Abstract
During lymphocyte antigen receptor gene assembly, DNA cleavage by the Rag proteins generates pairs of coding and signal ends that are normally joined into coding joints and signal joints, respectively, by the classical non-homologous end-joining (NHEJ) pathway of DNA double strand break (DSB) repair. Coding and signal ends can also be aberrantly joined to each other, generating hybrid joints, through NHEJ or through NHEJ-independent pathways, such as Rag-mediated transposition. Hybrid joints do not participate in the formation of functional antigen receptor genes and can alter the configuration of antigen receptor loci in ways that limit subsequent productive rearrangements. The formation of these non-functional hybrid joints occurs rarely in wild type lymphocytes, demonstrating that mechanisms exist to limit both the NHEJ-dependent and the NHEJ-independent joining of a signal end to a coding end. In contrast to wild type cells, hybrid joint formation occurs at high levels in Atm-deficient lymphocytes, suggesting that Atm functions to limit the formation of these aberrant joints. Here we show that hybrid joint formation in Atm-deficient cells requires the NHEJ proteins Artemis, DNA-PKcs and Ku70, demonstrating that Atm functions primarily by modulating the NHEJ-dependent, and not the NHEJ-independent, joining of coding ends to signal ends.
Keywords: Gene rearrangement, Gene Regulation, Immunodeficiency Diseases, B cells, T cells
Introduction
The formation of a complete lymphocyte antigen receptor gene requires that the second exon be assembled from component V, J and, in some cases, D gene segments (1). This assembly occurs through the V(D)J recombination reaction, which is initiated by the endonuclease formed by the Rag-1 and -2 proteins, hereafter referred to as Rag (2). Rag introduces DNA double strand breaks (DSBs) at the border of a pair of gene segments and their flanking recombination signals (RSs), leading to the formation of two blunt phosphorylated signal ends and two hairpin-sealed coding ends (2). Proteins of the classical non-homologous end-joining (NHEJ) pathway of DNA DSB repair normally process and join the signal end pair into a signal joint and the coding end pair into a coding joint (3). Coding joint formation results in either the deletion or inversion of the genomic region between the recombining gene segments. The classical NHEJ factors required for V(D)J recombination include XRCC4, DNA Ligase IV, XLF, Artemis and the DNA-PK complex, which is composed of a catalytic subunit (DNA-PKcs), Ku70 and Ku80 (3–5). DNA-PKcs and Artemis are required primarily for coding joint formation, due to their activity in opening hairpin-sealed coding ends, whereas all of the other NHEJ factors, often referred to as the core NHEJ factors, are required for both signal and coding joint formation (3–5).
Signal ends and coding ends can also be joined aberrantly in ways that cannot lead to the formation of a functional antigen receptor gene. In this regard, the originally appended coding end and signal end can be rejoined, generating an open-and-shut joint, or a coding end can be joined to the signal end generated by cleavage at the other gene segment, generating a hybrid joint (6, 7). Although hybrid joints can form through classical NHEJ, these joints can also be formed in ways that bypass the requirement for classical NHEJ factors (8–12). For example, the truncated “core” Rags can catalyze hybrid joint formation through a transposition-like reaction (11–13). However, full length Rag proteins do not efficiently catalyze hybrid joint formation in vivo, suggesting that the non-core regions of the Rag proteins inhibit this transposition activity (11). Whether the non-core regions have intrinsic activities that inhibit transposition or whether they serve as targets for trans-acting factors that promote this inhibitory function is not known. Moreover, hybrid joint formation could also be catalyzed through alternative end-joining pathways of DNA DSB repair (14–18). Importantly, in wild type developing lymphocytes, the formation of these non-functional hybrid joints, through either NHEJ-dependent or NHEJ-independent pathways, occurs at very low levels.
Deficiency in the ataxia telangiectasia mutated (Atm) serine threonine kinase leads to lymphopenia and lymphoid malignancies with chromosomal translocations involving antigen receptor genes (19, 20). This is due, in part, to the function of Atm in the repair of Rag-mediated DSBs (21–24). In this regard, we have previously shown that, in Atm-deficient lymphocytes undergoing V(D)J recombination, there is an accumulation of un-repaired coding ends that are frequently resolved aberrantly as chromosomal translocations or large chromosomal deletions or inversions (22). In addition, there is a marked increase in hybrid joint formation during inversional rearrangements (22). We have proposed that the accumulation of un-repaired coding ends and the increase in hybrid joint formation could both be explained by a requirement for Atm to stabilize DNA DSB complexes generated after Rag-mediated DNA cleavage (22). These defects, however, could also reflect distinct requirements for Atm in promoting the NHEJ-dependent joining of coding ends and in prohibiting hybrid joint formation by NHEJ-independent pathways, such as Rag-mediated transposition. To distinguish between these possibilities, here we determine whether hybrid joints generated in Atm-deficient cells form through NHEJ-dependent or NHEJ-independent joining pathways.
Materials and Methods
Mice
All mice were bred and maintained under specific pathogen-free conditions at the Washington University School of Medicine and were handled in accordance to the guidelines set forth by the Division of Comparative Medicine of Washington University.
Cell lines and culture conditions
Artemis−/−, Artemis−/−:Atm−/−, and Ku70−/− v-abl-transformed pre-B cells were generated by culturing bone marrow of 3–5 week old mice with the pMSCV v-abl retrovirus, as described previously (22). Cells were generated from at least two mice of each genotype, which all expressed an Eμ-Bcl-2 transgene. These cells (106/mL) were transduced with the pMX-INV retrovirus by centrifugation at 1800 rpm for 90 minutes. Cells containing pMX-INV were isolated by fluorescence activated cell sorting based on expression of the human CD4 (hCD4). hCD4 expression was detected using phycoerythrin-conjugated anti-hCD4 (Pharmingen). The wild type, Atm−/− and scid abl pre-B cell lines containing pMX-INV have been described previously (22). Cells were treated with 3µM STI571 (Novartis) for the indicated times at 106 cells/ml. KU-55933 (Sigma) was used at 15µM.
Southern blot and PCR analyses
Genomic DNA was isolated and Southern blot analyses were carried out using the indicated restriction enzymes and the C4 probe as previously described (22). PCR analyses for hybrid joint formation during pMX-INV rearrangement in abl pre-B cells, and during Vβ14 and Vδ5 rearrangement in thymocytes, were performed as previously described (22). IL2 gene PCR was carried out as a DNA loading control as previously described (22).
Results
Increased hybrid joint formation in Atm-deficient abl pre-B cells
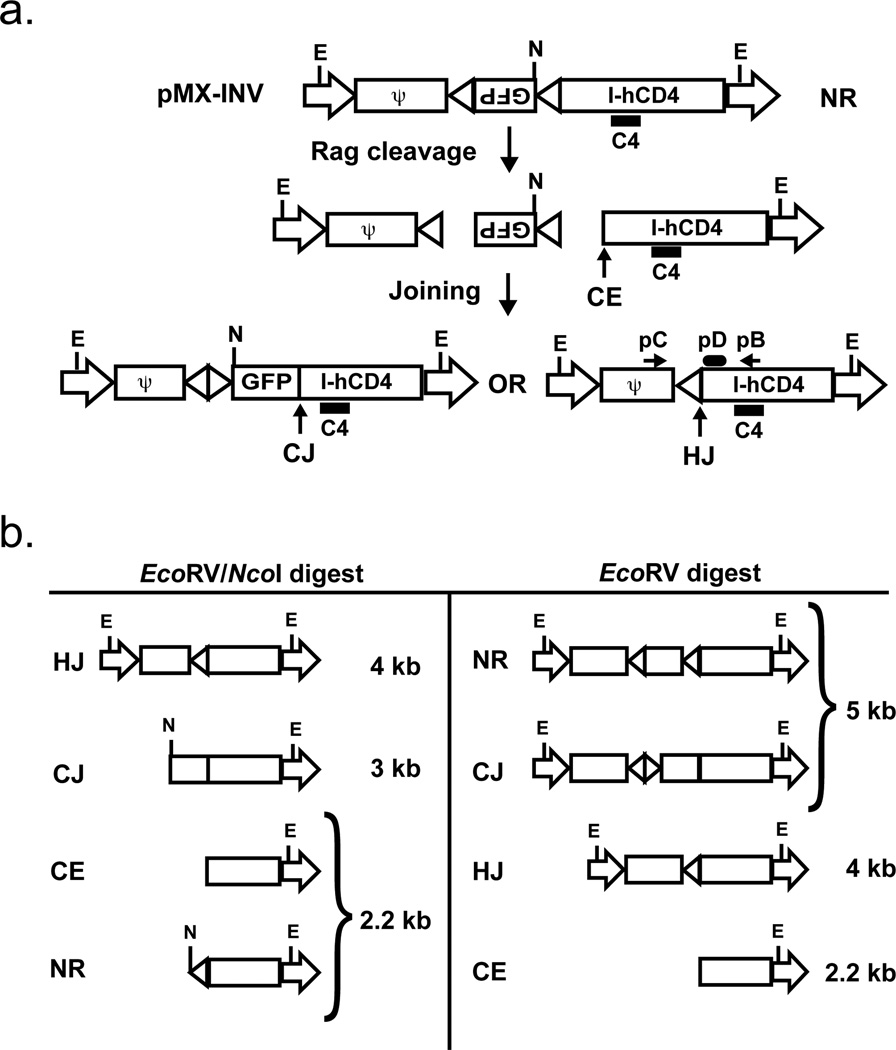
We have recently established an experimental approach whereby chromosomal V(D)J recombination can be analyzed in murine v-abl transformed pre-B cells, hereafter referred to as abl pre-B cells (22). Rag gene expression and G1 cell cycle arrest can be induced in these cells upon inhibition of v-abl kinase activity with STI571 (22, 25). This leads to robust rearrangement of the endogenous IgLκ locus and introduced retroviral recombination substrates, such as pMX-INV (Fig. 1) (22). pMX-INV has a pair of RSs that are oriented such that normal rearrangement occurs by inversion, generating a signal joint and coding joint that remain in the chromosome (Fig. 1a). pMX-INV also has an IRES-human CD4 cDNA cassette that permits the flow cytometric purification of cells containing chromosomal pMX-INV integrants (22).
Figure 1. Schematic of pMX-INV and rearrangement products.
a. Schematic showing the non-rearranged (NR), coding joined (CJ) and hybrid joined (HJ) configurations of the pMX-INV retroviral recombination substrate. The IRES-human CD4 cDNA, the GFP cDNA and the recombination signals (triangles) are shown. The relative positions of the EcoRV (E) and NcoI (N) restriction sites are shown. Also indicated is the 3’ coding end (CE) generated by Rag cleavage. The relative positions of the pC and pB oligonucleotide primers used to amplify hybrid joints are shown, as is the pD oligonucleotide used to probe these PCR products. The relative position of the C4 probe used for Southern blot analysis is also shown. b. Schematic of the different C4 probing DNA fragments generated by digestion of pMX-INV in different configurations (NR, CJ, HJ and CE) with EcoRV or EcoRV/NcoI.
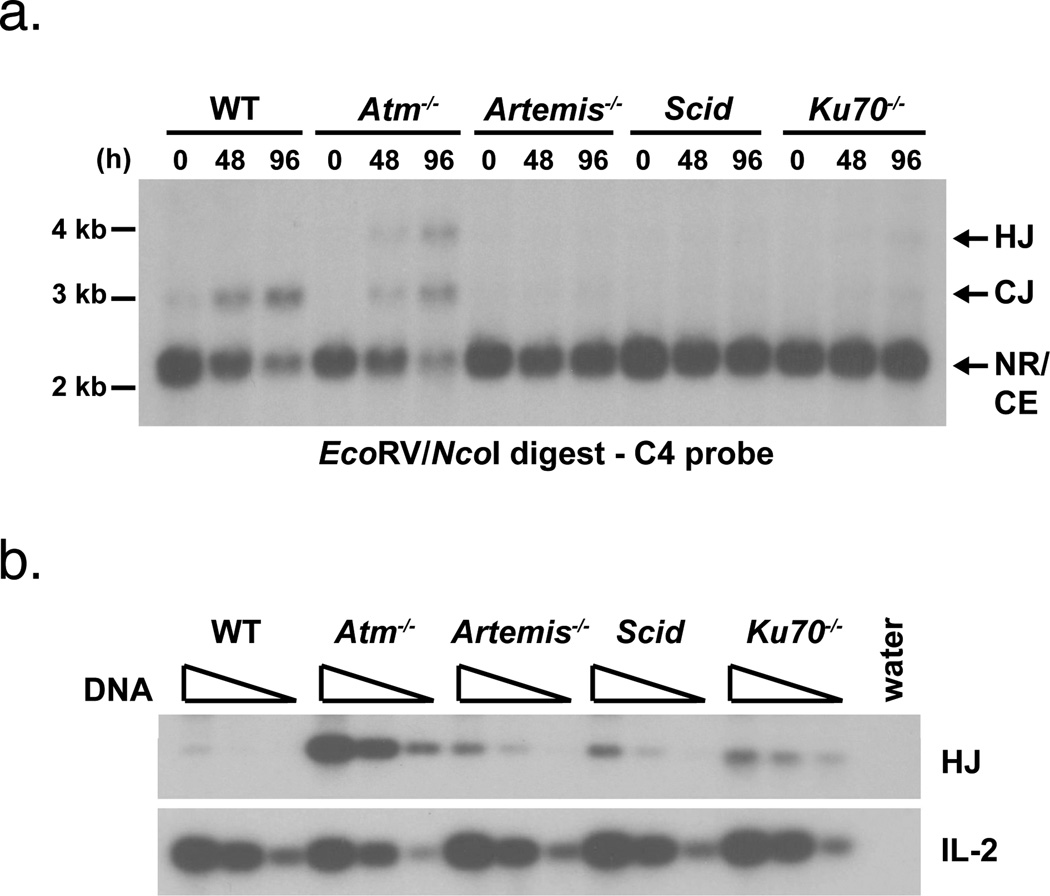
Induction of Rag in wild type abl pre-B cells leads to robust pMX-INV recombination (Fig. 2a) (22). Most of the rearrangement products are coding and signal joints, with hybrid joint formation being very rare (Fig. 2) (22). However, high levels of pMX-INV hybrid joints form after induction of V(D)J recombination in Atm-deficient abl pre-B cells (Fig. 2) (22). Sequence analyses revealed that these hybrid joints are diverse in nature with features, such as N and P nucleotide additions and nucleotide loss, suggestive of joining by NHEJ (22). Importantly, hybrid joints and coding joints in Atm-deficient abl pre-B cells form with similar kinetics and at similar levels, demonstrating that both are formed through efficient joining pathways in these cells.
Figure 2. Hybrid joint formation in wild type, Atm-deficient and NHEJ-deficient abl pre-B cells.
a. Southern blot analysis of pMX-INV coding and hybrid joint formation on EcoRV/NcoI digested genomic DNA from wild type (WT), Atm−/−, Artemis−/−, Scid and Ku70−/− abl pre-B cells treated with STI571 for the indicated time (hours, h). Fragments of the correct size for pMX-INV NR, CJ, HJ and CE are indicated. The position of the molecular weight markers is shown. Results are representative of at least two experiments on each of two independent cell lines. b. PCR analysis of pMX-INV hybrid joint formation in the samples from (a) treated with STI571 for 96 hours. The IL-2 gene PCR is shown as a DNA loading control. The PCRs were performed on serial 4-fold dilutions of genomic DNA.
Hybrid joints are formed at low levels in NHEJ-deficient abl pre-B cells
Atm could prevent hybrid joint formation through either NHEJ-dependent or NHEJ-independent pathways. To determine whether NHEJ-independent pathways can mediate hybrid joining in abl pre-B cells, we generated abl pre-B cells deficient in both core (Ku70) and non-core (Artemis and DNA-PKcs) NHEJ proteins and transduced these cells with pMX-INV. The induction of Rag in Ku70−/−, Artemis−/− and Scid abl pre-B cells led to DNA cleavage at pMX-INV and a marked accumulation of un-repaired coding ends, but low levels of coding joint formation, as would be expected given the NHEJ deficiencies (Figs. 2a, 3a and 4a). Although pMX-INV hybrid joints also formed in Ku70−/−, Artemis−/− and Scid abl pre-B cells, the level of hybrid joint formation was approximately 20-fold lower than that observed in Atm-deficient cells (Fig. 2). Thus, NHEJ-independent pathways do mediate hybrid joint formation, although at very low levels, in NHEJ-deficient abl pre-B cells.
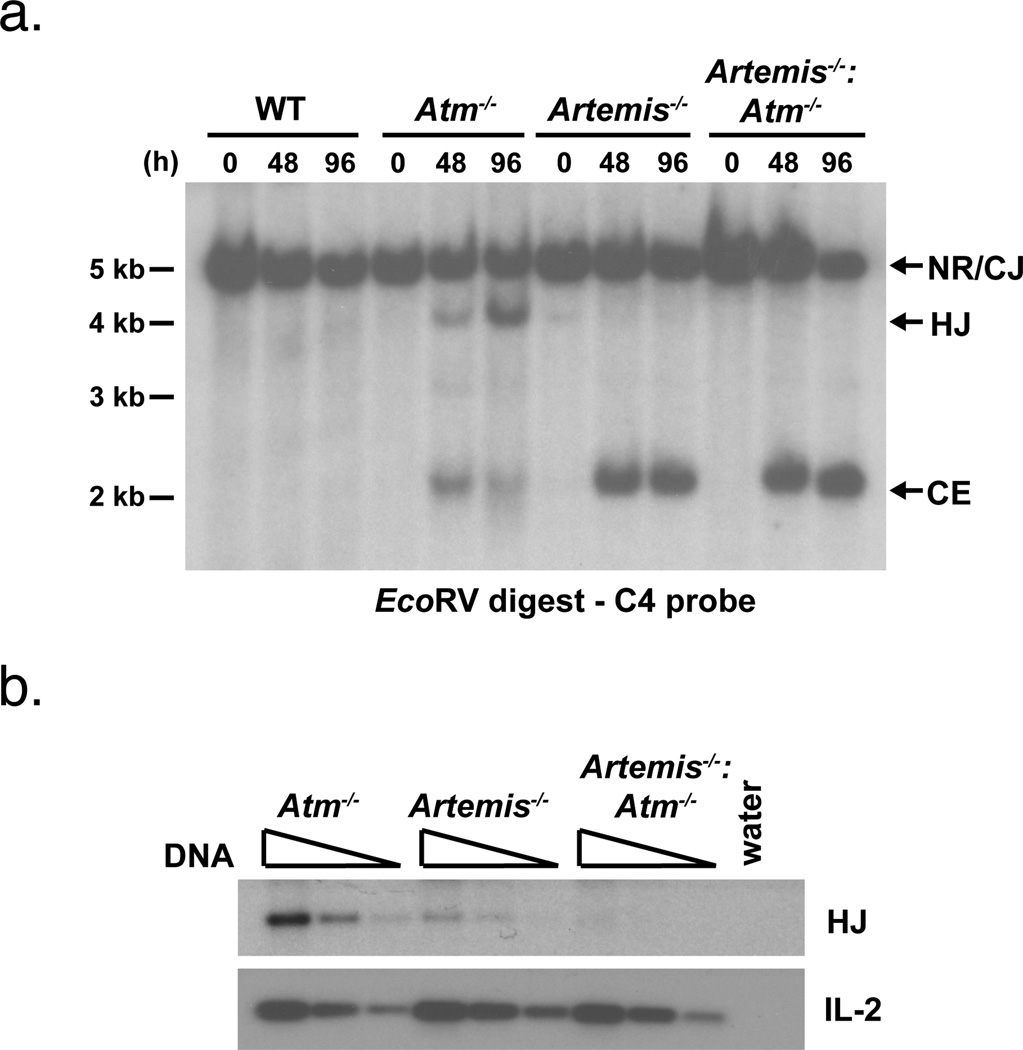
Figure 3. Hybrid joint formation in Atm-deficient cells is Artemis-dependent.
a. Southern blot analyses of EcoRV digested, probe C4 hybridized genomic DNA from abl pre-B cells treated with STI571 for the indicated period of time (h). Results are representative of at least two experiments on each of two independent cell lines. b. PCR analysis of pMX-INV hybrid joint formation in the samples from (a) treated with STI571 for 48 hours. The IL-2 gene PCR is shown as a DNA loading control. The PCRs were performed on serial 4-fold dilutions of genomic DNA.
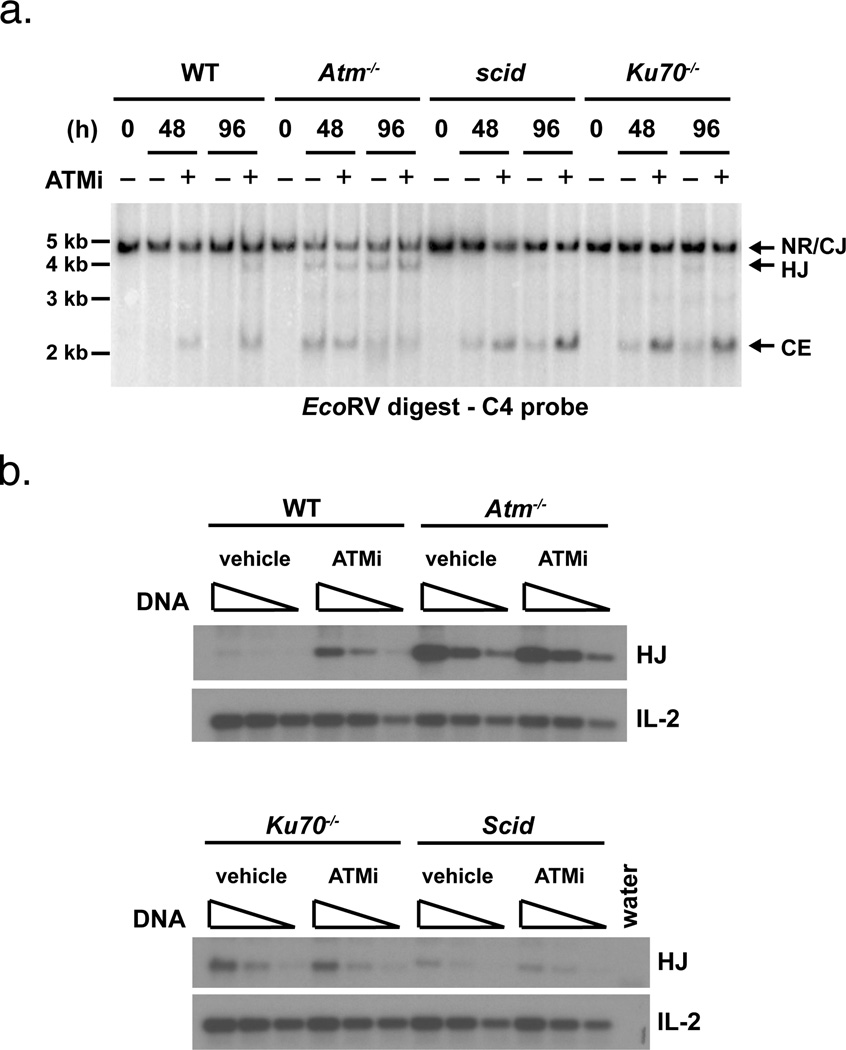
Figure 4. Hybrid joint formation in Atm-deficient cells is dependent on DNA-PKcs and Ku70.
a. Southern blot analyses of EcoRV digested, probe C4 hybridized genomic DNA from abl pre-B cells treated with STI571 for the indicated period of time (h) with the Atm inhibitor KU-55933 (ATMi) in DMSO (+) or DMSO alone (−). Results are representative of two experiments. b. PCR analysis of pMX-INV hybrid joint formation in the samples from (a) treated with STI571 for 48 hours. Vehicle indicates cells that were treated with DMSO alone. The IL-2 gene PCR is shown as a DNA loading control. The PCRs were performed on serial 4-fold dilutions of genomic DNA.
Hybrid joint formation in Atm-deficient cells is dependent on the NHEJ protein Artemis
The low level of hybrid joint formation in NHEJ-deficient cells could be due to the suppression of NHEJ-independent pathways by Atm. In this regard, we reasoned that if Atm prevents hybrid joint formation primarily by modulating the function of NHEJ-independent pathways, then abl pre-B cells deficient in both Atm and the NHEJ protein Artemis (Artemis−/−:Atm−/−) should form hybrid joints at a level similar to that observed in Atm−/− cells. However, in striking contrast to Atm−/− cells, pMX-INV hybrid joints form at a very low level in Artemis−/−:Atm−/− abl pre-B cells (Fig. 3). Thus, the efficient formation of hybrid joints in Atm-deficient abl pre-B cells is dependent on the NHEJ protein Artemis.
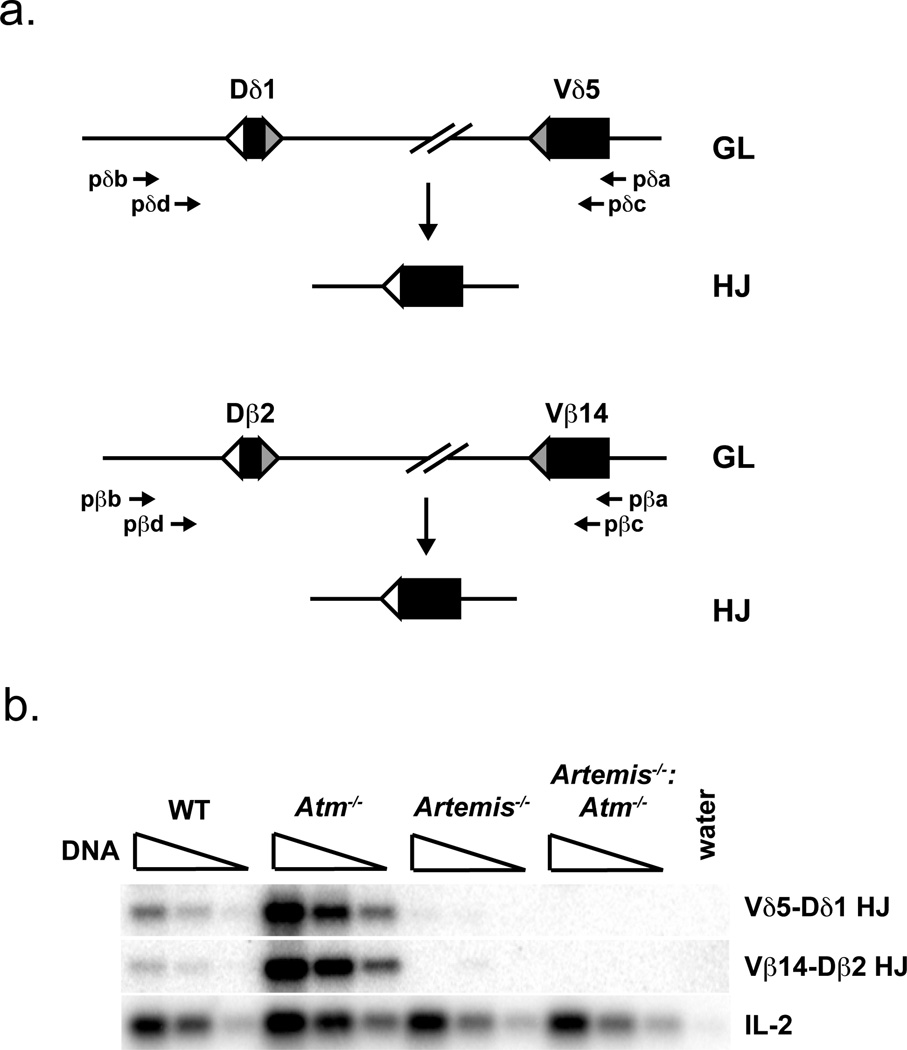
To confirm that Artemis is also required for hybrid joint formation in developing Atm-deficient lymphocytes in vivo, we assayed rearrangements at the T cell receptor (TCR) β and δ loci in developing thymocytes. To this end, CD4−/CD8−/CD25+ (CD25+ DN) thymocytes were purified by flow cytometric cell sorting from wild type, Atm−/−, Artemis−/− and Artemis−/−:Atm−/− mice. In wild type CD25+ DN thymocytes, we find low levels of hybrid joint formation involving the Vβ14 and Vδ5 gene segments, which both rearrange by inversion (Fig. 5). In contrast, hybrid joints involving these V gene segments are readily detected in Atm−/− CD25+ DN thymocytes, reaching levels that are 20-fold higher than those observed in wild type CD25+ DN thymocytes (Fig. 5). Importantly, these hybrid joints were not detected in Artemis−/−:Atm−/− CD25+ DN thymocytes (Fig. 5). Thus, as was observed in Atm−/− abl pre-B cells, the robust hybrid joint formation observed in developing Atm−/− lymphocytes is also dependent on Artemis.
Figure 5. Hybrid joint formation in endogenous TCR loci requires Artemis.
a. Schematic of the Vδ5 and Dδ1, and Vβ14 and Dβ2, gene segments before (germline, GL) and after (hybrid joint, HJ) hybrid joint formation. The oligonucleotides used to amplify Vδ5-Dδ1 hybrid joints and Vβ14-Dβ2 hybrid joints are shown. b. PCR analysis of Vδ5-Dδ1 hybrid joints and Vβ14-Dβ2 hybrid joints carried out on genomic DNA from CD25+ DN thymocytes purified from mice of the indicated genotypes. The IL-2 gene PCR is shown as a DNA loading control. The PCRs were performed on serial 4-fold dilutions of genomic DNA. Results are representative of analysis of two mice from each genotype.
Hybrid joint formation in Atm-deficient cells is dependent on Ku70 and DNA-PKcs
We wished to determine whether hybrid joint formation in Atm-deficient abl pre-B cells was dependent on NHEJ factors in addition to Artemis. Mice with combined deficiency in Atm and DNA-PKcs or Ku70 exhibit early embryonic lethality, precluding the generation of abl pre-B cells with combined deficiencies in Atm and either DNA-PKcs or Ku70 (26). Thus, an alternate approach was needed to determine whether DNA-PKcs and Ku70 are required for hybrid joint formation in cells deficient in Atm activity. In this regard, treatment of wild type abl pre-B cells with the Atm kinase inhibitor, KU-55933, resulted in an increase in pMX-INV hybrid joint formation and an accumulation of un-repaired coding ends, demonstrating that inhibition of Atm kinase activity recapitulates the Atm−/− phenotype. (Fig. 4) (22). Induction of Rag expression in Scid or Ku70−/− abl pre-B cells leads to an accumulation of un-repaired coding ends and a low level of coding and hybrid joint formation (Figs. 2 and 4). Notably, treatment of Scid or Ku70−/− abl pre-B cells with KU-55933 leads to a slight increase in un-repaired coding ends, possibly due to the blunting of Atm-dependent pathways that mediate cell death in response to un-repaired DNA DSBs (Fig. 4a). However, treatment of Scid or Ku70−/− abl pre-B cells with KU-55933 did not lead to the increase in hybrid joint formation that was observed in wild type abl pre-B cells (Fig. 4). Thus, formation of hybrid joints in abl pre-B cells deficient in Atm kinase activity is dependent on the DNA-PKcs and Ku70 NHEJ factors.
Discussion
Our findings demonstrate that Atm functions to restrict hybrid joint formation through NHEJ-dependent pathways of DNA DSB repair. In this regard, we have shown that, like coding joints, the efficient generation of hybrid joints is dependent upon the Artemis, DNA-PKcs and Ku70 NHEJ proteins in both Atm-sufficient and Atm-deficient lymphocytes (Fig. 6). Notably, the overall level of joining (coding and hybrid joints) in Atm-deficient cells is similar to the level of coding joint formation in wild type abl pre-B cells. Thus, NHEJ activity per se is relatively intact during V(D)J recombination in Atm-deficient lymphocytes, suggesting that the increased hybrid joint formation is due to defects in processes other than those that directly mediate joining.
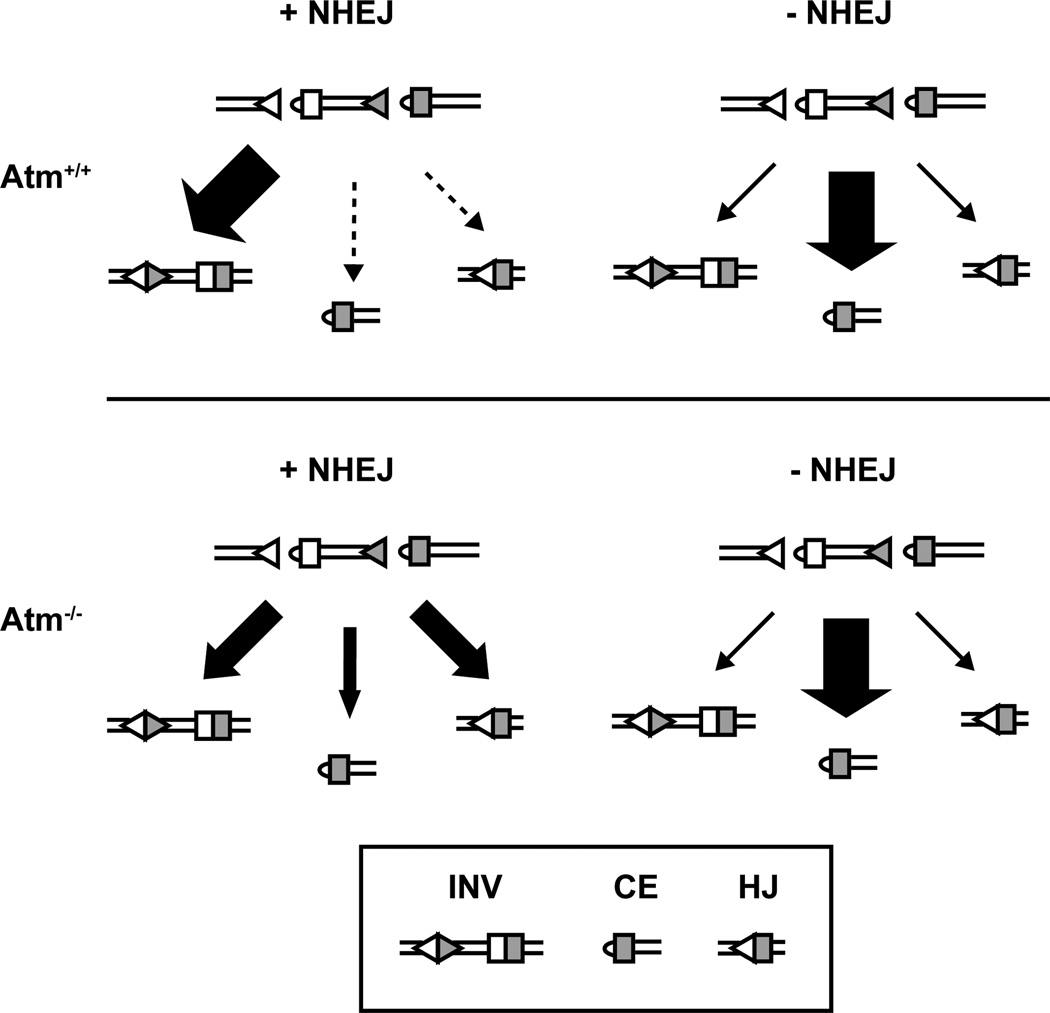
Figure 6. The resolution of Rag DSBs in NHEJ-sufficient or NHEJ-deficient cells in the presence or absence of Atm signaling.
The schematic shows the products generated after initiation of an inversional rearrangement in each genetic background used in this study. In NHEJ-sufficient cells, Atm signaling is required to prevent formation of hybrid joints and accumulation of un-repaired DNA ends. Lack of Atm signaling in NHEJ-deficient cells, however, does not lead to an increase in the formation of hybrid joints. Triangles represent recombination signals (RSs); rectangles represent coding segments. Size of arrows denotes relative frequency of formation of each product; dotted line indicates that the outcome is near the limit of detection. INV: normal coding and signal joints; CE: coding end; HJ: hybrid joint.
Several findings are inconsistent with the notion that Atm performs a significant function in preventing NHEJ-independent hybrid joint formation through processes such as Rag-mediated transposition. The formation of hybrid joints by Rag-mediated transposition can occur through the joining of a signal end and a hairpin-sealed coding end, without a requirement for any additional proteins other than Rag-1 and Rag-2 (12, 13). Thus, hybrid joint formation by Rag-mediated transposition is not dependent upon the non-core NHEJ proteins Artemis and DNA-PKcs, whose primary known function during V(D)J recombination is in opening hairpin-sealed coding ends (9, 27–29). However, here we show that the majority of the hybrid joints that form in Atm-deficient cells are dependent on both DNA-PKcs and Artemis, consistent with the notion that their formation requires NHEJ hairpin opening activity.
Furthermore, hybrid joints formed by Rag-mediated transposition usually exhibit minimal joint diversity consisting of the addition of, at most, two P-nucleotides (8, 29, 30). However, sequence analysis of hybrid joints generated in Atm deficient cells reveals the full spectrum of joint diversity including nucleotide loss and N-and P-nucleotide addition (22). It is conceivable that NHEJ-independent hybrid joint formation could also occur through an alternative end-joining pathway (14–18). However, when active, this pathway can mediate the efficient repair of Rag-mediated DSBs in DNA-PKcs-deficient cells, yet we find that most hybrid joints that form in Atm-deficient cells are dependent on DNA-PKcs. Together, our data clearly demonstrate that the majority of hybrid joints that form during V(D)J recombination in Atm-deficient lymphocytes require NHEJ activity. Thus, Atm functions to modulate the NHEJ repair of Rag-mediated DSBs in a way that significantly limits the formation of hybrid joints during rearrangements that occur by inversion.
During the repair of genotoxic DSBs, NHEJ generally rejoins the two DNA ends generated at a single DSB. However, during V(D)J recombination, NHEJ must join DNA ends that are generated at two distinct DSBs. In this regard, the coding ends from two distinct DSBs are joined to generate a coding joint, and the signal ends from these two breaks are joined to generate a signal joint. Atm could function in a way that directs the correct joining of DNA ends generated by Rag during lymphocyte antigen receptor gene assembly. However, the increased hybrid joint formation in Atm-deficient cells is only observed during rearrangements that occur by inversion, not during those that occur by deletion (22). Thus, if Atm directs the appropriate joining of DNA ends during V(D)J recombination, the requirement for this function is restricted to rearrangements that occur by inversion.
Rather than invoke distinct Atm regulated pathways that prevent the accumulation of un-repaired coding ends and the formation of hybrid joints exclusively during inversional rearrangements, we have proposed that both of these defects are due to a requirement for Atm to promote stability of DNA end complexes generated after Rag-mediated cleavage (22). Cleavage by the Rag proteins generates two DNA DSBs that divide the chromosome into three non-continuous segments: the centromeric chromosomal end, the telomeric chromosomal end and an intervening chromosomal segment. Signal ends flank the intervening chromosomal segment when the rearrangement occurs by deletion, whereas this segment is flanked by a coding end and a signal end when the rearrangement occurs by inversion. Loss of the intervening chromosomal segment during inversional rearrangements would leave chromosomal coding and signal ends that, upon joining, would form a hybrid joint. In contrast, during rearrangements by deletion, loss of the signal end flanked intervening chromosomal segment would leave chromosomal coding ends that, upon joining, would form a coding joint.
In addition, defects in the stability of post-cleavage complexes in Atm-deficient cells would also lead to the loss of chromosomal coding ends. These coding ends could persist un-repaired or be resolved aberrantly as chromosomal translocations and large chromosomal deletions and inversions. Thus, a requirement for Atm to stabilize DSB complexes in a way that facilitates their repair through the NHEJ pathway could explain both the accumulation of un-repaired coding ends and the increase in hybrid joint formation observed in Atm-deficient lymphocytes. This notion is supported by our current data demonstrating that hybrid joint formation in Atm-deficient cells is dependent on classical NHEJ. Atm could function directly to stabilize DNA DSB complexes after Rag-mediated DNA cleavage; however, it seems more likely that Atm promotes stability by activating downstream proteins that perform this function. In this regard, it is notable that inhibition of Atm kinase activity, with KU-55933, is sufficient to recapitulate the defects in V(D)J recombination observed in Atm−/− cells.
The inhibition of hybrid joint formation during inversional rearrangements has important implications for efficient antigen receptor gene assembly. In this regard, inversional rearrangements involving V gene segments occur in the TCRβ and TCRδ loci. Moreover, in the IgLκ locus, half of the Vκ to Jκ rearrangements occur by inversion. Hybrid joint formation during these rearrangements would result in large deletions of the TCR or IgLκ loci that could severely limit, or even prevent, subsequent rearrangements that would generate functional antigen receptor genes. Thus, by inhibiting hybrid joint formation during inversional rearrangements, Atm functions to preserve the integrity of antigen receptor loci in a manner that optimizes the generation of functional antigen receptor genes.
Acknowledgments
B.P.S is supported by the National Institutes of Health grants AI47829 and AI49934 and American Cancer Society grant RSG-05-070-01-LIB.
Abbreviations used in this paper
- CJ
Coding joint
- HJ
hybrid joint
- CE
coding end
- NR
non-rearranged
- E
EcoRV
- N
NcoI
- Atm
ataxia telangiectasia mutated
- DSB
double strand break
- NHEJ
non-homologous end joining
- RS
recombination signal
References
- 1.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 2.Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG. The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu Rev Immunol. 2000;18:495–527. doi: 10.1146/annurev.immunol.18.1.495. [DOI] [PubMed] [Google Scholar]
- 3.Rooney S, Chaudhuri J, Alt FW. The role of the non-homologous end-joining pathway in lymphocyte development. Immunol Rev. 2004;200:115–131. doi: 10.1111/j.0105-2896.2004.00165.x. [DOI] [PubMed] [Google Scholar]
- 4.Buck D, Malivert L, de Chasseval R, Barraud A, Fondaneche MC, Sanal O, Plebani A, Stephan JL, Hufnagel M, le Deist F, Fischer A, Durandy A, de Villartay JP, Revy P. Cernunnos, a novel nonhomologous end-joining factor, is mutated in human immunodeficiency with microcephaly. Cell. 2006;124:287–299. doi: 10.1016/j.cell.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Ahnesorg P, Smith P, Jackson SP. XLF interacts with the XRCC4-DNA ligase IV complex to promote DNA nonhomologous end-joining. Cell. 2006;124:301–313. doi: 10.1016/j.cell.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Morzycka-Wroblewska E, Lee FE, Desiderio SV. Unusual immunoglobulin gene rearrangement leads to replacement of recombinational signal sequences. Science. 1988;242:261–263. doi: 10.1126/science.3140378. [DOI] [PubMed] [Google Scholar]
- 7.Lewis SM, Hesse JE, Mizuuchi K, Gellert M. Novel strand exchanges in V(D)J recombination. Cell. 1988;55:1099–1107. doi: 10.1016/0092-8674(88)90254-1. [DOI] [PubMed] [Google Scholar]
- 8.Bogue MA, Wang C, Zhu C, Roth DB. V(D)J recombination in Ku86-deficient mice: distinct effects on coding, signal, and hybrid joint formation. Immunity. 1997;7:37–47. doi: 10.1016/s1074-7613(00)80508-7. [DOI] [PubMed] [Google Scholar]
- 9.Bogue MA, Jhappan C, Roth DB. Analysis of variable (diversity) joining recombination in DNAdependent protein kinase (DNA-PK)-deficient mice reveals DNA-PK-independent pathways for both signal and coding joint formation. Proc Natl Acad Sci U S A. 1998;95:15559–15564. doi: 10.1073/pnas.95.26.15559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghavan SC, Tong J, Lieber MR. Hybrid joint formation in human V(D)J recombination requires nonhomologous DNA end joining. DNA Repair (Amst) 2006;5:278–285. doi: 10.1016/j.dnarep.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Sekiguchi JA, Whitlow S, Alt FW. Increased accumulation of hybrid V(D)J joins in cells expressing truncated versus full-length RAGs. Mol Cell. 2001;8:1383–1390. doi: 10.1016/s1097-2765(01)00423-3. [DOI] [PubMed] [Google Scholar]
- 12.Melek M, Gellert M, van Gent DC. Rejoining of DNA by the RAG1 and RAG2 proteins. Science. 1998;280:301–303. doi: 10.1126/science.280.5361.301. [DOI] [PubMed] [Google Scholar]
- 13.Agrawal A, Eastman QM, Schatz DG. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. doi: 10.1038/29457. [see comments]. [DOI] [PubMed] [Google Scholar]
- 14.Verkaik NS, Esveldt-van Lange RE, van Heemst D, Bruggenwirth HT, Hoeijmakers JH, Zdzienicka MZ, van Gent DC. Different types of V(D)J recombination and end-joining defects in DNA double-strand break repair mutant mammalian cells. Eur J Immunol. 2002;32:701–709. doi: 10.1002/1521-4141(200203)32:3<701::AID-IMMU701>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 15.Kabotyanski EB, Gomelsky L, Han JO, Stamato TD, Roth DB. Double-strand break repair in Ku86- and XRCC4-deficient cells. Nucleic Acids Res. 1998;26:5333–5342. doi: 10.1093/nar/26.23.5333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth DB, Wilson JH. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986;6:4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan CT, Boboila C, Souza EK, Franco S, Hickernell TR, Murphy M, Gumaste S, Geyer M, Zarrin AA, Manis JP, Rajewsky K, Alt FW. IgH class switching and translocations use a robust non-classical end-joining pathway. Nature. 2007;449:478–482. doi: 10.1038/nature06020. [DOI] [PubMed] [Google Scholar]
- 18.Corneo B, Wendland RL, Deriano L, Cui X, Klein IA, Wong SY, Arnal S, Holub AJ, Weller GR, Pancake BA, Shah S, Brandt VL, Meek K, Roth DB. Rag mutations reveal robust alternative end joining. Nature. 2007;449:483–486. doi: 10.1038/nature06168. [DOI] [PubMed] [Google Scholar]
- 19.Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 20.Lavin MF, Shiloh Y. The genetic defect in ataxia-telangiectasia. Annu Rev Immunol. 1997;15:177–202. doi: 10.1146/annurev.immunol.15.1.177. [DOI] [PubMed] [Google Scholar]
- 21.Huang CY, Sharma GG, Walker LM, Bassing CH, Pandita TK, Sleckman BP. Defects in coding joint formation in vivo in developing ATM-deficient B and T lymphocytes. J Exp Med. 2007;204:1371–1381. doi: 10.1084/jem.20061460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bredemeyer AL, Sharma GG, Huang CY, Helmink BA, Walker LM, Khor KC, Nuskey B, Sullivan KE, Pandita TK, Bassing CH, Sleckman BP. ATM stabilizes DNA double-strand-break complexes during V(D)J recombination. Nature. 2006;442:466–470. doi: 10.1038/nature04866. [DOI] [PubMed] [Google Scholar]
- 23.Vacchio MS, Olaru A, Livak F, Hodes RJ. ATM deficiency impairs thymocyte maturation because of defective resolution of T cell receptor {alpha} locus coding end breaks. Proc Natl Acad Sci U S A. 2007;104:6323–6328. doi: 10.1073/pnas.0611222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matei IR, Gladdy RA, Nutter LM, Canty A, Guidos CJ, Danska JS. ATM deficiency disrupts TCR{alpha} locus integrity and the maturation of CD4+CD8+ thymocytes. Blood. 2006 doi: 10.1182/blood-2006-05-020917. [DOI] [PubMed] [Google Scholar]
- 25.Muljo SA, Schlissel MS. A small molecule Abl kinase inhibitor induces differentiation of Abelson virus-transformed pre-B cell lines. Nat Immunol. 2003;4:31–37. doi: 10.1038/ni870. [DOI] [PubMed] [Google Scholar]
- 26.Sekiguchi J, Ferguson DO, Chen HT, Yang EM, Earle J, Frank K, Whitlow S, Gu Y, Xu Y, Nussenzweig A, Alt FW. Genetic interactions between ATM and the nonhomologous end-joining factors in genomic stability and development. Proc Natl Acad Sci U S A. 2001;98:3243–3248. doi: 10.1073/pnas.051632098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Y, Pannicke U, Schwarz K, Lieber MR. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell. 2002;108:781–794. doi: 10.1016/s0092-8674(02)00671-2. [DOI] [PubMed] [Google Scholar]
- 28.Goodarzi AA, Yu Y, Riballo E, Douglas P, Walker SA, Ye R, Harer C, Marchetti C, Morrice N, Jeggo PA, Lees-Miller SP. DNA-PK autophosphorylation facilitates Artemis endonuclease activity. Embo J. 2006;25:3880–3889. doi: 10.1038/sj.emboj.7601255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han JO, Steen SB, Roth DB. Ku86 is not required for protection of signal ends or for formation of nonstandard V(D)J recombination products. Mol Cell Biol. 1997;17:2226–2234. doi: 10.1128/mcb.17.4.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han JO, Erskine LA, Purugganan MM, Stamato TD, Roth DB. V(D)J recombination intermediates and non-standard products in XRCC4-deficient cells. Nucleic Acids Res. 1998;26:3769–3775. doi: 10.1093/nar/26.16.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]