Abstract
Crystallin proteins are responsible for maintaining lens transparency and allowing the lens to focus light undistorted onto the retina. The α-crystallins are the major lens crystallins, and function as both structural proteins and chaperones to protect all lens proteins from damage leading to lens deterioration. Because lens crystallin proteins do not turn over, the damage they accumulate can lead to cataracts, the world’s leading cause of blindness. Photosensitizing porphyrins can accumulate in the eye through either endogenous metabolism or through therapeutic or diagnostic procedures. Porphyrin buildup exacerbates lens aging through increased levels of singlet oxygen, resulting in protein polymerization and amino acid residue alteration. Tryptophans oxidize to kynurenine and N-formylkynurenine (NFK) causing irreversible changes in the refractive index of the normally transparent lens, leading to development of cataracts. Additionally, NFK is itself a photosensitizer, and its presence exacerbates lens deterioration. This work uses anti-NFK antiserum to study porphyrin-facilitated photooxidation of α-crystallin tryptophan residues. In vitro experiments show that four biologically interesting porphyrins mediate α-crystallin polymerization and accumulation of both protein radicals and NFK. Confocal microscopy of cultured human lens epithelial cells indicates that while all four porphyrins photosensitize cellular proteins, not all oxidize the tryptophans of cellular α-crystallin to NFK.
INTRODUCTION
The predominant proteins of the vertebrate eye lens are the crystallins, with α-crystallin comprising up to 40-50% of all lens protein (1,2), and the two similar subunits, αA and αB, occurring in a ratio of approximately 3:1. While both subunits are found in other organs, αB-crystallin is far more ubiquitous, acting as a heat shock protein/chaperone in the lens and in other tissues (1,2). Alpha-crystallin, thus, plays a dual role in the lens: 1) It assembles into an orderly arrangement of protein fibers that results in a transparent lens and 2) It acts as a chaperone to maintain the integrity of all lens proteins, including itself and the other crystallins (1,2). Interest in the role of α-crystallin as a chaperone, combined with studies of protein aggregation in disease development, have lead to the observation that increased levels of αB-crystallin are associated with neurological disorders such as Alexander’s disease, Creutzfeldt-Jacob disease, Alzheimers, Parkinsons (2).
The human lens continues to grow throughout our lifetime, although it grows more slowly with age. Progenitor lens epithelial cells differentiate into lens fiber cells which, at maturity, lack all intracellular membrane-bound organelles (3). Although new lens proteins continue to be produced, α-crystallin lens proteins do not turn over, and the lens retains all the α-crystallin it has synthesized. Damage to these essential proteins, therefore, is cumulative and can result in the buildup of altered amino acid residues leading to deleterious alterations in the refractive index and ultimately opacification (cataractogenesis) of the normally transparent lens (4). Despite advances in lens replacement technology, the cataracts thus produced remain the leading cause of blindness in the US and the world (5).
Porphyrins can accumulate in the eye and in other organs due to metabolic errors or when injected for diagnosis of cancer or as part of photodynamic cancer therapy (6-8). Porphyria cutanea tarda, for example, is a disorder resulting from low levels of one of the heme biosynthetic enzymes, leading to the buildup in the blood of the heme porphyrin intermediate uroporphyrin III (9). The subsequent dermal accumulation of this porphyrin leads to increased photosensitivity (10). Along with the skin, the eyes are the organs most exposed to light, and therefore the skin and the eyes are the organs most likely to suffer photooxidative damage. Although the cornea cuts off all radiation below 295 nm, this still allows wavelengths capable of photosensitizing porphyrins to reach the lens, which absorbs radiant energy between 295 nm and 400 nm. In vitro studies with different porphyrins have shown them to be efficient generators of singlet oxygen (1O2) which can cause polymerization of α-crystallin and can react with and modify amino acid residues (11). Additionally, some porphyrins can bind to lens proteins, thereby enhancing the potential for harmful interactions (11-13).
Photosensitized protein damage is understood to occur through two major and distinct routes. Type I reactions involve direct ionization of the protein through absorption of UV radiation by the protein itself or by a bound or adjacent chromophore, resulting in the generation of radicals. Type II, indirect, reactions occur via an energy transfer from the excited chromophore to molecular oxygen, resulting in the production of 1O2, which then reacts with the protein. The differentiation, however, between, radical (Type I) and non-radical, 1O2-mediated (Type II) reactions resulting from photosensitization is complicated by the long-lived hydroperoxides that can be produced in 1O2-exposed proteins and cells (14,15). Metal ions and UV radiation can catalyze the decomposition of these hydroperoxides to radicals (16,17) thereby increasing the potential for radical-mediated, but non-Type I, interactions (14,15,18).
While tyrosine, cysteine and methionine also react with 1O2 at biologically relevant rates, histidine and tryptophan have the highest 1O2 rate constants for reaction with protein side-chains at physiological pH (17). Uniquely amongst the amino acids, tryptophan can act as both a physical and chemical quencher of 1O2, with the relative contributions of the two routes being affected by various factors (16). Reaction products of tryptophan and tryptophan residues with 1O2 include hydroperoxides, hydrotryptophans, kynurenine and N-formylkynurenine (NFK). Identification of tryptophan, and other, oxidation end-products frequently involves time-consuming and technologically intense methods such as mass spectrometry (19-21) and HPLC (22,23) for identification of stable end-products. Unfortunately, the sample handling required for these kinds of analyses has the potential to generate artifacts (24), producing misleading data.
We recently published a more generally approachable, less technologically complex immunological method for detection of NFK residues in proteins (25). The use of our rabbit polyclonal anti-NFK antiserum has allowed us to detect NFK in photooxidized single proteins and protein mixtures, in human SOD1 oxidized by carbonate anion radical and to visualize NFK in cultured human HaCaT kerotinocyte cells (25,26).
In this study we investigate the role of NFK accumulation in the photobiology of cataract development. As photosensitizing agents we chose porphyrins that are of biological interest due to their roles in either a disease state (porphyria cutanea tarda) or their potential as agents for photodynamic therapy (6-9). We used UVA radiation to examine porphyrin photosensitizing effects on the accumulation of NFK in α-crystallin in vitro and in human lens epithelial (HLE) cell culture because UVA wavelengths best overlap those wavelengths absorbed by the human lens. In vitro photosensitization was performed in both the presence and absence of the 1O2 quencher azide and the radical spin trap/scavenger DMPO. Confocal microscopy was used to assess the extent of NFK colocalization with α-crystallin in photosensitized human epithelial cell culture.
MATERIALS AND METHODS
Materials
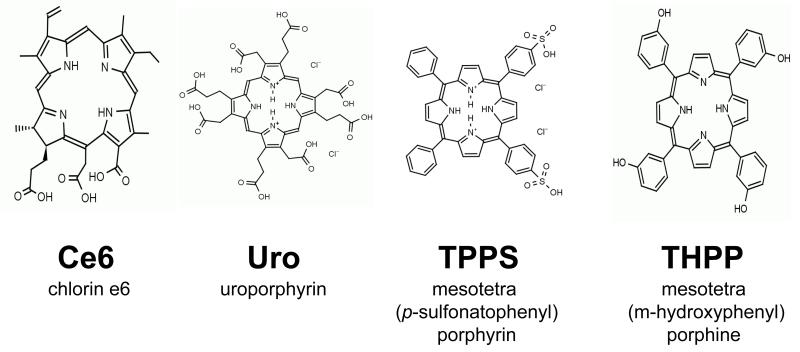
Bovine α-crystallin, TPPS (mesotetra (p-sulfonatophenyl) porphyrin) and uroporphyrin were purchased from Sigma Chemical Co. (St. Louis, MO). Ce6 (chlorin e6) and THPP (mesotetra (m-hydroxyphenyl) porphine) were purchased from Porphyrin Products (Logan, UT). Stock solutions of 1 mM TPPS, Uro and Ce6 were made in chelex-treated, 100 mM phosphate buffer, pH 7.4, while a 1 mM stock solution of THPP was dissolved in 95% ethanol. The structure of the four porphyrins used in this study are shown in Fig. 1.
Figure 1.
Chemical structure of porphyrins used in this study.
UVA irradiation
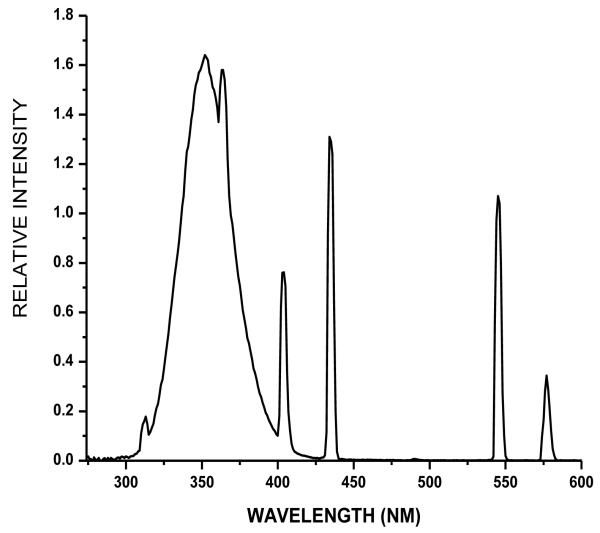
Solutions (0.5 ml) of 1 mg/ml bovine α-crystallin in chelex-treated, 100 mM phosphate buffer, pH 7.4, were placed in individual wells of a 24-well microtiter plate (Corning Incorporated, Corning, NY) with and without the addition of 1 or 10 μM porphyrin. The samples were then either kept in the dark (0 min, 0 J cm−1) or were irradiated with UVA for 10, 20 or 40 min at irradiances of 2.5, 5.0 and 10 J cm−1, respectively, as measured with a YSI-Kettering Model 65A Radiometer (Yellow Springs Instrument Co., Yellow Springs, OH). The UVA radiation source was composed of four parallel fluorescent UVA lamps (Houvalite F20T12BL-HO; National Biological Co. Twinsburg, OH). The emission spectrum of the UVA radiation was measured with a spectroradiometer (LuzChem Research, Inc., Ottawa, ON, Canada) (Fig. 2). Parallel experiments were performed with inhibitors and spin trap using 10 mM sodium azide, 200 mM DMPO individually or in combination.
Figure 2.
Emission spectrum of UVA radiation source with four parallel fluorescent UVA lamps.
Protein electrophoresis and western analysis
Duplicate gels containing 13 μg aliquots of α-crystallin were electrophoresed under reducing conditions. One gel of each pair was stained with Coomassie blue while the other was transferred to nitrocellulose for western analysis. Anti-NFK westerns were performed as previously described (25,26). Anti-DMPO westerns were performed using 10 μg/ml chicken polyclonal anti-DMPO (27).
Spectroscopic measurements
Absorption spectra were recorded on a Cary 100-Bio UV visible spectrophotometer. Fluorescence measurements were done on a FluoroLog 3 spectrofluorometer (Horiba Jobin Yvon, Edison, NY). Four hundred nm was chosen as the excitation wavelength because all samples absorb strongly at that wavelength.
Cell culture
An extended lifespan human lens epithelial cell line (HLE B-3) was used in these studies (28). Cells were grown in Eagle’s MEM (Sigma) containing 2 mm l-glutamine, 50 μg mL−1 gentamicin and 20% FBS in an atmosphere of 5% CO2/95% air at 37°C. Cells were fed three times a week and after attaining confluence were passaged using trypsin (0.125%)-EDTA (0.5 mM). For experiments with HLE cells, the cells were incubated for 18 h at 37°C in the dark with porphyrin photosensitizer, and, after washing with PBS were irradiated with UVA for 15 min (3.75 J cm−1) after which sodium azide was added to a final concentration of 10 mM.
Confocal microscroscopy
HLE B-3 cells (28) were seeded onto 35 mm plates containing 1.5 mm thick coverslips (MatTek, Ashland MA) and grown to near confluence. Control and treated cells were fixed and then stained simultaneously with rabbit polyclonal anti-NFK (1:250 dilution) and both mouse monoclonal anti-αA-crystallin and mouse monoclonal anti-αB-crystallin (1:500 dilutions) (Santa Cruz Biotechnology Inc., Santa Cruz, CA). After washing the cells were then stained simultaneously with 1:1000 dilutions of Alexa Fluor 488 anti-mouse and Alexa Fluor 568 anti-rabbit antibodies. For visualization of nuclei, cells were stained for 10 min in a solution of 1 μg/ml DAPI (Thermo Scientific, Rockford, IL).
RESULTS
Photosensitization of bovine α-crystallin
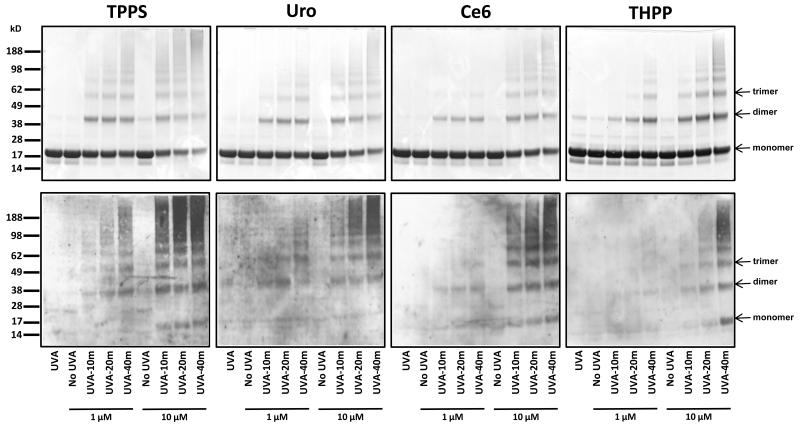
Bovine α-crystallin was mixed with 1 or 10 μM porphyrin photosensizer (Fig. 1) and exposed to increasing durations of UVA irradiation (0, 10 min, 20 min, 40 min). Control (porphyrin minus) aliquots of protein were also irradiated for 40 min with UVA. Samples were then subjected to SDS-PAGE electrophoresis for parallel examination of subunit cross-linking by Coomassie blue staining and tryptophan residue oxidation by anti-NFK western blotting. Coomassie staining of the samples showed that photosensitization with all four porphyrins resulted in polymerization of α-crystallin (Fig. 3, top panel). TPPS and Uro were most efficient in promoting photo-cross linking of lens protein, forming trimers and higher molecular weight polymers at a concentration of 1 μM after only 10 or 20 minutes of UVA exposure. Both THPP and Ce6 also promoted polymerization to high molecular weight polymers, but required more extensive photosensitization, achieved through either a higher concentration of porphyrin or longer UVA exposure.
Figure 3.
Photosensitization of α-crystallin by porphyrins promotes cross-linking and accumulation of NFK. Aliquots of α-crystallin were incubated without addition of porphyrin or with 1 μM and 10 μM of the indicated porphyrins and were either kept in the dark (no UVA) or were exposed to 10, 20 or 40 min of UVA irradiation as described in the materials and methods. For each porphyrin duplicate gels containing 13 μg of protein per lane were subjected to SDS-PAGE electrophoresis under reducing conditions and one gel was stained with Coomassie blue (top panels) and the other was transferred to nitrocellulose for western analysis with anti-NFK antiserum (bottom panels).
The anti-NFK western blots roughly parallelled the Coomassie gels (Fig. 3, bottom panel). The efficacy of α-crystallin photosensitization as measured by tryptophan conversion to NFK (TPPS>Uro>Ce6>THPP) corroborates the results from protein staining (Fig. 3). Where there is little or no cross-linking, as in the UVA only and the dark/photosensitizer controls, there is little or no detectable NFK. NFK accumulation was detected in dimers, trimers and higher molecular weight α-crystallin polymers in all or nearly all the bands seen in the Coomassie-stained gels. And, with the exception of the Uro-photooxidized samples, NFK was also present at above background levels in at least some of the α-crystallin monomers.
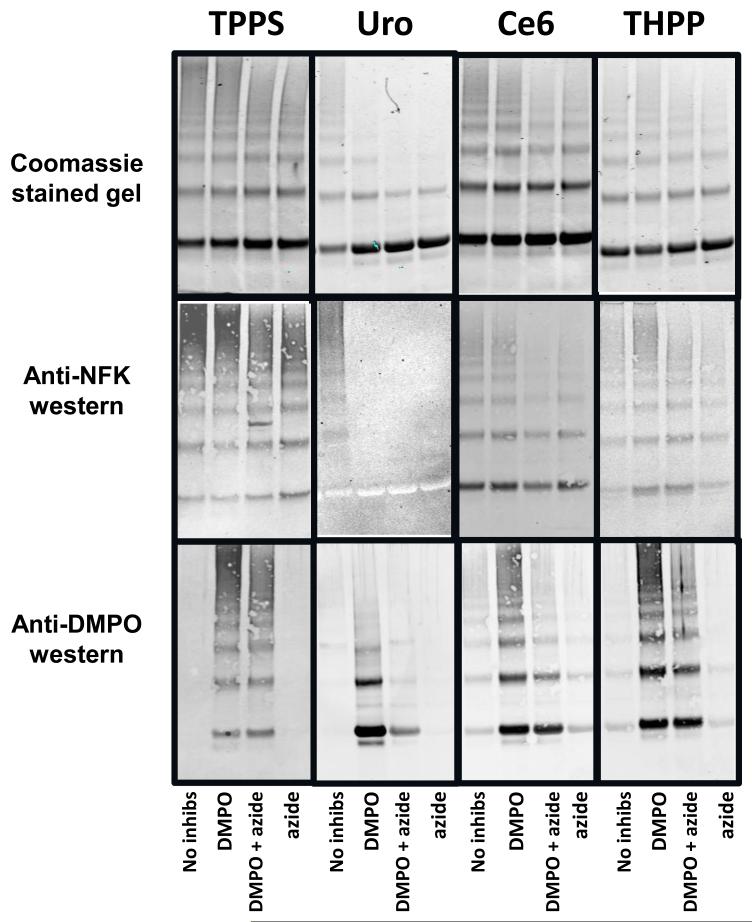
Parallel irradiations were also performed in the presence of the 1O2 quencher sodium azide, the radical scavenger/spin trap DMPO, or both (Fig. 4). This experiment used only the maximum photosensitization conditions used in Fig.1, i.e. 10 μM porphyrin and 40 min UVA irradiation. Coomassie staining of SDS-PAGE gels (top panel) showed that, although neither azide nor DMPO alone or in combination completely inhibited photo-induced cross-linking, azide was somewhat more effective than DMPO.
Figure 4.
Photosensitization of α-crystallin by porphyrins in the presence of the singlet oxygen quencher azide and the radical spin trap/scavenger DMPO. Aliquots of α-crystallin containing 10 μM of the indicated porphyrin, and containing either no further additions or 10 mM NaN3 or 200 mM DMPO or both, as indicated, were exposed to 40 min of UVA irradiation as described in the materials and methods. For each porphyrin three duplicate gels containing 13 μg of protein per lane were subjected to SDS-PAGE electrophoresis under reducing conditions. One gel was stained with Coomassie blue (top panels), and two were transferred to nitrocellulose for western blot analysis with anti-NFK (middle panels) and anti-DMPO (bottom panels) antisera
Anti-NFK blots western blots (Fig. 4 middle panel) showed that the effect of azide on NFK accumulation generally echoed that of the effect of azide on cross-linking. Azide reduced NFK accumulation in the Ce6 and THPP samples, but produced little, if any, decrease in the TPPS samples. The Uro reactions were most drastically effected, with NFK accumulation becoming undetectable. Anti-DMPO westerns (Fig. 4, bottom panel) showed that addition of DMPO allowed the trapping of α-crystallin radicals in all photosensitized samples. These western blots also showed that supplementation of azide to the DMPO-containing, Uro-photosensitized samples substantially reduced radical adduct production. Azide supplementation of the comparable DMPO-containing, Ce6- and THPP-photosensitized samples resulted in a less marked, but still detectable, decrease in DMPO adduct accumulation.
Absorption and fluorescence spectra of porphyrins with and without α-crystallin
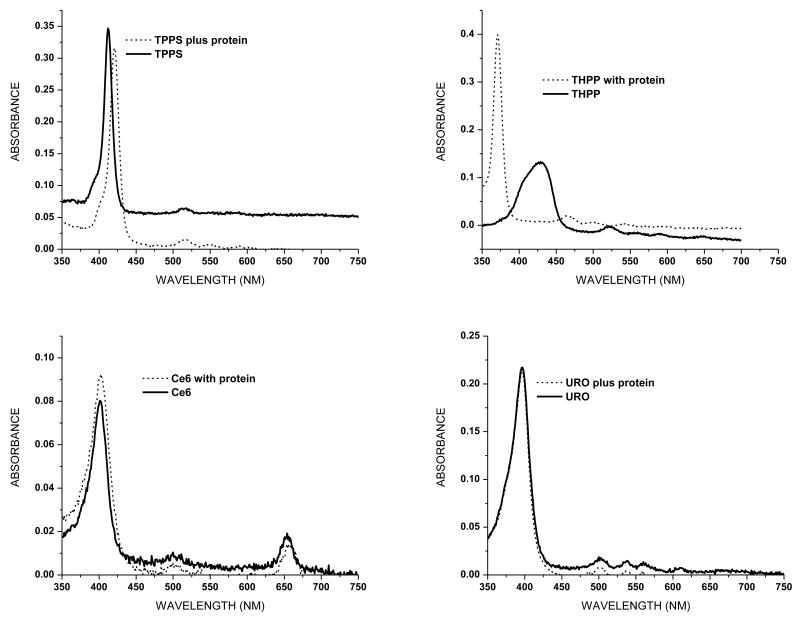
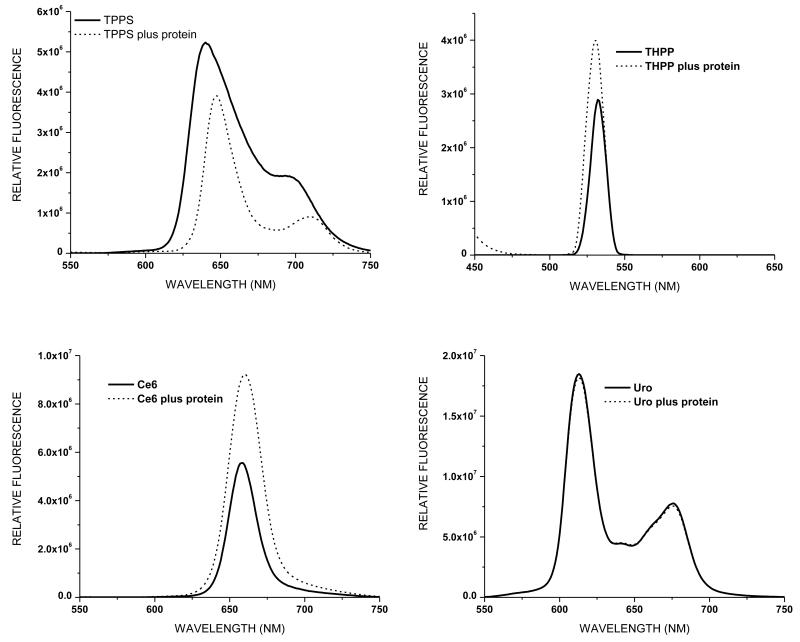
Binding of porphyrins to α-crystallin would increase their potential for producing 1O2-mediated protein damage. To assess binding between the porphyrins in this study and α-crystallin we determined porphyrin absorption and fluorescence spectra in the absence and presence of the protein. The absorption spectra of the 1 μM porphyrin samples, both with and without 1 mg/ml α-crystallin, are shown in Fig. 5. The presence of the lens protein has no effect on the absorption peak of either Ce6 or Uro, while the absorption peak of TPPS is slightly red shifted in the presence of α-crystallin, consistent with previously published work (11). The absorption spectrum of THPP is most altered by the presence of α-crystallin, with a large blue shift and both a narrowing and heightening of the peak. Fluorescence emission spectra of the same samples excited at a wavelength of 400 nm are shown in Fig. 6. While the fluorescence spectrum of Uro is unaffected by the presence of α-crystallin protein the spectra of the other porphyrins are altered in both amplitude and emission maxima. The emission spectrum of TPPS is both reduced and the peak red-shifted. Both Ce6 and THPP show enhanced fluorescence in the presence of α-crystallin, with the emission peak of Ce6 being slightly red-shifted while the emission spectrum of THPP is very slightly blue-shifted.
Figure 5.
Absorption spectra of porphyrins with and without 1 mg/ml α-crystallin. A solution of 1 mg/ml was used to zero the spectrophotometer.
Figure 6.
Fluorescence spectra of porphyrins with and without 1 mg/ml α-crystallin. Samples were excited at a wavelength of 400 nm.
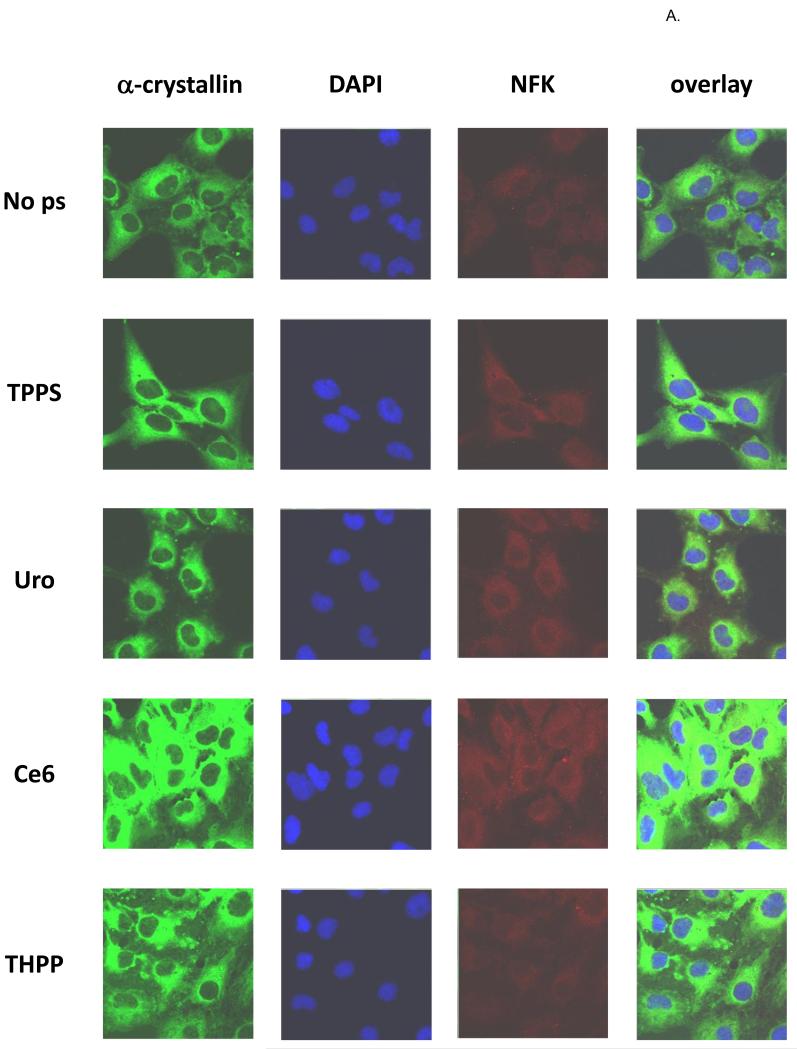
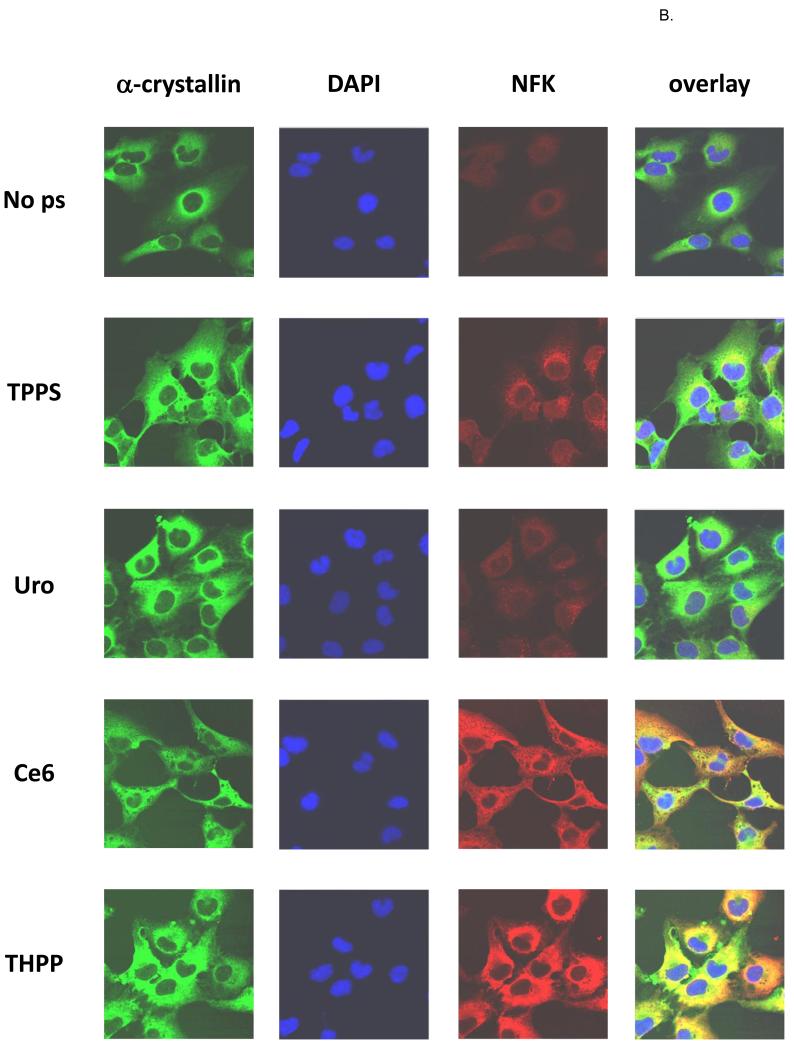
Detection of NFK in HLE cells and colocalization with α-crystallin
HLE cells were incubated for 18 h in the dark with porphyrin photosensitizers, and after washing with PBS were exposed to UVA or were kept in the dark. After fixation the cells were stained with anti-α-crystallin (green), DAPI (blue), and anti-NFK (red) (Fig. 7A-dark and Fig. 7B-UVA). As expected, all of the cells regardless of porphyrin treatment or UVA exposure contain obvious cytoplasmic accumulations of α-crystallin. Also as expected, the “dark” cells contain little detectable NFK. The porphyrin-containing, UVA exposed cells, however, clearly contain NFK. The Uro-treated cells accumulate the lowest amount of NFK, followed by cells treated with TPPS and Ce6 and THPP. While the UVA irradiated TPPS and Uro treated cells accumulate NFK, only the Ce6 and THPP treated cells show clear and extensive colocalization of NFK with α-crystallin.
Figure 7.
Confocal fluorescent microscopy of (A) dark-incubated and (B) UVA-irradiated HLE cells. Nearly confluent HLE-B3 cells were treated overnight with or without 10 μM porphyrins as indicated and after washing were kept in the dark. Fixed cells were simultaneously stained with rabbit anti-NFK and mouse anti-αA and anti-αB-crystallin followed by staining with Alexa Fluor anti-rabbit 568 and anti-mouse 488 and then with DAPI. α-crystallin (green), DAPI (blue), NFK (red), and overlay of all three.
DISCUSSION
This work used a combination of in vitro and cell culture experiments to study the effect of four biologically interesting porphyrins (Fig. 1) and UVA exposure on lens α-crystallin. Coomassie staining showed that neither UVA radiation alone nor addition of porphyrin alone results in α-crystallin polymerization (Fig. 3). Increasing photosensitization, however, by either increased UVA exposure in the presence of a porphyrin or increased porphyrin concentration in the presence of UVA radiation, does result in more extensive protein polymerization. The NFK westerns show that NFK accumulation parallels protein polymerization, which also increases with increasing photosensitization. Of the porphyrins used, TPPS was the most potent photosensitizer in vitro, causing both more extensive polymerization and NFK accumulation at the lowest concentration of porphyrin and shortest duration of UVA exposure.
To differentiate between the contributions of Type I (radical) and Type II (1O2) reactions to the protein modifications observed in Fig. 3 we used the 1O2 quencher sodium azide and the radical scavenger/spin trap DMPO (Fig. 4). Azide reduced cross-linking of α-crystallin more effectively than DMPO, indicating that Type II reactions were primarily responsible for increased polymerization of the protein. Decrease in NFK accumulation roughly paralleled the differences in cross-linking, again suggesting that 1O2 was responsible for tryptophan residue conversion to NFK. The anti-DMPO western blots, however, showed that Type I reactions also play a role in porphyrin-mediated photosensitization of α-crystallin. Radicals are trapped by DMPO in all photosensitized samples, and, in the samples irradiated in the presence of Uro, radical trapping/scavenging reduces both cross-linking and NFK accumulation. In addition to Type I radical-mediated events there is also evidence for the production of radicals from 1O2 generated protein peroxides which can, in the presence of metals or UV radiation, decompose to radicals (16,17). This UV radiation catalyzed decomposition of protein peroxides to radicals could explain the decrease in DMPO radical adduct production seen in photosensitized samples containing both DMPO and azide (Fig. 4, bottom panel).
Absorbance (Fig. 5) and fluorescence (Fig. 6) spectra were obtained to assess binding between protein and porphyrin. The absorbance and fluorescence maxima of both TPPS and THPP are shifted in the presence of α-crystallin, indicated binding between porphyrin and protein. Ce6 appears to participate in a less robust interaction with α-crystallin, as the spectra are altered in amplitude but show a less pronounced shift in wavelength. Uro, however, appears to have no interaction with α-crystallin as neither spectrum is altered in the presence of the protein (Figs. 5 and 6) (11). Porphyrin interaction with α-crystallin influences the extent of 1O2-mediated polymerization seen in our in vitro experiments (Figs. 3 and 4). The porphyrins that interact the most strongly with the protein (TPPS and THPP) are also more resistant to azide quenching.
Recently, Medinas et al (29) described the characterization of a tryptophan dimer resulting from the carbonate-dependent peroxidase activity of human SOD1 (30,31). Interestingly, NFK formation and this novel mode of cross-linking and appear mutually exclusive. When they used reaction conditions focused on optimizing hSOD1 dimer yield Medinas et al (29) detected no NFK in the dimer. Additionally, our original publication describing the anti-NFK antibody (25) showed that while increasing H2O2 concentration up to 10 mM increased NFK accumulation in SOD1, dimer formation was maximal at 0.5 to 1.0 mM H2O2. It is unlikely, therefore, that the protein polymers seen in Fig. 3 are due to tryptophan dimerization. There is, however, evidence that dityrosine formation occurs in crystallin proteins via a radical-mediated process (32). And, in addition to the 8 tyrosine residues found in the two subunits of α-crystallin, there are also a total of 16 histidine residues which can form His-His and His-Lys crosslinks (16).
The confocal microscopy analysis (Fig. 7) shows that in HLE cells the extent of porphyrin photosensitization is further influenced by their differential subcellular localization. In this experiment, while all four porphyins promote accumulation of NFK, substantial NFK accumulation that colocalizes with α-crystallin is only present in Ce6- and THHP-photosensitized cells. Thus, while TPPS promotes abundant oxidation of tryptophan to NFK in vitro, in HLE cells, where the acidic TPPS accumulates in lysosomes (33,34), there is little interaction between the porphyrin and the protein and therefore less photosensitized damage. THPP and Ce6, on the other hand, localize in HLE cells in the same compartment as α-crystallin, enabling physical interactions between protein and porphyrin, allowing opportunity for extensive NFK accumulation in α-crystallin protein molecules. The capacity of each porphyrin to photosensitize α-crystallin is a result of a combination of factors, including absorption of radiant energy, 1O2 quantum yield, and ability to bind to α-crystallin and/or to co-localize with α-crystallin in HLE cells.
Previous studies have examined photosensitization of α-crystallin by porphyrins and other biologically relevant substances (11,20-22,35). Porphyrins (TPPS, Uro, hematoporphyin) and other photosensitizers such as hypericin, an ingredient of the herb St. John’s wort used to treat depression, and xanthurenic acid, a metabolite of tryptophan, all facilitated polymerization of α-crystallin under UVA irradiation and also promoted the oxidation of specific amino acid residues such as histidine, methionine, tyrosine and tryptophan. In this work we demonstrated that the porphyrin-mediated photooxidation of α-crystallin that leads to protein polymerization also leads to accumulation of NFK in monomers, dimers and higher molecular weight species. Additionally, we were able to detect the oxidation of cellular tryptophan residues to NFK in human lens epithelial cells, and to visualize the colocalization of NFK with α-crystallin in photosensitized cells.
The absorption spectrum of the human lens changes with age. The adult human lens absorbs most light and radiation between wavelengths of 295 to 400 nm due to the presence of 3-OH kynurenine and its glucoside, (36,37) while the aged human lens absorbs longer wavelengths of light into the blue visible region due to a change in lenticular chromophores (20). Photosensitizers which absorb wavelengths of light above 400-450 can damage both the lens and retina. Because NFK is itself a 1O2-generating photosensitizer, accumulation of NFK in lens proteins serves to exacerbate the potential for light-mediated damage. Furthermore, the long-lived triplet state of a photosensitizer can react with reducing substrates to produce radicals in type I reactions (18), and these radicals are also capable of altering amino acid side chains as well as the protein backbone.
Although the other eye lens crystallins (beta- and gamma-crystallins) represent a smaller percentage of the total protein of the lens, they are also susceptible to oxidation of tryptophan residues to NFK (38). Because α-crystallin is a chaperone protein in the lens and other tissues, these observations may have implications that extend beyond eye functioning and health to areas related to protein folding and stress physiology. Indeed, there has been growing interest in the effect of post-translational modifications on α-crystallin efficiency as a chaperone (39-42). Abraham et al (39) observe an inverse correlation between glycation of lysine residues of and chaperone efficiency. The availability and accessibility of NFK immunological detection will facilitate the unraveling of the potential effect of tryptophan residue oxidation to NFK on chaperone activity.
In this study we used immunological techniques to detect N-formylkynurenine (NFK), a tryptophan oxidation product, in α-crystallin using an anti-NFK antibody. Here we show UVA irradiated human lens epithelial cells containing the porphyrin THPP (mesotetra (m-hydroxyphenyl) porphine). Fluorescent confocal analysis was used to visualize (clockwise, starting at bottom left) nuclei (blue), NFK (green), and α-crystallin (red), the major lens protein. The final panel (bottom, right) shows the overlay image of the preceding three, and illustrates the potential for irreversible oxidative photodamage that porphyrin accumulation can inflict on the critical eye lens protein α-crystallin. Unirradiated porphyrin-containing cells do not accumulate NFK.
Acknowledgments
The authors sincerely thank B. Jean Corbett and Mary Mason for their valuable help in the preparation of this manuscript. This work was supported by the Intramural Research Program of the National Institutes of Health and the National Institute of Environmental Health Sciences 52 (Z01 ES050139-13).
REFERENCES
- 1.Andley UP. Crystallins in the eye: Function and pathology. Prog. Retin. Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Horwitz J. Alpha-crystallin. Exp. Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 3.Andley UP. The lens epithelium: Focus on the expression and function of the α-crystallin chaperones. Int. J. Biochem. Cell Biol. 2008;40:317–323. doi: 10.1016/j.biocel.2007.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benedek GB. Theory of transparency of the eye. Appl. Optics. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 5.Rao GN, Khanna R, Payal A. The global burden of cataract. Curr. Opin. Ophthamol. 2011;22:4–9. doi: 10.1097/ICU.0b013e3283414fc8. [DOI] [PubMed] [Google Scholar]
- 6.Chin WWL, Heng PWS, Thong PSP, Bhuvaneswari R, Hirt W, Kuenzel S, Soo KC, Olivo M. Improved formulation of photosensitizer chlorin e6 polyvinylpyrrolidone for fluorescence diagnostic imaging and photodynamic therapy of human cancer. Eur. J. Pharm. Biopharm. 2008;69:1083–1093. doi: 10.1016/j.ejpb.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Peng Q, Moan J, Ma L-W, Nesland JM. Uptake, localization, and photodynamic effect of meso-tetra(hydroxyphenyl)porphine and its corresponding chlorin in normal and tumor tissues of mice bearing mammary carcinoma. Cancer Res. 1995;55:2620–2626. [PubMed] [Google Scholar]
- 8.Sacchini V, Melloni E, Marchesini R, Luini A, Bandieramonte G, Spinelli P, Cascinelli N. Preliminary clinical studies with PDT by topical TPPS administration in neoplastic skin lesions. Lasers Surg. Med. 1987;7:6–11. doi: 10.1002/lsm.1900070103. [DOI] [PubMed] [Google Scholar]
- 9.Lambrecht RW, Thapar M, Bonkovsky HL. Genetic aspects of porphyria cutanea tarda. Semin. Liver Dis. 2007;27:99–108. doi: 10.1055/s-2006-960173. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson J. Drug-induced photosensitivity. In: Ferguson J, Dover JS, editors. Photodermatology. Manson Publishing; 2006. pp. 66–71. [Google Scholar]
- 11.Roberts JE, Dillon J. In vitro studies on the photosensitized oxidation of lens proteins by porphyrins. Photochem. Photobiol. 1987;46:683–688. doi: 10.1111/j.1751-1097.1987.tb04832.x. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JE, Atherton SJ, Gaillard ER, Dillon J. Age-related changes in the human lens as monitored by detection of porphyrin excited states. Photochem. Photobiol. 1995;62:339–341. doi: 10.1111/j.1751-1097.1995.tb05278.x. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JE, Kinley JS, Young AR, Jenkins G, Atherton SJ, Dillon J. In vivo and photophysical studies on photooxidative damage to lens proteins and their protection by radioprotectors. Photochem. Photobiol. 1991;53:33–38. doi: 10.1111/j.1751-1097.1991.tb08464.x. [DOI] [PubMed] [Google Scholar]
- 14.Gracanin M, Hawkins CL, Pattison DI, Davies MJ. Singlet-oxygen-mediated amino acid and protein oxidation: Formation of tryptophan peroxides and decomposition products. Free Radic. Biol. Med. 2009;47:92–102. doi: 10.1016/j.freeradbiomed.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Wright A, Hawkins CL, Davies MJ. Photo-oxidation of cells generates long-lived intracellular protein peroxides. Free Radic. Biol. Med. 2003;34:637–647. doi: 10.1016/s0891-5849(02)01361-8. [DOI] [PubMed] [Google Scholar]
- 16.Davies MJ. Singlet oxygen-mediated damage to proteins and its consequences. Biochem. Biophys. Res. Commun. 2003;305:761–770. doi: 10.1016/s0006-291x(03)00817-9. [DOI] [PubMed] [Google Scholar]
- 17.Davies MJ. The oxidative environment and protein damage. Biochim. Biophys. Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Davies MJ. Reactive species formed on proteins exposed to singlet oxygen. Photochem. Photobiol. Sci. 2004;3:17–25. doi: 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- 19.Finley EL, Dillon J, Crouch RK, Schey KL. Identification of tryptophan oxidation products in bovine α-crystallin. Protein Sci. 1998;7:2391–2397. doi: 10.1002/pro.5560071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts JE, Finley EL, Patat SA, Schey KL. Photooxidation of lens proteins with xanthurenic acid: A putative chromophore for cataractogenesis. Photochem. Photobiol. 2001;74:740–744. doi: 10.1562/0031-8655(2001)074<0740:polpwx>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Schey KL, Patat S, Chignell CF, Datillo M, Wang RH, Roberts JE. Photooxidation of lens α-crystallin by hypericin (active ingredient in St. John’s wort) Photochem. Photobiol. 2000;72:200–203. doi: 10.1562/0031-8655(2000)072<0200:polcbh>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.McDermott M, Chiesa R, Roberts JE, Dillon J. Photooxidation of specific residues in α-crystallin polypeptides. Biochemistry. 1991;30:8653–8660. doi: 10.1021/bi00099a023. [DOI] [PubMed] [Google Scholar]
- 23.Roberts JE, Roy D, Dillon J. The photosensitized oxidation of the calf lens main intrinsic protein (MP26) with hematoporphyrin. Curr. Eye Res. 1985;4:181–185. doi: 10.3109/02713688509000848. [DOI] [PubMed] [Google Scholar]
- 24.Perdivara I, Deterding LJ, Przybylski M, Tomer KB. Mass spectrometric identification of oxidative modifications of tryptophan residues in proteins: Chemical artifact or post-translational modification? J. Am. Soc. Mass Spectrom. 2010;21:1114–1117. doi: 10.1016/j.jasms.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ehrenshaft M, de Oliveira Silva S, Perdivara I, Bilski P, Sik RH, Chignell CF, Tomer KB, Mason RP. Immunological detection of N-formylkynurenine in oxidized proteins. Free Radic. Biol. Med. 2009;46:1260–1266. doi: 10.1016/j.freeradbiomed.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehrenshaft M, Bonini MG, Feng L, Chignell CF, Mason RP. Partial colocalization of oxidized, N-formylkynurenine-containing proteins in mitochondria and golgi of keratinocytes. Photochem. Photobiol. 2010;86:752–756. doi: 10.1111/j.1751-1097.2010.00718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruggiero C, Ehrenshaft M, Cleland E, Stadler K. High fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am J Physiol Endocrinol Metab. 2011;300:E1047–E1058. doi: 10.1152/ajpendo.00666.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andley UP, Rhim JS, Chylack LT, Jr., Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest. Ophthalmol. Vis. Sci. 1994;35:3094–3102. [PubMed] [Google Scholar]
- 29.Medinas DB, Gozzo FC, Santos LFA, Iglesias AH, Augusto O. A ditryptophan cross-link is responsible for the covalent dimerization of human superoxide dismutase 1 during its bicarbonate-dependent peroxidase activity. Free Radic. Biol. Med. 2010;49:1046–1053. doi: 10.1016/j.freeradbiomed.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Andrekopoulos C, Joseph J, Chandran K, Karoui H, Crow JP, Kalyanaraman B. Bicarbonate-dependent peroxidase activity of human Cu,Zn-superoxide dismutase induces covalent aggregation of protein: Intermediacy of tryptophan-derived oxidation products. J. Biol. Chem. 2003;278:24078–24089. doi: 10.1074/jbc.M302051200. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Joseph J, Crow J, Kalyanaraman B. Mass spectral evidence for carbonate-anion-radical-induced posttranslational modification of tryptophan to kynurenine in human Cu, Zn superoxide dismutase. Free Radic. Biol. Med. 2004;37:2018–2026. doi: 10.1016/j.freeradbiomed.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 32.Guptasarma P, Balasubramanian D. Dityrosine formation in the proteins of the eye lens. Curr. Eye Res. 1992;11:1121–1125. doi: 10.3109/02713689209015084. [DOI] [PubMed] [Google Scholar]
- 33.Strauss WSL, Gschwend MH, Sailer R, Schneckenburger H, Steiner R, Ruck A. Intracellular fluorescence behaviour of meso-tetra(4-sulphonatophenyl)porphyrin during photodynamic treatment at various growth phases of cultured-cells. J. Photochem. Photobiol. B: Biol. 1995;28:155–161. doi: 10.1016/1011-1344(94)07082-y. [DOI] [PubMed] [Google Scholar]
- 34.Wessels JM, Strauss W, Seidlitz HK, Ruck A, Schneckenburger H. Intracellular localization of meso-tetraphenylporphine tetrasulphonate probed by time-resolved and microscopic fluorescence spectroscopy. J. Photochem. Photobiol. B: Biol. 1992;12:275–284. doi: 10.1016/1011-1344(92)85029-t. [DOI] [PubMed] [Google Scholar]
- 35.Roberts JE, Atherton SJ, Dillon J. Detection of porphyrin excited states in the intact bovine lens. Photochem. Photobiol. 1991;54:855–857. doi: 10.1111/j.1751-1097.1991.tb02102.x. [DOI] [PubMed] [Google Scholar]
- 36.Dillon J, Atherton SJ. Time resolved spectroscopic studies on the intact human lens. Photochem. Photobiol. 1990;51:465–468. doi: 10.1111/j.1751-1097.1990.tb01738.x. [DOI] [PubMed] [Google Scholar]
- 37.Dillon J, Wang R-H, Atherton SJ. Photochemical and photophysical studies on human lens constituents. Photochem. Photobiol. 1990;52:849–854. doi: 10.1111/j.1751-1097.1990.tb08692.x. [DOI] [PubMed] [Google Scholar]
- 38.Balasubramanian D, Du X, Zigler JS., Jr. The reaction of singlet oxygen with proteins, with special reference to crystallins. Photochem. Photobiol. 1990;52:761–768. doi: 10.1111/j.1751-1097.1990.tb08679.x. [DOI] [PubMed] [Google Scholar]
- 39.Abraham EC, Huaqian J, Aziz A, Kumarasamy A, Datta P. Role of the specifically targeted lysine residues in the glycation dependent loss of chaperone activity of αA- and αB-crystallins. Mol. Cell. Biochem. 2008;310:235–239. doi: 10.1007/s11010-007-9685-1. [DOI] [PubMed] [Google Scholar]
- 40.Clark JI, Huang Q-L. Modulation of the chaperone-like activity of bovine α–crystallin. Proc. Natl. Acad. Sci. USA. 1996;93:15185–15189. doi: 10.1073/pnas.93.26.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sgarbossa A, Youssef T, Lenci F. Photosensitized structural modifications of the lens protein α-crystallin: Do all modifications impair chaperone-like activity? Photochem. Photobiol. 2003;77:567–571. doi: 10.1562/0031-8655(2003)077<0567:psmotl>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 42.Youssef T, Kassem M, Abdella T, Harith MA, Lenci F. Photosensitized effects of rose bengal on structure and function of lens protein “alpha crystallin”. Photochem. Photobiol. 2009;85:1306–1313. doi: 10.1111/j.1751-1097.2009.00613.x. [DOI] [PubMed] [Google Scholar]