Abstract
The management of head and neck mucosal dysplasia and microinvasive carcinoma is an appealing strategy to prevent the development of invasive carcinomas. While surgery remains the standard of care, photodynamic therapy (PDT) offers several advantages including the ability to provide superficial yet wide field mucosal ablative treatment. This is particularly attractive where defining the extent of the dysplasia can be difficult. PDT can also retreat the mucosa without any cumulative fibrotic complications affecting function. To date, clinical experience suggests that this treatment approach can be effective in obtaining a complete response for the treated lesion but long term follow-up is limited. Further research efforts are needed to define not only the risk of malignant transformation with PDT but to develop site specific treatment recommendations that include the fluence, fluence rate and light delivery technique.
Keywords: head and neck premalignant dysplasia, microinvasive carcinoma, photodynamic therapy, PDT, review
I. Introduction
The management of head and neck squamous cell carcinoma (HNSCC) has been the focus of significant study in the past 30-40 years seeking to improve local-regional disease control rates while striving to preserve function in the head and neck. These efforts have demonstrated that modest but suboptimal improvements in survival can be achieved with various non-surgical treatment approaches that were used as strategies to preserve speech and swallow function (1, 2). However, it is now clear that the survival gains with these approaches paradoxically come with an increased risk of treatment-related injury to speech and swallow function (3, 4). This vexing competition between optimizing local-regional control rates and function preservation can still be an issue even with the development of organ-preservation surgical approaches.
As such, ongoing efforts to prevent the development of invasive HNSCC are clearly desired given that dysplasia is a clear histologic precursor lesion with definable clinical features. At present, the challenges include: 1) the need to identify more reliable markers for the risk of malignant progression (5), 2) optimizing the characterization and surveillance of such precursor lesions and 3) the development of less invasive and more effective therapies without the morbidity and cumulative function loss that comes typically with multiple surgical resections of dysplasia (6). These challenges highlight the need to better identify and better treat a wide mucosal area, often due to the challenges in defining the extent of the lesion, but to only a superficial depth.
For these reasons, the application of photodynamic therapy (PDT) for head and neck dysplasia is particularly appealing as this couples the administration of a photosensitizing agent with activating non-ionizing light that is capable of treating a specific yet wide mucosal area. Adding to its appeal is the ability to limit the depth of any normal tissue injury and the ability to retreat a mucosal region without any cumulative normal tissue injury. We summarize the work to date that supports an emerging role for PDT in the management of head and neck dysplasia and the relevant treatment parameters influencing the outcome of this treatment modality.
II. Natural History of Premalignant Dysplasia of the Mucosa and Current Management Approaches
Leukoplakia is a common diagnosis with a reported prevalence rate of 1-5% (7). It is a clinical definition that refers to any white mucosal lesion that cannot be rubbed off and is typically a diagnoses of exclusion. This clinical entity may reflect a spectrum of histologic diagnosis ranging from para-keratosis to dysplasia and microinvasive carcinoma. While there is no clear consensus on the definition for microinvasive carcinoma in the head and neck, it is accepted that this represents invasion of the basement membrane at the very least. Of the various possible non-invasive histologic diagnoses, dysplasia has the greatest clinical significance due to the risk of malignant transformation.
Mucosal dysplasia has been the subject of significant pathologic classification especially as it pertains to the histologic grade. This is largely due to the recognition that increasing dysplasia grade is associated with a higher malignant transformation rate (MTR). However, dysplasia grade is subject to significant clinical variability both in terms of its identification and classification and its natural history to malignant progression. To address this heterogeneity, there continues to be significant interest in identifying a molecular signature for the risk of malignant progression.
In an effort to establish a baseline risk assessment for an evidence based approach to the surveillance and management of oral dysplasia, Mehanna et al recently reported the results of a systematic review of the literature (6). In a meta-analysis of 14 observational studies limited due to the paucity of randomized trials, a mean overall MTR was 12.1% (95% confidence interval [CI]: 8.1%, 17.9%) with a mean time to malignant transformation (TMT) of 4.3 years. The MTR was significantly grade dependent as analyzed by grouping mild-moderate vs. severe dysplasia and carcinoma in-situ (p < 0.008) (6). This grouping was an attempt to reduce the impact of rater variability in the grading. For high grade dysplasia and carcinoma in-situ, the risk was 24.1% (CI: 13.3, 39.5) compared to a risk of 10.3% (CI: 6.1, 16.8) for mild-moderate grade disease. Several other investigatoers have also reported on an association between MTR and high grade dysplasia (8, 9). In a recent multivariate analysis of 114 patients, Yang et al noted that lesions demonstrating a propensity for relapse after surgical resection were more at risk for relapse than the presence of high grade dysplasia (10).
While higher rates of malignant transformation appear to be associated with higher grades of dysplasia, the finding of malignant transformation even with lower grades would suggest that these lesions require at least ongoing surveillance (8). Whetehr or not malignant transformation occurs as an end product of a spectrum of molecular and genetic perturbations characterized by progressive grades of dysplasia has not been definitively established. In fact, allelic loss of heterozygosity at the 3p and 9p chromosome has been shown to be elevated in mild/moderate dysplastic and hyperplastic lesions showing malignant progression (11).
Mehanna et al also demonstrated that therapy with surgical excision was able to significantly reduce the MTR even when adjusting for the grade of the dysplasia when compared to patients who underwent observation (6). The MTR for patients observed was 14.6% versus 5.4% when treated with surgery (p = 0.003) (6) supporting a need to continue ongoing surveillance and prophylactic management of dysplasia. These findings have also been independently confirmed (9). Even so, the risk of relapse with surgical resection has been reported to be as high as 20-30% (12-14).
Despite the risk of malignant transformation, any therapy remains prophylactic at this time and must balance any treatment toxicity with possible benefits. While the MTR is associated with the grade of dysplasia, it remains impossible to accurately predict which lesion will progress emphasizing the need to temper any effective therapy with its risk of toxicities. While more accurate biomarkers predicting malignant progression may help to identify a high risk group in which more aggressive therapy can then be considered and justified, a recent systematic review concluded that further longitudinal evaluation of various promising biomarkers was still needed (5, 15).
Currently, surgical resection is viewed as the only effective management option (16, 17). As outlined above, surgical resection can still be associated with a significant risk of relapse and transformation to invasive carcinoma. In part, this is due again to the competing issues of function preservation and the desire to achieve wide surgical margins for optimal oncologic results. For example, in some situations of extensive but superficial dysplasia of the oral tongue, significant resection of the musculature may be required due to concerns of unidentified areas of invasive carcinoma. Compounding this is the recognition that surrounding apparently normal appearing mucosa can harbor dysplastic cells and the ability to resect with an additional safety margin is limited by functional considerations especially in the larynx site.
Another challenge is the patient who experiences recurrent dysplasia. With recurrent lesions, the need to ensure that any biopsies exclude the development of invasive carcinomas necessitates even further and sometimes serial wide excisions, with each procedure resulting in more scarring and organ dysfunction. These complications can ultimately compromise organ function or at the very least, complicate the successful administration of photodynamic therapy for reasons described below.
Using malignant transformation as the main endpoint, Lodi and colleagues concluded that no effective therapy existed based on a Cochrane review of randomized trials that utilized approaches ranging from therapy to observation or placebo (16). Due to the selection criteria, this analysis resulted in the exclusion of all surgical approaches due to the absence of any randomized control trials to date. Amongst chemopreventive medical therapies such as vitamin A and retinoids, effective resolution of the target lesion was identified but high relapse rates and toxicities were noted. As such, the role of chemoprevention continues to be an active area of investigation.
External beam radiotherapy (EBRT) has been used in the management of dysplasia and carcinoma in-situ (CIS) especially for the larynx (14). Sadri and colleagues noted that EBRT appeared to have superior local control rates for the treatment of severe dysplasia / CIS of the larynx compared to surgical procedures (14). These investigators suggest that this may be due to the wider field of therapeutic effect compared to the removal of a visible lesion. However, this wide field radiotherapeutic effect comes at the risk of acute and long-term injury to the surrounding and underlying normal tissues.
In summary, dysplasia is a common histologic diagnosis in the head and neck that poses a clinically significant risk for malignant transformation. While a higher risk is associated with higher grades of dysplasia, there is significant clinical variability such that any treatment for the dysplasia must carefully balance the treatment toxicities. Treatment for dysplasia appears to have an impact on the risk of malignant transformation. Despite this, it is also prudent to advocate for the elimination of existing risk factors such as ongoing tobacco exposure which is associated with an increased risk of dysplasia relapse (18). Hence, ongoing efforts to develop not only more effective therapies but ones with reduced side-effects that can offer wide field superficial treatment are needed. We review the attributes of photodynamic therapy and the promising role it may play in the management of head and neck mucosal dysplasia.
III. Mechanism of Action of Photodynamic Therapy and its Treatment Implications
Photodynamic therapy (PDT) requires three fundamental elements to be present for effective cytotoxicity: oxygen, photosensitizer and an activating light source of the appropriate wavelength. As this is an oxygen dependent process, the need for an effective and functional tumor vasculature is an important consideration. Practically, this requires careful consideration of how the head and neck mucosa is handled during its exposure for PDT. For example, we have recently described the use of a balloon diffuser light source as a technique to illuminate the larynx (19). While this offered a more stable and conformal illumination for this anatomic site (Figure 1) (19), the balloon diffuser must be carefully inflated in apposition to the glottic mucosa to not compromise its vascular supply.
Figure 1. The Placement of a Balloon Diffuser with a Radial Diffusing Light Source for the Treatment of Glottic Dysplasia.

Figure 1A demonstrates the placement of the balloon diffuser with adjacent fibers measuring the fluence rate. The balloon was carefully inflated taking care to gently compress the adjacent glottic without distorting the mucosa and its potential vasculature (1B).
PDT damage of the tissue vasculature can also compromise oxygen levels during treatment. Furthermore, oxygen concentration can be affected by the rate of oxygen consumption during the light activation as the reactive oxidative species is generated (explained below). Studies have demonstrated that while baseline oxygenation is an important predictor of the success of PDT, the change in oxygen levels during PDT may be more important. Specifically, animals that demonstrate larger relative increases (i.e. smaller decreases) in the tissue hemoglobin oxygen saturation during illumination experience a more durable treatment response (20). While a favorable oxygen response (ie. maintaining sufficient oxygen levels during the activation of the photosensitizer) is in part influenced by the tumor biology and its vasculature, the rate in which the light energy is delivered (fluence rate, defined as the light power per unit area incident on a point from all directions (21)) is an important and modifiable therapeutic consideration.
Assuming that the sensitizer molecules in the illuminated tissue are either isotropic absorbers or are randomly distributed in orientation, the fluence rate is directly proportional to the rate of excitation of the sensitizer. High fluence rates result in rapid rates of oxygen consumption which outstrip the oxygen supply by the tumor microcirculation. This can limit the cytotoxicity that can be achieved for the total amount of light energy (light dose or fluence) that is being delivered (22). Low fluence rates have been investigated as a means of maintaining tumor oxygenation during PDT assuming that the tumor blood flow remains intact (23).
Low fluence rate has been shown to increase damage to mouse skin in conjunction with trends toward more efficient photobleaching (24). In an orthotopic model of rat glioma low fluence rate increased the extent of necrosis and improved animal survival (25). In clinical studies, low fluence rate has additionally been found to lessen the pain associated with PDT using topical 5-aminolevulinic acid (5-ALA) (26).
Fractionation of the treatment light is another approach that has been used to increase PDT cytotoxicity through its effects on the local tissue microenvironment, e.g. oxygen or photosensitizer concentrations. This is an active area of clinical investigation especially for 5-ALA which is the pro-drug for the photosensitizer protoporphyrin IX (PpIX). Approaches that have been investigated include the addition of a long (e.g., 2 h) interruption in light delivery based on the hypothesis that additional PpIX will be synthesized from ALA during this time interval. However, studies have shown that any increases gained in PpIX concentration during the dark interval are small (<10% of that measured pre-illumination) (27). This finding led Star et al (28) to suggest that gains in therapy response with the addition of a long dark interval may be attributed to mechanisms other than increased photosensitizer availability. For example, beneficial effects of light interruption on tissue oxygenation and blood flow may be contributing to improved outcome.
Alternatively, a short (e.g., 150 s) interruption in light delivery has also been studied based on the hypothesis that recovery from PDT-induced (treatment-limiting) hypoxia will occur (29, 30). In a systematic set of studies in the rat colon, Curnow et al (29) and Messmann et al (30) evaluated response to ALA-PDT (200 mg/kg, i.v., 25 J/cm2) and found that just one 150 second break in illumination after administering 20% of the total light dose produced more treatment-induced necrosis than multiple, evenly-spaced fractions of 50 or 150 seconds. Increasing the length of the break to 5 or 15 minutes provided no additional benefit. These results are consistent with findings that light interruption partially reversed the hypoxia created by ALA-PDT under the same set of conditions (31). Furthermore, reperfusion itself can contribute to tissue damage through ischemia-reperfusion injury, which has been shown to play a role in ALA-PDT (32). However, others have shown ALA-PDT to lead to increases in tissue oxygenation (33), which speaks to a need for tissue (patient) specific monitoring in order to optimally design fractionation schemes.
The vasculature not only affects the level of oxygenation but can also determine if there is adequate photosensitizer delivery and uptake in the target cells (34). This is influenced by the drug-light interval (DLI). To date, the optimum DLI has largely been based on studies determining the time interval to maximum uptake of drug in the target tumor and/or the achieving the maximum differential drug levels between tumor and normal tissue. The optimum DLI may also be influenced by the preferential location of activity of some photosensitizers.
For some photosensitizers, a significant component of its cytotoxicity may be targeting the tumor vasculature such as the case in the photosensitizers verteporfin (Visudyne®)(35) and motexafin lutetium (Antrin®)(36). A shorter DLI may result in higher vascular drug levels and greater vascular mediated damage. For some agents such as with Photofrin®, both vascular targeting and tumor cytotoxicity have been described with a longer DLI (37). With such agents, the potential to combine vascular and cellular targeting with PDT may significantly enhance the treatment results (38). For such combined targeting to be effective, it appears that the sequence of the targeting may be very important. In a series of experiments, Bin et al demonstrated that the initial targeting of the vasculature first with PDT treatment, followed by cellular targeting, was less effective and was associated with a reduction in the tissue oxygenation (38).
Exposure of a photosensitizer to a specific wavelength of light, typically administered with a laser light source or recently with light emitting diodes, results in its activation from a ground energy state to an excited state that is capable of initiating a cascade of redox reactions when the drug returns to its ground energy state. The majority of the cellular injury is believed to involve the generation of reactive oxygen species (ROS) through both direct and indirect chemical reactions generating especially singlet oxygen. As ROS are highly reactive and of very short half-lives limiting its radius of activity, the oxidation targets are governed by the sub-cellular localization of the photosensitizer. These targets may include protein and constituents of membranes. While the DNA can be a target, this appears to be less of a major target for the mechanism of action for PDT. While potentially mutagenic, clinical practice has not demonstrated cumulative normal tissue injury or a risk of developing PDT-induced secondary malignancies.
The calculation of the light dose delivered to a point in tissue and thus the amount of photosensitizer activation in the head and neck mucosa is complicated by several factors. Light delivered to concave and convex surfaces, commonly seen in the head and neck mucosa, can result in additional and significant light scattered from the tissue on adjacent and opposing mucosa. This multiple scattering can also result in a significant increase in the fluence rate, a phenomenon known as the integrating sphere effect (39). This effect varies with the geometric shape being treated and is an important consideration for head and neck PDT given the complex geometry and variation across the oral and pharyngeal lumen. In the oral cavity, a five-fold magnification of the fluence rate has been reported for treatments that utilized a microlens for light delivery (40). As a result, prescribed fluences calculated assuming a normally-incident field based solely on the source power and area of direct illumination is inadequate.
The picture becomes even more complicated when the target is below the tissue surface. The fluence rate at depth in tissue is related to the optical properties of the intervening tissue, which can be modeled using optical diffusion theory. Diffusion theory is based on the premise that light is scattered much more strongly than it is absorbed. Both the scattering and absorption spectra of tissue can have significant effects on the light distribution. The scattering spectrum depends primarily on the size and distribution of the source of scattering (cells, mitochondria, and connective tissue) (41), while absorption is dominated by hemoglobin. The effects of multiple scattering within the tissue can be of the same magnitude as the effects of scattering from the mucosal walls further contributing to the integrating sphere effect (39). It is therefore advantageous to base light dosimetry on direct measurements of fluence rate (42).
Cumulatively, the oxidative damage within the target cells, be it the tumor or the vasculature can induce several mechanisms of cytotoxicity including the induction of apoptosis (43, 44) and autophagy (which may be protective under specific circumstances as well) (45). Secondarily, the vascular targeting of the PDT can result in rheological effects contributing to tumor necrosis. This results in a significant amount of tumor tissue injury which is able to elicit a host immune response with the development of a tumor antigen-specific adaptive immune response (46, 47). As seen in other cytotoxic therapies, the PDT induced oxidative damage is able to elicit several signal transduction mediated stress responses that is reviewed by Moor (48).
IV. The Application of Photodynamic Therapy in the Management of Premalignant Head and Neck Dysplasia
Photodynamic therapy (PDT) is a treatment modality that uses laser light of specific wavelengths to activate photosensitizing agents in the presence of oxygen to generate selective cellular damage. To accomplish this, the light must be uniformly delivered providing a sufficient amount of light to fully activate the photosensitizers. Selectivity is achieved in part by selective uptake of photosensitizers in pre-malignant and malignant cells (49, 50), but more so, by the selective illumination of an area deemed to contain the target lesion.
PDT has the advantage of incorporating not only the target visible lesion but a margin of surrounding normal mucosa that may reflect this uncertainty in target delineation. This is also an appealing treatment option as postoperative adjuvant therapy in patients with positive dysplasia margins. Moreover, photosensitizers can be used in photodiagnosis to map subclinical regions of potential dysplastic cells (51). Wang et al demonstrated that with the topical application of 5-aminolevulinic acid (5-ALA, Levulan®), ALA-induced PpIX fluorescence spectroscopy could be used to significantly identify the premalignant lesions (dysplasia) in oral fibrotic mucosa when compared to hyperkeratotic and normal mucosa (51).
The most common way the activating laser light is administered is with a silica optical fiber containing a distal diffusing microlens that produces a uniform spot for surface illumination. It is a very good approach for small dysplastic lesions on the buccal mucosa, retromolar trigone, ventral and lateral oral tongue and the floor of mouth. However, this approach may not be ideal for all anatomic sites in the head and neck as the ability to achieve optimal light delivery or fluence can be affected by many factors including the size, geometry, accessibility of the area being treated and the depth of the lesion being treated.
Depending on the anatomic site, the exposure and the treatment time required, PDT may be administered as an outpatient procedure or may require general anesthesia. The later is particularly indicated for lesions in the pharynx and larynx. For cases requiring general anesthesia, the patient should be positioned supine and intubated with either an endotracheal or nasotracheal laser-resistant tube. Proper exposure of the lesion frequently requires the use of a mouth gag, cheek retraction, and tongue retraction. Additionally, the laser must be secured in a manner that allows for flexible movements, fine adjustments, and ultimately the ability to lock the laser in the appropriate position. While it is possible for the physician to hold the laser throughout the treatment, pneumatic and robotic scope holders may be readily adapted to hold the laser with great success and improved the accuracy in the light delivery.
The penetrating depth of any surface light is limited by its light absorption within tissue, which is wavelength-dependent. In general, this limits the effective treatment depth to several millimeters (52) which is usually sufficient for dysplastic mucosal lesions. Light absorption can be improved by reducing surface secretions which can contribute to its reflection. However, lesions with thick keratin layers and microinvasive carcinomas may benefit from a single plane of interstitial light fibers placed through 18 gauge angiocatheters. This approach has been used for lesions more than 3 mm in thickness (53). When this is used, it is important to place the light fibers beyond the dysplasia due to the light anisotropy that occurs at the end of each light fiber. This technique also addresses the technical limitations associated with achieving a uniform light fluence with a microlens across a large mucosal surface that cannot be flattened and made perpendicular to the microlens. This is a particular challenge for large lateral and ventral tongue dysplasia where illumination with a microlens placed intraorally and perpendicular to the mucosa may not be physically possible. Rather, the light source must often be placed anterior to the oral cavity with light falling across the lateral tongue surface. Typically, the posterior tongue is under-illuminated.
When the tongue dysplasia also extends to the adjacent floor of mouth, the added concavity of the mucosal target creates additional technical illumination challenges. Placement of an interstitial planar implant may still be technically possible but may be limited by how anterior the dysplasia lies to the mandible which can interfere with the entry placement of trocars or angiocatheters that are used to place the light fibers. Alternatively, surface contact illumination with a cylindrical balloon diffuser may be considered gently conforming the mucosal surfaces to the diffuser balloon (Figure 2). Key to this technique is to ensure complete mucosal contact with the balloon diffuser. Similar surface contact illumination techniques have been described for the nasopharynx (54) and recently for the larynx (19).
Figure 2. The Placement of a Balloon Diffuser with a Radial Diffusing Light Source for the Treatment of Extensive Oral Tongue Dysplasia.
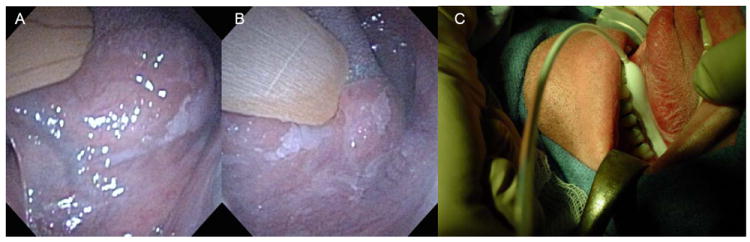
Biopsy proven severe dysplasia is seen in the anterior (A) and the posterior lateral and ventral left oral tongue that extends to the floor of mouth. Figure 2C demonstrates a longitudinal balloon diffuser inflated and placed in the floor of mouth with the lateral tongue mucosa rolled in contact with the balloon surface.
These applicator approaches have the advantage of ensuring reproducible and stable light delivery and are particularly important to consider when planning head and neck PDT. For small illuminated targets, ensuring stable light delivery can have a significant impact on the fluence delivered such as the case when the microlens is placed down a laryngoscope for treatment of the larynx. Tan et al reported that fluctuations in fluence rates with a handheld microlens fiber can result in coefficients of variation up to 21% at the center of a 2 cm diameter illuminated spot and 63% at the periphery, compared to <3% and 9%, respectively, when the fiber was fixed (40). Therefore, slight movements of the diffuser lens fiber on a small target such as the true vocal cords, could result in substantial fluctuation of fluence.
While the use of applicators facilitates reproducible and stable light fluence, applicators do not eliminate the impact of light scatter in the illuminated region. Scattered light can increase both the total fluence and the fluence rate that is delivered with its biologic implications (see above). The build-up factors for the fluence rate can be substantial depending on the anatomic geometry. For the oral cavity, build-up factors up to 4 fold have been reported when a microlens has been used (40). In the nasopharynx, even with the use of a silicone applicator shaped to conform to the nasopharynx, similar average build-up factors of 3-4 fold have been reported (54). However, the increase in the fluence rates is heterogeneous and even for the nasopharyngeal applicator the build-up factor was reported to be as high as 7-fold. Nyst et al demonstrated that this build-up can be reduced by the selective application of black silicone patch to the inferior nasopharynx.
More recently, our group has begun to investigate the role of transoral robotic surgical (TORS) technology as a strategy to facilitate PDT in the oropharynx. To date, this anatomic site has not been a focus for head and neck PDT due to the limited access with traditional transoral approaches. With the application of robotic surgical technology, transoral robotic surgery for oropharyngeal carcinomas has been shown to not only be feasible but effective (55, 56). In the first robotic PDT of the oropharynx (Figure 3), we demonstrated that the robotic arms not only allow access to the oropharynx but facilitate very stable light delivery. Moreover, we demonstrated a significant 5-fold build up in the fluence rate, consistent with the light scattering geometry of the oropharyngeal mucosa.
Figure 3. Transoral Robotic Surgery Photodynamic Therapy (TORS-PDT).


Figure 3A demonstrates the intraoperative arrangement of the robotic arms holding the laser light source and a fiber measuring the fluence rate along with a light filter cap placed over the robotic camera (3B).
Scatter can also result in light being delivered to the mucosa adjacent to the illuminated region resulting in unnecessary mucositis. Several strategies may be adopted to absorb this scattered light depending on the anatomic site that is being treated. These include painting the mucosa with methylene blue or the placement of black thermal resistant materials such as wax that can be customed shaped around the edges of the illuminated region. Alternatively, saline soaked surgical white packing allowing it to be molded to the illuminated region may be used. This is an especially attractive and flexible approach when treating the pharynx where access is limited and where the packing may be placed and molded with long forceps. Whether or not surface scatter into the illuminated region is increased has not been systematically evaluated to date.
V. Clinical Results with Photodynamic Therapy for Premalignant Head and Neck Dysplasia
While various photosensitizers including porfirmer sodium (Photofrin®) (53, 57, 58), mTHPC (Foscan®) (59-61) and aminolevulinic acid (Levulan®) (62-66) have been reported for the treatment of dysplasia, there has been very few concerted studies for the management of dysplasia alone (58, 64). Table 1 summarizes the results for patients with dysplasia only in these reports. Many of these reports included the treatment of various early stages of invasive carcinoma and leukoplakia but without dysplasia (53, 59, 62, 63). These institutional experiences have primarily been retrospective in design with the exception of two prospective studies enrolling only patients with histologic evidence of dysplasia (58, 64). The results have all been based on calculated light irradiance for the area treated and have primarily used a laser microlens light source with surface illumination.
Table 1.
Summary of Studies Treating Head and Neck Mucosal Dysplasia with Photodynamic Therapy.
| Study | Photosensitizer | Prospective? | Number of Patients | Prescribed Light Dose | Dose Rate | Light Technique | Outcome |
|---|---|---|---|---|---|---|---|
| Biel et al. (53) | Photofrin | No | Oral cavity: 2 | Oral cavity: 50- 75 J/cm2 | 150 mW/cm2 | Surface (microlens) | Oral cavity: 2 CR |
| Larynx: 3 | Larynx: 80 J/cm2 | Larynx: 3 CR 1 | |||||
| Grant et al. (57) | Photofrin | No | 11 | 50-100 J/cm2 | ---- | Surface (microlens) | 10 CR |
| Rigual et al. (58) | Photofrin | Yes | Oral cavity: 9 | 50 J/cm2 | Not specified | Surface (microlens) | Oral cavity: 9 2 |
| Larynx: 3 | Larynx: 3 | ||||||
| Copper et al. (59) | Foscan | No | 3 | 20 J/cm2 | 100 mW/cm2 | Surface (microlens) | 3 CR |
| Savary et al. (60) | Foscan | Yes | 5 | 7-75 J/cm2 | 150 mW/cm2 | Surface (microlens) | 5 CR |
| Fan et al. (61) | Foscan | Yes | 3 | 5-10 J/cm2 | ≤ 250 mW/cm2 | Surface (microlens) | 3 CR |
| Chen et al. (62) | Levulan 3 | No | 2 | 100 J/cm2 | 100 mW/cm2 | Surface (LED) | 2 CR |
| Sieron et al. (63) | Levulan 3 | No | 2 | 100 J/cm2 | 150 mW/cm2 | Surface (microlens) | 2 CR |
| Yu et al. (64) | Levulan 3 | Yes | 46 | 100 J/cm2 | 100 mW/cm2 | Surface (microlens, n=26 and LED, n=20) | Microlens: 25 CR |
| LED: 17 CR | |||||||
| Kubler et al. (65) | Levulan 3 | No | 12 | 100 J/cm2 | 100 mW/cm2 | Surface (microlens) | 6 CR 4 |
| Fan et al. (66) | Levulan | No | 12 | Group 1: 200 J/cm2 fractionated | <250 mW/cm2 | Surface (microlens) | 7 with pathologic CR |
| Group 2: 100 J/cm2 fractionated |
For oral cavity lesions, a mean follow-up of 51 months noted 5 invasive relapses. For larynx lesions, a mean follow-up of 91 months noted 10 invasive relapses.
3 subsequent oral cavity dysplasia relapses
topical administration
one patient with a complete response following a second PDT treatment
CR: complete response (clinical)
mTHPC is currently approved by the European Union for the palliative management of advanced head and neck invasive carcinomas failing prior therapies. It is a potent selective photosensitizer and is typically administered at a dose of 0.15 mg/kg body weight with a drug to light intervarl of 90-110 hours. Photoactivation is with an incident light dose of 20 J/cm2 typically delivered at an incidence of 100 mW/cm2 to the surface. Hence, it has the advantage of a short illumination time affording even intraoral treatment of the tonsil and soft palate sites without the need for general anesthesia. For dysplasia, high activity has been documented based on complete responses for the target lesion.
Recently, Rigual et al reported the results of a prospective study of porfimer sodium for the treatment of moderate to severe dysplasia and early T1 invasive oral and laryngeal carcinomas (58). For dysplasia, a single fluence of 50 J/cm2 and for carcinoma a fluence of 75 J/cm2 was prescribed and administered. With a mean follow-up of 15 months (7-52 months) 24 of 26 evaluable subjects had a complete response. Among patients with dysplasia, all 12 patients had a complete response but 3 of 9 subjects with dysplasia of the oral cavity relapsed. In contrast, all 3 laryngeal dysplasia remained clinically free of relapse at the time of the reporting. The authors speculated that the relapses in the oral cavity may have been due to the keratin layer limiting sufficient light penetration with light reflectance. This concern is consistent with our experience for oral cavity lesions where the ability to achieve a perpendicular mucosal surface can be difficult especially with fibrotic complications from past surgical resections.
Aminolevulinic acid (ALA) has been the drug (a photosensitizer precursor) most extensively studied for dysplasia and primarily studied as a topical application (62-65). Various histologic diagnosis of leukoplakia including dysplasia and various keratoses have been studied (62-66). In general, the experience has demonstrated that ALA is limited in its depth of activity, generally sufficient for 1.5 mm thick lesions (66), and when applied topically, requires several treatments to achieve a clinical complete response. More treatments are needed to achieve a complete response for lesions with a thicker keratin layer reflecting, in part, the limited depth of drug surface absorption along with the dilution effects of saliva with a topical administration (64). Studies suggest that systemic ALA may be more effective than topical administration (67). No significant adverse toxicities including late fibrotic complications have been reported (58, 64).
Yu et al has also reported the results of a mature prospective study of ALA administered topically as a 20% solution. The prescription was 100 J/cm2 at a 100 mW/cm2. Two cohorts were defined by whether they were treated with a standard laser microlens source or a LED light source (Table 1). With a mean follow of 32 months (16-72 months), the complete response rates were 96% and 85% respectively. However, relapses rates of 20% and 29% were reported in each respective cohort. On average, patients required multiple topical applications and treatments due to the limited surface absorption with thicker lesions significantly requiring more treatments. As such, preclinical studies demonstrating that systemic ALA may be more effective than topical ALA are important to consider in the clinical development of PDT treatment for dysplasia.
Systemic ALA for dysplasia at its maximum tolerated dose of 60 mg/kg has been reported by Fan et al (66). Two fluence dose cohorts were studied, one at 200 J/cm2 in 2 equal fractions and one at 100 J/cm2 in 2 unequal fractions. However, the fluence rate was significant and noted to be <250 mW/cm2 with multiple potentially overlapping illumination fields used for some patients to achieve adequate illumination due to the anatomic constraints already discussed in the oral cavity. This poses a considerable risk of very high fluence rates even without considering the impact of light scatter that may have an adverse hypoxic effect on the efficacy of the treatment. Not surprisingly, only a 58% complete response rate was reported for dysplastic lesions.
Collectively, these institutional experiences are consistent in demonstrating that PDT can safely remove the dysplastic lesions at a high rate likely in more than 80% of cases. The optimal dose is unclear as no studies have measured the effective fluence rate that increases due to light scatter. As such, we have initiated a dose escalation study of systemic ALA PDT for the treatment of dysplastic lesion in the head and neck based on measured delivered fluence and a target fluence rate of 100 mW/cm2 (Table 2). The experience to date also lacks the reporting of actuarial treatment outcome with mature follow-up. As many patients with dysplasia can have a field effect with multiple lesions, it becomes important to characterize relapses based as in-field or out of field relapses. It therefore becomes difficult to conclude with confidence that PDT can afford long-term control rates. More so, not characterized to date is the risk of subsequent malignant transformation both within and outside of the illuminated field.
Table 2.
University of Pennsylvania Phase 1 Dose Escalation Study Schema.
| Cohort | Light Dose (J/cm 2) | Fractionation |
|---|---|---|
| 1 | 50 | no |
| 2 | 50 | yes |
| Randomization begins | ||
| 3a | 100 | no |
| 3b | 100 | yes |
| 4a | 150 | no |
| 4b | 150 | yes |
| 5a | 200 | no |
|
| ||
| 5b | 200 | yes |
VI. Conclusions
In summary, PDT has been shown to have significant activity in eradicating dysplastic mucosal cells of the head and neck. Ongoing research efforts including photosensitizer drug development, light delivery techniques and devices and the development of real time monitoring during the treatment that offer the promise of optimizing current treatment outcomes. As a management strategy to prevent the development of invasive carcinomas, it has several advantages including the ability to treat a broad surface area superficially without the risk of developing fibrotic and functional debilitating complications. Moreover, the ability to repeat treatments without cumulative toxicities offers the promise that this treatment approach may one day be considered as first line therapy in patients demonstrating pre-malignant mucosal disease.
Acknowledgments
This study was supported in part by the National Institutes of Health (R01-CA-129554), American Cancer Society (IRG-78-002-28) and T32-CA-009677. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflict of Interests: None
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pignon J-P, le Maître A, Bourhis J. Meta-Analyses of Chemotherapy in Head and Neck Cancer (MACH-NC): An Update. International Journal of Radiation Oncology*Biology*Physics. 2007;69(2, Supplement 1):S112–S4. doi: 10.1016/j.ijrobp.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 2.Bourhis J, Overgaard J, Audry H, Ang KK, Saunders M, Bernier J, et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: a meta-analysis. Lancet. 2006 Sep 2;368(9538):843–54. doi: 10.1016/S0140-6736(06)69121-6. [DOI] [PubMed] [Google Scholar]
- 3.Langendijk JA, Doornaert P, Rietveld DH, Verdonck-de Leeuw IM, Leemans CR, Slotman BJ. A predictive model for swallowing dysfunction after curative radiotherapy in head and neck cancer. Radiother Oncol. 2009 Feb;90(2):189–95. doi: 10.1016/j.radonc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008 Jul 20;26(21):3582–9. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith J, Rattay T, McConkey C, Helliwell T, Mehanna H. Biomarkers in dysplasia of the oral cavity: a systematic review. Oral Oncol. 2009 Aug;45(8):647–53. doi: 10.1016/j.oraloncology.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Mehanna HM, Rattay T, Smith J, McConkey CC. Treatment and follow-up of oral dysplasia - a systematic review and meta-analysis. Head & neck. 2009 Dec;31(12):1600–9. doi: 10.1002/hed.21131. [DOI] [PubMed] [Google Scholar]
- 7.Stefano P. Pooled estimate of world leukoplakia prevalence: a systematic review. Oral oncology. 2003;39(8):770–80. doi: 10.1016/s1368-8375(03)00102-7. [DOI] [PubMed] [Google Scholar]
- 8.Weller MD, Nankivell PC, McConkey C, Paleri V, Mehanna HM. The risk and interval to malignancy of patients with laryngeal dysplasia; a systematic review of case series and meta-analysis. Clin Otolaryngol. 2010 Oct;35(5):364–72. doi: 10.1111/j.1749-4486.2010.02181.x. [DOI] [PubMed] [Google Scholar]
- 9.Al-Dakkak I. Oral dysplasia and risk of progression to cancer. Evidence-based dentistry. 2010;11(3):91–2. doi: 10.1038/sj.ebd.6400745. [DOI] [PubMed] [Google Scholar]
- 10.Yang SW, Wu CJ, Lee YS, Chen TA, Tsai CN. Postoperative recurrence as an associated factor of malignant transformation of oral dysplastic leukoplakia. ORL; journal for oto-rhino-laryngology and its related specialties. 2010;72(5):280–90. doi: 10.1159/000318874. [DOI] [PubMed] [Google Scholar]
- 11.Rosin MP, Cheng X, Poh C, Lam WL, Huang Y, Lovas J, et al. Use of allelic loss to predict malignant risk for low-grade oral epithelial dysplasia. Clin Cancer Res. 2000 Feb;6(2):357–62. [PubMed] [Google Scholar]
- 12.van der Waal I, Schepman KP, van der Meij EH, Smeele LE. Oral leukoplakia: a Clinicopathological review. Oral Oncology. 1997;33(5):291–301. doi: 10.1016/s1368-8375(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 13.Damm M, Sittel C, Streppel M, Eckel HE. Transoral CO2 Laser for Surgical Management of Glottic Carcinoma in Situ. The Laryngoscope. 2000;110(7):1215–21. doi: 10.1097/00005537-200007000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Sadri M, McMahon J, Parker A. Management of laryngeal dysplasia: a review. Eur Arch Otorhinolaryngol. 2006 Sep;263(9):843–52. doi: 10.1007/s00405-006-0078-y. [DOI] [PubMed] [Google Scholar]
- 15.Pitiyage G, Tilakaratne WM, Tavassoli M, Warnakulasuriya S. Molecular markers in oral epithelial dysplasia: review. J Oral Pathol Med. 2009 Nov;38(10):737–52. doi: 10.1111/j.1600-0714.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 16.Lodi G, Sardella A, Bez C, Demarosi F, Carrassi A. Interventions for treating oral leukoplakia. Cochrane Database Syst Rev. 2006;(4) doi: 10.1002/14651858.CD001829.pub3. CD001829. [DOI] [PubMed] [Google Scholar]
- 17.Epstein JB, Gorsky M, Fischer D, Gupta A, Epstein M, Elad S. A survey of the current approaches to diagnosis and management of oral premalignant lesions. Journal of the American Dental Association (1939) 2007 Dec;138(12):1555–62. doi: 10.14219/jada.archive.2007.0104. quiz 614. [DOI] [PubMed] [Google Scholar]
- 18.Hamadah O, Thomson PJ. Factors affecting carbon dioxide laser treatment for oral precancer: a patient cohort study. Lasers Surg Med. 2009 Jan;41(1):17–25. doi: 10.1002/lsm.20733. [DOI] [PubMed] [Google Scholar]
- 19.Grossman C, Zhu T, Finlay J, Dimofte A, Malloy K, O’Malley B, Jr, et al. Targeted laryngeal photodynamic therapy with a balloon diffusing light source. Photodiagnosis Photodyn Ther. 2010 Sep;7(3):158–61. doi: 10.1016/j.pdpdt.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HW, Putt ME, Emanuele MJ, Shin DB, Glatstein E, Yodh AG, et al. Treatment-induced changes in tumor oxygenation predict photodynamic therapy outcome. Cancer Res. 2004 Oct 15;64(20):7553–61. doi: 10.1158/0008-5472.CAN-03-3632. [DOI] [PubMed] [Google Scholar]
- 21.Ishimaru A. Wave propagation and scattering in random media. New York: IEEE Press; 1997. [Google Scholar]
- 22.Henderson BW, Gollnick SO, Snyder JW, Busch TM, Kousis PC, Cheney RT, et al. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004 Mar 15;64(6):2120–6. doi: 10.1158/0008-5472.can-03-3513. [DOI] [PubMed] [Google Scholar]
- 23.Busch TM, Xing X, Yu G, Yodh A, Wileyto EP, Wang HW, et al. Fluence rate-dependent intratumor heterogeneity in physiologic and cytotoxic responses to Photofrin photodynamic therapy. Photochem Photobiol Sci. 2009 Dec;8(12):1683–93. doi: 10.1039/b9pp00004f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Middelburg TA, van Zaane F, de Bruijn HS, van der Ploeg-van den Heuvel A, Sterenborg HJ, Neumann HA, et al. Fractionated Illumination at Low Fluence Rate Photodynamic Therapy in Mice. Photochem Photobiol. Jun 10; doi: 10.1111/j.1751-1097.2010.00760.x. [DOI] [PubMed] [Google Scholar]
- 25.Angell-Petersen E, Spetalen S, Madsen SJ, Sun CH, Peng Q, Carper SW, et al. Influence of light fluence rate on the effects of photodynamic therapy in an orthotopic rat glioma model. J Neurosurg. 2006 Jan;104(1):109–17. doi: 10.3171/jns.2006.104.1.109. [DOI] [PubMed] [Google Scholar]
- 26.Cottrell WJ, Paquette AD, Keymel KR, Foster TH, Oseroff AR. Irradiance-dependent photobleaching and pain in delta-aminolevulinic acid-photodynamic therapy of superficial basal cell carcinomas. Clin Cancer Res. 2008 Jul 15;14(14):4475–83. doi: 10.1158/1078-0432.CCR-07-5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson DJ, de Bruijn HS, Star WM, Sterenborg HJ. Dose and timing of the first light fraction in two-fold illumination schemes for topical ALA-mediated photodynamic therapy of hairless mouse skin. Photochemistry & Photobiology. 2003;77(3):319–23. doi: 10.1562/0031-8655(2003)077<0319:DATOTF>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Star WM, van’t Veen AJ, Robinson DJ, Munte K, de Haas ER, Sterenborg HJ. Topical 5-aminolevulinic acid mediated photodynamic therapy of superficial basal cell carcinoma using two light fractions with a two-hour interval: long-term follow-up. Acta Derm Venereol. 2006;86(5):412–7. doi: 10.2340/00015555-0129. [DOI] [PubMed] [Google Scholar]
- 29.Curnow A, McIlroy BW, Postle-Hacon MJ, MacRobert AJ, Bown SG. Light dose fractionation to enhance photodynamic therapy using 5-aminolevulinic acid in the normal rat colon. Photochem Photobiol. 1999 Jan;69(1):71–6. [PubMed] [Google Scholar]
- 30.Messmann H, Mlkvy P, Buonaccorsi G, Davies CL, MacRobert AJ, Bown SG. Enhancement of photodynamic therapy with 5-aminolaevulinic acid-induced porphyrin photosensitisation in normal rat colon by threshold and light fractionation studies. Br J Cancer. 1995 Sep;72(3):589–94. doi: 10.1038/bjc.1995.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curnow A, Haller JC, Bown SG. Oxygen monitoring during 5-aminolaevulinic acid induced photodynamic therapy in normal rat colon. Comparison of continuous and fractionated light regimes. Journal of Photochemistry & Photobiology B - Biology. 2000;58(2-3):149–55. doi: 10.1016/s1011-1344(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 32.Curnow A, Bown SG. The role of reperfusion injury in photodynamic therapy with 5-aminolaevulinic acid--a study on normal rat colon. British Journal of Cancer. 2002 Mar 18;86(6):989–92. doi: 10.1038/sj.bjc.6600178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pogue BW, O’Hara JA, Goodwin IA, Wilmot CJ, Fournier GP, Akay AR, et al. Tumor PO(2) changes during photodynamic therapy depend upon photosensitizer type and time after injection. Comparative Biochemistry & Physiology Part A, Molecular & Integrative Physiology. 2002;132(1):177–84. doi: 10.1016/s1095-6433(01)00545-1. [DOI] [PubMed] [Google Scholar]
- 34.Korbelik M, Krosl G. Cellular levels of photosensitisers in tumours: the role of proximity to the blood supply. Br J Cancer. 1994 Oct;70(4):604–10. doi: 10.1038/bjc.1994.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen B, Crane C, He C, Gondek D, Agharkar P, Savellano MD, et al. Disparity between prostate tumor interior versus peripheral vasculature in response to verteporfin-mediated vascular-targeting therapy. Int J Cancer. 2008 Aug 1;123(3):695–701. doi: 10.1002/ijc.23538. [DOI] [PubMed] [Google Scholar]
- 36.Busch TM, Wang HW, Wileyto EP, Yu G, Bunte RM. Increasing Damage to Tumor Blood Vessels during Motexafin Lutetium-PDT through Use of Low Fluence Rate. Radiation research. 2010 Sep;174(3):331–40. doi: 10.1667/RR2075.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li LB, Luo RC. Effect of drug-light interval on the mode of action of Photofrin photodynamic therapy in a mouse tumor model. Lasers in medical science. 2009 Jul;24(4):597–603. doi: 10.1007/s10103-008-0620-9. [DOI] [PubMed] [Google Scholar]
- 38.Chen B, Pogue BW, Hoopes PJ, Hasan T. Combining vascular and cellular targeting regimens enhances the efficacy of photodynamic therapy. International journal of radiation oncology, biology, physics. 2005 Mar 15;61(4):1216–26. doi: 10.1016/j.ijrobp.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 39.Star W. The relationship between integrating sphere and diffusion theory calculations of fluence rate at the wall of a spherical cavity. Phys Med Biol. 1995;40:1–8. doi: 10.1088/0031-9155/40/1/001. [DOI] [PubMed] [Google Scholar]
- 40.Tan IB, Oppelaar H, Ruevekamp MC, Veenhuizen RB, Timmers A, Stewart FA. The importance of in situ light dosimetry for photodynamic therapy of oral cavity tumors. Head Neck. 1999 Aug;21(5):434–41. doi: 10.1002/(sici)1097-0347(199908)21:5<434::aid-hed9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 41.Mourant JR, Freyer JP, Hielscher AH, Eick AA, Shen D, Johnson TM. Mechanisms of light scattering from biological cells relevant to noninvasive optical-tissue diagnosis. Appl Opt. 1998;37:3586–93. doi: 10.1364/ao.37.003586. [DOI] [PubMed] [Google Scholar]
- 42.Vulcan TG, Zhu TC, Rodriguez CE, Hsi A, Fraker DL, Baas P, et al. Comparison between isotropic and nonisotropic dosimetry systems during intraperitoneal photodynamic therapy. Lasers in surgery and medicine. 2000;26(3):292–301. doi: 10.1002/(sici)1096-9101(2000)26:3<292::aid-lsm7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 43.Zaidi SI, Oleinick NL, Zaim MT, Mukhtar H. Apoptosis during photodynamic therapy-induced ablation of RIF-1 tumors in C3H mice: electron microscopic, histopathologic and biochemical evidence. Photochemistry and photobiology. 1993 Dec;58(6):771–6. doi: 10.1111/j.1751-1097.1993.tb04969.x. [DOI] [PubMed] [Google Scholar]
- 44.Oleinick NL, Morris RL, Belichenko I. The role of apoptosis in response to photodynamic therapy: what, where, why, and how. Photochem Photobiol Sci. 2002 Jan;1(1):1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 45.Kessel D, Vicente MG, Reiners JJ., Jr Initiation of apoptosis and autophagy by photodynamic therapy. Autophagy. 2006 Oct-Dec;2(4):289–90. doi: 10.4161/auto.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korbelik M, Dougherty GJ. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999 Apr 15;59(8):1941–6. [PubMed] [Google Scholar]
- 47.Gollnick SO, Brackett CM. Enhancement of anti-tumor immunity by photodynamic therapy. Immunologic research. Mar;46(1-3):216–26. doi: 10.1007/s12026-009-8119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moor AC. Signaling pathways in cell death and survival after photodynamic therapy. Journal of photochemistry and photobiology. 2000 Aug;57(1):1–13. doi: 10.1016/s1011-1344(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 49.Hahn SM, Putt ME, Metz J, Shin DB, Rickter E, Menon C, et al. Photofrin uptake in the tumor and normal tissues of patients receiving intraperitoneal photodynamic therapy. Clin Cancer Res. 2006 Sep 15;12(18):5464–70. doi: 10.1158/1078-0432.CCR-06-0953. [DOI] [PubMed] [Google Scholar]
- 50.Stepp H, Beck T, Pongratz T, Meinel T, Kreth FW, Tonn J, et al. ALA and malignant glioma: fluorescence-guided resection and photodynamic treatment. J Environ Pathol Toxicol Oncol. 2007;26(2):157–64. doi: 10.1615/jenvironpatholtoxicoloncol.v26.i2.110. [DOI] [PubMed] [Google Scholar]
- 51.Wang CY, Tsai T, Chiang CP, Chen HM, Chen CT. Improved diagnosis of oral premalignant lesions in submucous fibrosis patients with 5-aminolevulinic acid induced PpIX fluorescence. J Biomed Opt. 2009 Jul-Aug;14(4):044026. doi: 10.1117/1.3200934. [DOI] [PubMed] [Google Scholar]
- 52.Taroni P, Pifferi A, Torricelli A, Comelli D, Cubeddu R. In vivo absorption and scattering spectroscopy of biological tissues. Photochem Photobiol Sci. 2003 Feb;2(2):124–9. doi: 10.1039/b209651j. [DOI] [PubMed] [Google Scholar]
- 53.Biel MA. Photodynamic therapy treatment of early oral and laryngeal cancers. Photochemistry and photobiology. 2007 Sep-Oct;83(5):1063–8. doi: 10.1111/j.1751-1097.2007.00153.x. [DOI] [PubMed] [Google Scholar]
- 54.Nyst HJ, van Veen RL, Tan IB, Peters R, Spaniol S, Robinson DJ, et al. Performance of a dedicated light delivery and dosimetry device for photodynamic therapy of nasopharyngeal carcinoma: phantom and volunteer experiments. Lasers Surg Med. 2007 Sep;39(8):647–53. doi: 10.1002/lsm.20536. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein GS, O’Malley BW, Jr, Snyder W, Sherman E, Quon H. Transoral Robotic Surgery: Radical Tonsillectomy. Arch Otolaryngol Head Neck Surg. 2007 Dec 1;133(12):1220–6. doi: 10.1001/archotol.133.12.1220. [DOI] [PubMed] [Google Scholar]
- 56.O’Malley BW, Jr, Weinstein GS, Snyder W, Hockstein NG. Transoral robotic surgery (TORS) for base of tongue neoplasms. The Laryngoscope. 2006 Aug;116(8):1465–72. doi: 10.1097/01.mlg.0000227184.90514.1a. [DOI] [PubMed] [Google Scholar]
- 57.Grant WE, Hopper C, Speight PM, Macrobert AJ, Bown SG. Photodynamic therapy of malignant and premalignant lesions in patients with ‘field cancerization’ of the oral cavity. The Journal of laryngology and otology. 1993 Dec;107(12):1140–5. doi: 10.1017/s0022215100125496. [DOI] [PubMed] [Google Scholar]
- 58.Rigual NR, Thankappan K, Cooper M, Sullivan MA, Dougherty T, Popat SR, et al. Photodynamic therapy for head and neck dysplasia and cancer. Arch Otolaryngol Head Neck Surg. 2009 Aug;135(8):784–8. doi: 10.1001/archoto.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Copper MP, Triesscheijn M, Tan IB, Ruevekamp MC, Stewart FA. Photodynamic therapy in the treatment of multiple primary tumours in the head and neck, located to the oral cavity and oropharynx. Clin Otolaryngol. 2007 Jun;32(3):185–9. doi: 10.1111/j.1365-2273.2007.01441.x. [DOI] [PubMed] [Google Scholar]
- 60.Savary JF, Monnier P, Fontolliet C, Mizeret J, Wagnieres G, Braichotte D, et al. Photodynamic therapy for early squamous cell carcinomas of the esophagus, bronchi, and mouth with m-tetra (hydroxyphenyl) chlorin. Arch Otolaryngol Head Neck Surg. 1997 Feb;123(2):162–8. doi: 10.1001/archotol.1997.01900020042006. [DOI] [PubMed] [Google Scholar]
- 61.Fan KF, Hopper C, Speight PM, Buonaccorsi GA, Bown SG. Photodynamic therapy using mTHPC for malignant disease in the oral cavity. Int J Cancer. 1997 Sep 26;73(1):25–32. doi: 10.1002/(sici)1097-0215(19970926)73:1<25::aid-ijc5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 62.Chen HM, Yu CH, Tu PC, Yeh CY, Tsai T, Chiang CP. Successful treatment of oral verrucous hyperplasia and oral leukoplakia with topical 5-aminolevulinic acid-mediated photodynamic therapy. Lasers Surg Med. 2005 Aug;37(2):114–22. doi: 10.1002/lsm.20214. [DOI] [PubMed] [Google Scholar]
- 63.Sieron A, Adamek M, Kawczyk-Krupka A, Mazur S, Ilewicz L. Photodynamic therapy (PDT) using topically applied delta-aminolevulinic acid (ALA) for the treatment of oral leukoplakia. J Oral Pathol Med. 2003 Jul;32(6):330–6. doi: 10.1034/j.1600-0714.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 64.Yu CH, Lin HP, Chen HM, Yang H, Wang YP, Chiang CP. Comparison of clinical outcomes of oral erythroleukoplakia treated with photodynamic therapy using either light-emitting diode or laser light. Lasers Surg Med. 2009 Nov;41(9):628–33. doi: 10.1002/lsm.20841. [DOI] [PubMed] [Google Scholar]
- 65.Kubler A, Haase T, Rheinwald M, Barth T, Muhling J. Treatment of oral leukoplakia by topical application of 5-aminolevulinic acid. International journal of oral and maxillofacial surgery. 1998 Dec;27(6):466–9. doi: 10.1016/s0901-5027(98)80040-4. [DOI] [PubMed] [Google Scholar]
- 66.Fan KF, Hopper C, Speight PM, Buonaccorsi G, MacRobert AJ, Bown SG. Photodynamic therapy using 5-aminolevulinic acid for premalignant and malignant lesions of the oral cavity. Cancer. 1996 Oct 1;78(7):1374–83. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1374::AID-CNCR2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 67.Ma G, Ikeda H, Inokuchi T, Sano K. Effect of photodynamic therapy using 5-aminolevulinic acid on 4-nitroquinoline-1-oxide-induced premalignant and malignant lesions of mouse tongue. Oral Oncol. 1999 Jan;35(1):120–4. doi: 10.1016/s1368-8375(98)00066-9. [DOI] [PubMed] [Google Scholar]


