [Google Scholar]
- 99.Langeron O, Masso E, Huraux C, et al. Prediction of difficult mask ventilation. Anesthesiology. 2000;92:1229–36. doi: 10.1097/00000542-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 100.Rose D, Cohen MM. The airway: problems and predictions in 18,500 patients. Can J Anaesth. 1994;41:372–83. doi: 10.1007/BF03009858. [DOI] [PubMed] [Google Scholar]
- 101.Brodsky J, Lemmens HJ, Brock-Utne JG, et al. Morbid obesity and tracheal intubation. Anesth Analg. 2002;94:732–6. doi: 10.1097/00000539-200203000-00047. [DOI] [PubMed] [Google Scholar]
- 102.Mashour G, Kheterpal S, Vanaharam V, et al. The extended Mallampati score and a diagnosis of diabetes mellitus are predictors of difficult laryngoscopy in the morbidly obese. Anesth Analg. 2008;107:1919–23. doi: 10.1213/ane.0b013e31818a9946. [DOI] [PubMed] [Google Scholar]
- 103.Kheterpal S, Han R, Tremper KK, et al. Incidence and predictors of difficult and impossible mask ventilation. Anesthesiology. 2006;105:885–91. doi: 10.1097/00000542-200611000-00007. [DOI] [PubMed] [Google Scholar]
- 104.Cattano D, Melnikov V, Khalil Y, et al. An evaluation of the rapid airway management positioner in obese patients undergoing gastric bypass or laparoscopic gastric banding surgery. Obes Surg. 2010;20:1436–41. doi: 10.1007/s11695-009-9885-8. [DOI] [PubMed] [Google Scholar]
- 105.Murgu S, Pecson J, Colt HG. Bronchoscopy during noninvasive ventilation: indications and technique. Respir Care. 2010;55:595–600. [PubMed] [Google Scholar]
- 106.Wong D, Wang J, Venkatraghavan L. Awake bronchoscopic intubation through an air-Q® with the application of BIPAP. Can J Anaesth. 2012;59:915–6. doi: 10.1007/s12630-012-9741-5. [DOI] [PubMed] [Google Scholar]
- 107.Rothfleisch R, Davis LL, Kuebel DA, et al. Facilitation of fiberoptic nasotracheal intubation in a morbidly obese patient by simultaneous use of nasal CPAP. Chest. 1994;106:287–8. doi: 10.1378/chest.106.1.287. [DOI] [PubMed] [Google Scholar]
- 108.Gander S, Frascarolo P, Suter M, et al. Positive end-expiratory pressure during induction of general anesthesia increases duration of nonhypoxic apnea in morbidly obese patients. Anesth Analg. 2005;100:580–4. doi: 10.1213/01.ANE.0000143339.40385.1B. [DOI] [PubMed] [Google Scholar]
- 109.Gupta A, Stierer T, Zuckerman R, et al. Comparison of recovery profile after ambulatory anesthesia with propofol, isoflurane, sevoflurane and desflurane: a systematic review. Anesth Analg. 2004;98:632–41. doi: 10.1213/01.ane.0000103187.70627.57. [DOI] [PubMed] [Google Scholar]
- 110.Cashman J, Dolin SJ. Respiratory and haemodynamic effects of acute postoperative pain management: evidence from published data. Br J Anaesth. 2004;93:212–23. doi: 10.1093/bja/aeh180. [DOI] [PubMed] [Google Scholar]
- 111.Gali B, Whalen FX, Schroeder DR, et al. Identification of patients at risk for postoperative respiratory complications using a preoperative obstructive sleep apnea screening tool and postanesthesia care assessment. Anesthesiology. 2009;110:869–77. doi: 10.1097/ALN.0b013e31819b5d70. [DOI] [PubMed] [Google Scholar]
- 112.Macintyre P, Loadsman JA, Scott DA. Opioids, ventilation and acute pain management. Anaesth Intensive Care. 2011;39:545–58. doi: 10.1177/0310057X1103900405. [DOI] [PubMed] [Google Scholar]
- 113.Ready L, Oden R, Chadwick HS, et al. Development of an anesthesiology-based postoperative pain management service. Anesthesiology. 1988;68:100–6. doi: 10.1097/00000542-198801000-00016. [DOI] [PubMed] [Google Scholar]
- 114.Vila HJ, Smith RA, Augustyniak MJ, et al. The efficacy and safety of pain management before and after implementation of hospital-wide pain management standards: is patient safety compromised by treatment based solely on numerical pain ratings? Anesth Analg. 2005;101:474–80. doi: 10.1213/01.ANE.0000155970.45321.A8. [DOI] [PubMed] [Google Scholar]
- 115.American Society of Anesthesiologists Task Force on Neuraxial Opioids. Horlocker TT, Burton AW, Connis RT, et al. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology. 2009;110:218–30. doi: 10.1097/ALN.0b013e31818ec946. [DOI] [PubMed] [Google Scholar]
- 116.Rennotte M, Baele P, Aubert G, et al. Nasal continuous positive airway pressure in the perioperative management of patients with obstructive sleep apnea submitted to surgery. Chest. 1995;107:367–74. doi: 10.1378/chest.107.2.367. [DOI] [PubMed] [Google Scholar]
- 117.El-Solh A, Aquilina A, Pineda L, et al. Noninvasive ventilation for prevention of post-extubation respiratory failure in obese patients. Eur Respir J. 2006;28:588–95. doi: 10.1183/09031936.06.00150705. [DOI] [PubMed] [Google Scholar]
- 118.Carr G, Mokhlesi B, Gehlbach BK. Acute cardiopulmonary failure from sleep-disordered breathing. Chest. 2012;141:798–808. doi: 10.1378/chest.11-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]