Abstract
Heavy drinking has increased in recent years and has been linked to numerous health-related risks, particularly in women. A number of factors may play a role in exacerbating the risks linked to heavy drinking, such as impulsivity, which itself is related to a number of risky behaviors. The present study investigated the effects of alcohol (0, 0.5, 0.75 g/kg) on impulsivity in female heavy drinkers (n = 23) and female light drinkers (n = 23) using a double-blind, placebo-controlled outpatient design; all women were tested during follicular phase of the menstrual cycle. Each session, participants completed a range of tasks including subjective measures of abuse liability, cognitive performance tasks, three behavioral impulsivity tasks, and a risk-taking task. Alcohol increased impulsivity on the Immediate and Delayed Memory Task (IMT and DMT) and Delay Discounting task. Heavy drinkers scored higher on impulsivity self-reports and were more impulsive on the IMT and the GoStop task than light drinkers. The high dose of alcohol further increased impulsive performance on the IMT and DMT in heavy drinkers. There were no group differences or alcohol effects on the Balloon Analogue Risk Task. Alcohol increased sedative-like effects more in light drinkers and increased stimulant-like effects and alcohol liking more in heavy drinkers. In summary, female heavy drinkers are less sensitive to the negative effects of alcohol, report more positive effects of alcohol, and are more impulsive than female light drinkers. Moreover, impulsive responding was exacerbated by alcohol drinking among female heavy drinkers, indicating that women who drink at this level are at increased risk for developing alcohol use disorders and engaging in other risky behaviors, particularly after drinking.
Keywords: impulsivity, alcohol, abuse liability, heavy drinking, women
Alcohol misuse (e.g., heavy and/or binge drinking) is prevalent (Grucza, Norberg, Bucholz, & Bierut, 2008; Helfand, Mukamal, & Mittleman, 2011; Substance Abuse and Mental Health Services Administration (SAMHSA), 2009; Center for Disease Control (CDC), 2010) and poses many health-related risks (e.g., Schuckit, 2009). For instance, the risk of developing drug and alcohol use disorders (Herd, 1993; Jackson, 2008; Maisto, Clifford, Stout, & Davis, 2007), heart disease (National Institute on Alcohol Abuse and Alcoholism (NIAAA), 2008; Roerecke et al., 2011), liver damage (NIAAA, 2008), and cancer (Chen, Rosner, Hankinson, Colditz, & Willett, 2011; NIAAA, 2008), and of engaging in sexual risk-taking (Hipwell, Stepp, Chung, Durand, & Keenan, 2012), are increased in individuals who misuse alcohol, particularly women. Despite this, there is little alcohol-related research focused on women, specifically with respect to well-controlled studies that attempt to elucidate factors that may contribute to these risks in women who drink.
One factor that has been repeatedly implicated in the initiation and continuation of drug and alcohol use is impulsivity (e.g., Hamilton, Ansell, Reynolds, Potenza, & Sinha, 2012; Nees et al., 2012; Quinn, Stappenbeck, & Fromme, 2011; Shin, Hong, & Jeon, 2012; see Aragues, Jurado, Quinto, & Rubio, 2011; Dick et al., 2010; Lejuez et al., 2010; Potenza & de Wit, 2010 for reviews). For example, self-reported impulsivity was greater in problem drinkers than controls (MacKillop, Mattson, MacKillop, Castelda, & Donovick, 2007), and greater alcohol use was associated with greater self-report of impulsivity in various alcohol using groups, such as social drinkers (Grau & Ortet, 1999; Henges & Marczinki, 2012; Waldeck & Miller, 1997) and comorbid marijuana and heavy alcohol users (Peters et al., 2012). Further, alcohol administration has been shown to increase impulsive performance on rapid-decision and/or continuous performance tasks in social drinkers (Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008; Dougherty, Marsh, Moeller, Chokshi, & Rosen, 2000; Fillmore & Weafer, 2011; Henges & Marczinksi, 2012; Mulvihill, Skilling, & Vogel-Sprott, 1997; Reynolds, Richards, & de Wit, 2006; Weafer & Fillmore, 2012), but not consistently on delay discounting tasks in problem drinkers or social drinkers (MacKillop, Mattson, MacKillop Castelda, & Donovick, 2007; Ortner, MacDonald, & Olmstead, 2003; Petry, 2001; Richards, Zhang, Mitchell, & de Wit, 1999; Reynolds, Richards, & de Wit, 2006), suggesting a differential interaction between alcohol and the various facets of impulsivity.
The majority of existing studies that have examined the interaction between alcohol and impulsivity have occurred in groups that combined men and women, combined individuals with various prestudy drinking levels, and/or did not control for other risk factors, all of which may influence impulsivity and/or alcohol’s effects. For instance, numerous studies have shown that individuals with a family history of alcoholism are more impulsive (e.g., Acheson, Richard, Mathias, & Dougherty, 2011) and report greater positive subjective effects of alcohol (e.g., Evans & Levin, 2011; Söderpalm & Söderpalm, 2011) than those who do not have a family history of alcoholism. A history of trauma has also recently been associated with greater impulsivity (Evans, Levin & Reed, submitted), risk-taking (Bornovalova, Gwadz, Kahler, Aklin, & Lejuez, 2008), and the development of alcohol use disorders (e.g., Enoch, 2011). While problem drinking is prevalent in individuals with a family history of alcoholism (Hinckers et al., 2006; Schuckit & Smith, 2011; Warner, White, & Johnson, 2007) and histories of trauma (Khoury, Tang, Bradley, Cubells, & Ressler, 2010; Magnusson et al., 2011), most studies have not assessed the presence of these other risk factors, particularly in women, making it difficult to ascertain whether heavy drinking alone is associated with greater impulsivity.
Therefore, the purpose of the present study was to examine the interaction between impulsivity and the response to alcohol among heavy (“at-risk”) drinking (HD) women compared with light-drinking (LD) women, without the potential confounds of other risk factors (e.g., history of trauma, family history of alcoholism). We hypothesized that HD women would (1) report greater positive subjective effects from alcohol, (2) exhibit greater baseline impulsivity based on both self-report and behavioral measures of impulsivity, and (3) be even more impulsive after alcohol, compared with LD women.
Method
Participants
Women who participated in this study responded to an advertisement in local newspapers recruiting female social and regular drinkers. Individuals were told that the purpose of the study was to determine the effects of various nonprescription and prescription drugs and alcohol on mood, vital signs, and ability to perform certain tasks across the menstrual cycle. The Institutional Review Board of the New York State Psychiatric Institute approved this study. Participants gave their written informed consent before beginning the study and were paid for their participation.
Seventy-one women (33 HD and 38 LD) met all inclusion/exclusion criteria and started the study, however 20 participants (35%) did not complete the entire study because of scheduling conflicts. In addition, five participants were not included in the final analyses as a result of hormone levels indicating that sessions were not conducted in the correct phase of the menstrual cycle (n = 2) or prospective drinking levels during the study were not within the inclusion criteria range for that group (n = 3). Therefore, a total of 46 women (23 HD women and 23 LD women) who met full study criteria and completed the study were used in these analyses. Heavy (“at-risk”) drinking was defined as ≥7 drinks/week, as per the NIAAA guidelines for women (NIAAA, 2009), and light drinking was defined as ≤6 drinks/week. Participants were interviewed on three occasions: first by the investigator during the initial consent meeting, then by the Master’s level clinical interviewer, and finally by the physician during the physical examination. Participants also completed the Daily Ratings Form (see Evans, Haney, Levin, Foltin, & Fischman, 1998 for details) each evening throughout the study to prospectively track alcohol use, mood, menstrual cycle length, and the onset of menstruation.
All women were medically and psychiatrically healthy based on a complete physical examination, a structured clinical interview, 12-lead electrocardiogram, clinical blood chemistries, and urinalyses. None of the participants were pregnant based on plasma levels of circulating chorionic gonadotropin hormone (hCG), or nursing, and no one had been pregnant or had an abortion within the previous six months. All women were normally cycling and were not using hormonal contraceptives or any other prescription medications. In addition, no one had any current Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM–IV–TR) Axis I psychiatric disorders (American Psychiatric Association, 2000) within the last year, including psycho-active substance (including alcohol) abuse or dependence (other than nicotine or caffeine), based on the Structured Clinical Interview for DSM–IV–TR (SCID I; First, Spitzer, Gibbon, & Williams, 1995) that was conducted by a trained Master’s level clinical interviewer. In terms of past psychiatric disorders, four women (three HD and one LD) had a past history of depression, nine women (four LD and five HD) had a past history of alcohol abuse, two HD women had a past history of alcohol dependence, and two HD women had a past history of marijuana abuse.
Women who abstained from alcohol or typically drank more than 20 drinks per week in the past year were excluded. Individuals who consumed >4 cups of coffee/day (the frequency and amount of all sources of caffeine use including coffee, tea, soft drinks and energy drinks was converted to cups of coffee/day for a standard reference) were also excluded. However, women who were cigarette smokers were not excluded, nor were women who used marijuana occasionally (<1 time/week). None of the participants suffered from premenstrual dysphoric disorder based on the Pre-menstrual Assessment Form (Halbreich, Endicott, & Schacht, 1982) and the modified Daily Ratings Form (Evans, Haney, Levin, Foltin, & Fischman, 1998).
Women were excluded if they had any major history of trauma (either during childhood or as adults) or a first-degree family history of alcoholism or substance abuse. This was done because the acute response to alcohol can differ as a function of family history of alcoholism (Evans & Levin, 2011; Pollock, 1992) and trauma history (Evans, Levin, & Reed, submitted). Early life trauma was determined by The Early Trauma Inventory (ETI; Bremner, Vermetten, & Mazure, 2000), and a family history of alcoholism and substance abuse was determined by The Family History Assessment Module and the Individual Assessment Module, both structured diagnostic instruments (Rice et al., 1995), and confirmed by conducting telephone interviews with first-degree biological relatives (see Evans & Levin, 2011 for details).
Procedures
Women participated as outpatients at the New York State Psychiatric Institute for five sessions lasting 7 hrs each. The first session was a practice session when they received placebo capsules and a placebo beverage and were trained on the various tasks and procedures. The testing phase consisted of three sessions scheduled during the follicular phase (Days 4 –10 after the onset of menstruation) of the menstrual cycle. Participants received alcohol (0.0, 0.50, and 0.75 g/kg) on separate days, with the dose order randomized within each group and across the HD and LD groups. The last session was a lottery session based on the Multiple Choice Procedure (described below). Data from the practice session and the lottery session were not used for data analysis.
Participants completed the modified Daily Ratings Form (see Evans & Levin, 2003 for details) each evening throughout the study to track changes in mood symptoms across the menstrual cycle, document the onset and duration of menstruation, and record the number of standard alcoholic beverages consumed. Participants were instructed to call the laboratory when they started menstruating. Sessions were scheduled during the follicular phase (e.g., Reed, Levin, & Evans, 2010) to eliminate potential variation in the subjective effects of alcohol as a function of menstrual cycle phase (e.g., Evans & Levin, 2011). Menstrual cycle phase was validated using hormone levels. Missed sessions were rescheduled during the follicular phase of the next menstrual cycle.
Experimental sessions
Participants reported to the laboratory at 0830 and remained until 1530. Participants were instructed not to eat breakfast before reporting to the laboratory and to refrain from using all psychoactive drugs (with the exception of tobacco, caffeinated products, and alcohol) for the duration of the study. Participants were instructed not to drink alcohol 24 hr before or after a session. Each session, a urine specimen was collected and analyzed for the presence of illicit drugs and a breath alcohol test was conducted (Alco-Sensor III, Intoximeters, Inc., St. Louis, MO). Four HD women had positive urine drug screens for marijuana; these were the same women who had reported occasional marijuana use during screening, which was not exclusionary. There were no other positive urine drug samples and no positive breath alcohol tests on any sessions.
In the mornings, blood samples were drawn for hormone assays; urine pregnancy tests were performed weekly. Participants ate a light breakfast (with a caffeinated beverage for those individuals who regularly consumed caffeine to avoid caffeine withdrawal), and then completed a baseline assessment battery (described below). After the baseline assessment battery, participants ingested two capsules and a beverage and completed the assessment battery at specified times (described below) for the remainder of the session. After the 3-hr assessment battery, participants were provided lunch.
At the end of each session, participants were required to pass a field sobriety test and were not allowed to leave the laboratory until breath alcohol concentrations were ≤20 mg/dl. If the participant was still impaired, she remained at the laboratory until the drug effects subsided. Participants were provided round-trip subway fare each session and were instructed not to drive, take any medications, or drink alcohol the remainder of the day.
Impulsivity
Three impulsivity self-reports, The Barratt Impulsiveness Scale, version 11 (BIS), the Impulsivity Questionnaire (IQ), and the Sensation-Seeking Scale (SSS), were completed during screening. (1) The BIS is a 30-item questionnaire that measures three dimensions of impulsivity: attentional, motor, and nonplanning and also generates a total impulsivity score (Patton, Stanford, & Barratt, 1995). (2) The IQ is a 54-item questionnaire that measures three dimensions of impulsivity: impulsiveness, venturesomeness, and emphathy, and also generates a total impulsivity score (Eysenck, Pearson, Easting, & Allsopp, 1985). (3) The SSS is a 40-item questionnaire that measures four dimensions of sensation seeking: thrill and adventure seeking, experience seeking, disinhibition, and boredom susceptibility and also generates a total sensation-seeking score (Zuckerman, Eysenck, & Eysenck, 1978).
Three measures of behavioral impulsivity (the Immediate Memory Task/Delayed Memory Task [IMT/DMT], the GoStop Task, and the Delay Discounting Task [DDT]) and one measure of risk-taking (the Balloon Analogue Risk Task (BART)) were assessed multiple times each session (Reed, Levin, & Evans, 2010).
The IMT/DMT is a continuous performance task that yields a number of measures related to response initiation (Dougherty & Marsh, 2003; Dougherty, Marsh, Moeller, Chokshi, & Rosen, 2002; Dougherty et al., 2003a). In the IMT, a series of five-digit numbers appeared successively on a computer monitor. Participants were instructed to respond when the stimulus on the monitor was identical to the one that preceded it. Each five-digit number appeared for 500 msec, and successive numbers were separated by a 500-msec intertrial interval. The DMT required the participant to remember a five-digit number and then compare it with another that was presented 3.5 sec later. During the 3.5-s interval, repetitive distracter stimuli (the five-digit No. 12345) were presented at the same rate and duration as the other stimuli. Participants were told to ignore the distracter stimuli and to remember and compare only the numbers spanning the distracter stimuli. The IMT/DMT consisted of one 5-min block of IMT followed by one 5-min block of DMT, with a 30-s rest period between blocks. The primary dependent measure for each of these two tasks was the IMT ratio and DMT ratio, respectively. The ratio is defined as the proportion of commission errors to correct detections (Dougherty, Marsh, & Mathias, 2002; Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008). The IMT/DMT was completed at baseline and 1, 2, and 4 hr after drug administration each session.
The GoStop task is a task that measures response inhibition (Dougherty, Mathias, & Marsh 2003b; Dougherty, Mathias, Marsh, & Jagar, 2005). In the GoStop task, a series of five-digit numbers presented in black on a white background, with randomly generated five-digit numbers appearing for 500 msec every 2 sec (500 msec on, 1500 msec off) were presented. Participants were told to respond when the number they saw was identical to the previous number. Half of all target trials feature a target-stop trial when the color of the matching target’s numerals changed from black to red at 50, 150, 250, and 350 msec after its presentation. Participants were instructed to respond to the identically matching numbers before the number disappeared from the screen, but not to respond to a number that turns red. The primary dependent measure was the 150-msec GoStop ratio, which is the number of response inhibition failures for the 150-msec delay relative to the number of responses to go trials (Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008). The GoStop task was completed at baseline and 1, 2, and 4 hr after drug administration each session.
The DDT, developed by Kirby and Marakovic (1996) and revised by Kirby, Petry, and Bickel (1999), consisted of a fixed set of 27 choices between smaller immediate rewards and larger delayed rewards; reward values ranged from $11 to $85 and delays ranged from 7 days to 6 months (Petry, Kirby, & Kranzler, 2002). The primary dependent measure is the k value, which determines the discount rate, or the steepness of the reduction in the present value of a reward with increases in delay to that reward (Kirby, Petry, & Bickel, 1999). Higher k values indicate higher levels of impulsivity. To encourage attentive responding, participants were informed that at the end of the last session, they would be given a one in six chance of receiving the reward that they chose on one of the trials. This was done by having participants role a die on the last session; if they rolled a 6, one question/response was selected out of a container of all of the questions/responses on all sessions, then they received whatever they chose in response to that question. If their choice had been an immediate amount of money, they received the money in cash immediately. If they selected delayed money, the money was given when the time had elapsed. The DDT was completed at baseline and 0.25, 1, 2, and 4 hr after drug administration each session.
The BART, developed by Lejuez et al. (2002), involved displaying a small blue balloon on a computer screen. Each pump inflated the balloon and was accompanied by an accrual of 5 cents; the average pump break point was 64 pumps. When the balloon was pumped past its individual explosion point, a “pop” sound was generated and all money in the temporary bank was lost, then the next uninflated balloon was displayed. However, at any point during each balloon trial, the participant could stop pumping the balloon and click the collect money button that transferred all money from the temporary bank to the permanent bank. Fifteen balloon trials were presented each time. The primary dependent measure was the number of adjusted pumps, defined as the average number of pumps excluding balloons that exploded (i.e., the average number of pumps on each balloon before money collection). To ensure consistent effort, participants were instructed that at the end of the study, they would receive a percentage of the actual money earned on this task. The BART was completed at baseline and 0.25, 1, 2, and 4 after drug administration each session.
Abuse-liability
(1) The Biphasic Alcohol Effects Scale (BAES; Martin, Earleywine, Musty, Perrine, & Swift, 1993) is a 14-item adjective rating scale to derive two subscales measuring stimulant (BAES Stimulant) and sedative (BAES Sedative) effects. The BAES was completed at baseline and 0.25, 1, 2, 3, 4, and 5 hr after drug administration each session. (2) The Drug Effects Questionnaire (DEQ; Reed, Levin, & Evans, 2010) asked participants to rate “good effects,” “bad effects,” “strength of the drug effect,” and “willing to take the drug again” on a five-point scale and “drug liking” on a nine-point scale. The DEQ was completed 0.25, 1, 2, 3, 4, and 5 hr after drug administration each session. (3) The Multiple Choice Procedure (Griffiths, Rush, & Puhala, 1996) was used to assess reinforcing effects and was also completed at 0.25, 1, 2, 3, 4, and 5 hr after drug administration. Six times each session participants made a series of nine discrete choices between the drug dose administered and various amounts of money, with the dollar value increasing from $0.25 to $64.00. Data from this procedure were analyzed as the maximum dollar amount that participants chose drug over money (i.e., the cross-over point). The last session was a lottery session and participants randomly selected a poker chip; each poker chip had a number that corresponded to one of their previous choices from Sessions 2–7. The choice corresponding to the number on the poker chip drawn was implemented and the session proceeded.
Other measures
The Beck Depression Inventory II (BDI II; Beck, Steer, Ball, & Ranieri, 1996) and the State Anxiety Inventory (STAI; Spielberger, Gorsuch, & Lushene, 1970) were completed at baseline and 2 and 4 hrs after drug administration. The Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971) questionnaire was completed at baseline and 0.25, 1, 2, 3, 4, and 5 hrs after drug administration.
The computerized Digit Symbol Substitution Test (DSST; McLeod, Griffiths, Bigelow, & Yingling, 1982; a measure of motor coordination and cognition) and the balance task (Evans & Levin, 2011; a measure of motor coordination) were completed at baseline and 0.25, 1, 2, 3, 4, and 5 hrs after drug administration. For the DSST, the primary dependent measures were total arrays attempted and total arrays correct; for balance the dependent measure was the total number of seconds the participant was able to balance (maximum of 60 sec).
Drug
A double-dummy design was used; participants ingested two capsules containing placebo and a beverage (0, 0.5, 0.75 g/kg alcohol) each session, with alcohol dose calculated based on the estimated total body water of each participant (see Evans & Levin, 2011). Participants were informed that they could receive placebo, over-the-counter medications, prescription medications, or alcohol. Placebo capsules (size 0 gelatin) were filled with lactose powder. The beverage consisted of tonic water and cranberry juice, with 100 proof Absolut vodka added to achieve the correct volume for each individualized alcohol dose. Participants had 5 min to consume the capsules and the entire beverage (placebo or alcohol) under the supervision of an investigator.
Data Analysis
Analyses were based on the 46 women who completed the study. t tests were used to compare demographic characteristics at screening between the HD and LD women. Peak effects were analyzed for all study measures using separate two-factor repeated measures analyses of variance (ANOVA) with Group (HD vs. LD) as the between-subjects factor and Dose (0, 0.50, 0.75 g/kg) as the within-subjects factors. Based on the time course, maximum breath alcohol levels were observed 1 hr after alcohol administration and maximal behavioral effects for the majority of measures were observed 1–2 hrs after alcohol administration. The peak value for each participant for each measure was determined and analyzed. The direction of the peak effect (maximum or minimum) was based on inspection of the time course data. Post hoc tests were conducted for significant main effects of group, dose, or group × dose interactions. For illustrative purposes, time course data are presented for selected primary measures. Results were considered statistically significant if p ≤ .05 and Huynh-Feldt corrections were used.
Results
Demographics
Table 1 shows that there were few significant differences in demographic variables between HD and LD women. However, as expected, HD women drank significantly more days per week, had more drinks per week and per occasion, had more heavy drinking days per week, and engaged in more binge drinking than LD women. There were no significant differences in other drug use between groups. HD women also reported significantly greater depressive symptoms on the BDI and state anxiety scores on the STAI than LD women. On the alcohol effects questionnaire (AEQ) that addresses alcohol outcome expectancies (data not shown), HD women reported significantly greater social and physical pleasure (p ≤ .05) and a trend in greater positive global changes in experience (p = .09) and arousal/interpersonal power (p = .08) than LD women in response to alcohol.
Table 1.
Demographic Characteristics of Study Participants
| LD (n = 23) | HD (n = 23) | |
|---|---|---|
| Age (yrs) | 28.2 (3.9) | 28.0 (5.2) |
| Race (Black/White/Hispanic/other) | 5/11/3/4 | 4/12/3/4 |
| Education (yrs) | 16.6 (1.8) | 15.9 (1.1) |
| Menstrual cycle length (days) | 28.5 (2.4) | 28.5 (1.9) |
| Prospective alcohol consumption | ||
| Alcohol drinks/week* | 2.9 (1.2) | 10.2 (2.7) |
| Absolute range (drinks/week) | 0.5–5.4 | 7.0–16.5 |
| Drinking days (%)* | 26.9 (13.5) | 62.1 (17.4) |
| Drinks/drinking, day* | 1.8 (0.6) | 2.7 (0.8) |
| Binge drinkers (n)* | 6 | 20 |
| Binge drinking days (%)* | 7.4 (17.5) | 24.6 (22.1) |
| Cigarette smokers (n) | 1 | 2 |
| Marijuana smokers (n) | 2 | 4 |
| Beck Depression Inventory* | 2.0 (3.3) | 4.1 (3.8) |
| Trait Anxiety Inventory | 31.7 (7.7) | 34.8 (5.5) |
| State Anxiety Inventory* | 28.8 (7.1) | 33.1 (7.1) |
Note. All demographics are presented as means ± SD unless otherwise denoted.
Significant difference between groups (p ≤ .05).
Hormone Levels
Based on prospective tracking of the menstrual cycle, all women had normal menstrual cycles that ranged from 23 to 34 days (mean of 29 days), with no differences between the two groups (see Table 1). There were no differences between HD and LD women with respect to estradiol levels (55.08 ± 13.44 pg/ml vs. 52.74. ± 4.77 pg/ml; p ≥ .05) or progesterone levels (0.98 ± 0.07 ng/ml vs. 0.82 ± 0.07 ng/ml; p ≥ .05); these levels are consistent with the follicular phase of the menstrual cycle.
Breath Alcohol Levels and Cardiovascular Effects
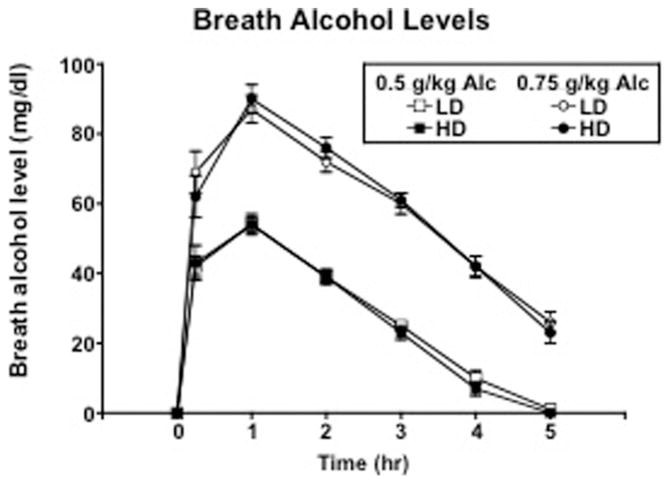
Figure 1 shows breath alcohol levels as a function of group, dose, and time. Breath alcohol levels peaked 1 hr after both doses of alcohol administration in both groups. Alcohol produced significant dose-related increases in peak breath alcohol levels [F(2, 88) = 751.95; p < .0001], reaching a maximum of 56 and 92 mg/dl following 0.50 and 0.75 g/kg alcohol, respectively. There were no differences in breath alcohol levels between the two groups. For cardiovascular effects (data not shown), there was a dose effect of alcohol [F(2, 88) = 8.94; p = .0003], where 0.75 g/kg alcohol produced significant increases in heart rate compared with placebo (p ≤ .05), with no differences between the two groups. There were no significant changes in systolic or diastolic blood pressure as a function of alcohol dose or between the two groups.
Figure 1.
Time course of breath alcohol levels as a function of group and alcohol dose. Error bars represent ± 1 SEM.
Impulsivity and Risk-Taking
Total scores on impulsivity and risk-taking self-reports are presented in Table 2. Overall, HD women reported being more impulsive and risk-taking than LD women. Self-report of impulsivity was significantly greater in HD women than LD women based on the attentional and nonplanning subscales of the BIS, as well as the total BIS scores (all ps ≤ 0.05). The impulsiveness subscale scores on the EIQ were also significantly greater in HD women than LD women (p ≤ .05). Lastly, scores of experience-seeking, disinhibition, boredom susceptibility, and total scores on the SSS were greater in the HD women than the LD women (all ps ≤ 0.05).
Table 2.
Impulsivity and Risk-Taking Self-Report Scores
| Self-report (mean ± SD) | LD | HD |
|---|---|---|
| Barratt Impulsiveness Scale (BIS) | ||
| Attentional* | 13.6 (3.5) | 17.1 (3.3) |
| Motor | 19.6 (3.3) | 21.2 (3.6) |
| Non-planning* | 24.6 (4.7) | 27.5 (3.9) |
| Total* | 57.8 (7.3) | 65.8 (7.1) |
| Eysenck Impulsivity Questionnaire (EIQ) | ||
| Impulsiveness* | 3.1 (2.3) | 5.2 (2.7) |
| Venturesomeness | 8.2 (5.6) | 6.7 (3.4) |
| Empathy | 10.0 (2.4) | 9.4 (2.8) |
| Total | 21.4 (4.2) | 21.3 (4.2) |
| Zuckerman Sensation-Seeking Scale (SSS) | ||
| Thrill and adventure seeking | 6.7 (2.8) | 5.7 (2.8) |
| Experience seeking* | 6.2 (2.1) | 7.3 (1.5) |
| Disinhibition* | 3.9 (2.1) | 6.2 (2.2) |
| Boredom susceptibility* | 2.2 (1.5) | 3.5 (2.1) |
| Total* | 18.9 (5.8) | 22.7 (5.5) |
Significant difference between groups (p ≤ .05).
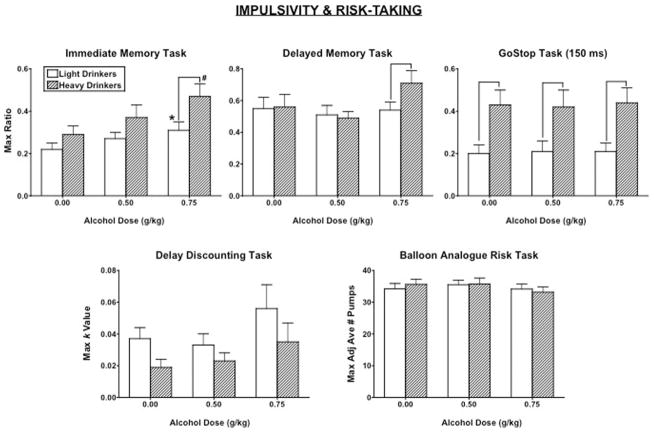
As a representation of the time course of alcohol-induced behavioral impulsivity, Figure 2 demonstrates the time course of the IMT ratio relative to breath alcohol levels after 0.75 g/kg alcohol in LD and HD women. For clarity, Figure 3 documents peak IMT, DMT, and GoStop ratios, DDT overall k value and BART adjusted number of pumps as a function of alcohol dose and group. Alcohol increased the IMT ratio [dose effect: F(2, 88) = 13.71, p < .0001], the DMT ratio [dose effect: F(2, 88) = 3.47, p = .04], and the DDT k value [dose effect: F(2, 88) = 4.70, p = .03]. Further, the IMT ratio [group effect: F(1, 44) = 4.29, p = .04] and the 150 ms GoStop ratio [group effect: F(1, 44) = 7.89, p = .007] were significantly greater in HD women than LD women. Specifically, the GoStop ratio was significantly greater in HD women than LD women regardless of alcohol dose, whereas the 0.75 g/kg alcohol dose produced a greater increase in the IMT ratio in HD women than LD women (p ≤ .05), with no group differences after placebo or 0.5 g/kg alcohol. On the DMT, there was a marginal interaction of alcohol dose and group [interaction: F(2, 88) = 2.52, p = .09]; the DMT ratio was also greater in HD women than LD women after 0.75g/kg alcohol (p ≤ .05). However, there was no significant group effect or group × alcohol dose interaction on the DDT. There were also no differences in risk-taking, as measured by the BART, between groups or as a function of alcohol dose (all ps > 0.05).
Figure 2.
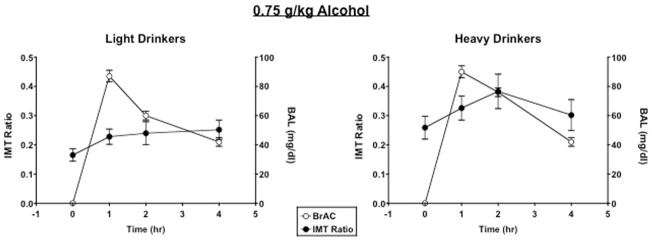
Time course of breath alcohol levels (BAL) and Immediate Memory Task (IMT) ratio in response to 0.75 g/kg alcohol in light drinkers and heavy drinkers. Error bars represent ± SEM.
Figure 3.
Peak Immediate Memory Task (IMT) ratio, Delayed Memory Task (DMT) ratio, 150 ms GoStop ratio, Delay Discounting Task (DDT) overall k value, and Balloon Analogue Risk Task (BART) adjusted number of pumps as a function of group and alcohol dose. * denotes a significant difference compared with 0.00 g/kg alcohol in the light drinkers (p ≤ .05). # denotes a significant difference compared with 0.00 g/kg alcohol in the heavy drinkers (p ≤ .05). denotes a significant difference between groups (p ≤ .05). Error bars represent + 1 SEM.
Abuse Liability Measures
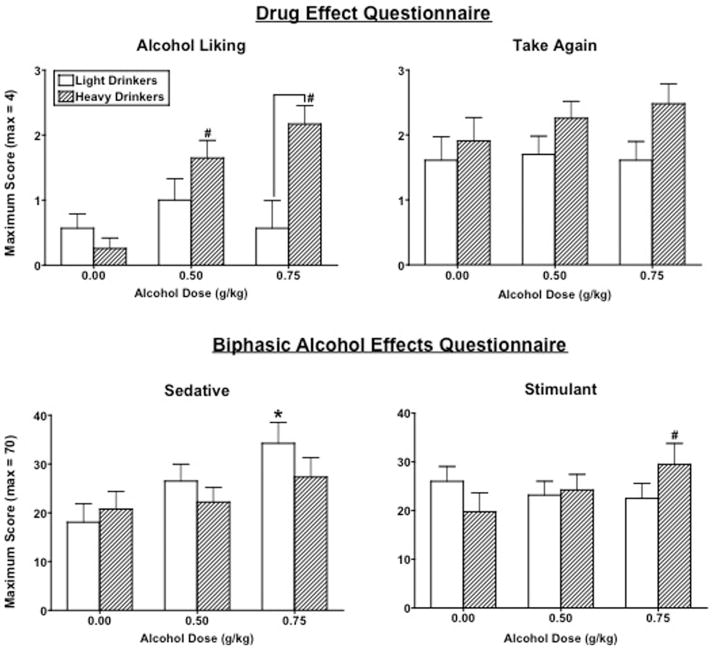
Figure 4 documents peak Drug Liking and Take Again ratings on the DEQ and Stimulant and Sedative scores on the BAES as a function of alcohol dose and group. Overall, alcohol significantly increased ratings of Good Drug Effect [dose effect: F(2, 88) = 17.12, p < .0001] (data not shown) similarly in both groups. However, alcohol significantly increased Drug Liking ratings only in the HD group [interaction: F(2, 88) = 6.31, p = .003]. Ratings of Take Again were also marginally higher [group effect: F(1, 44) = 3.10, p = .09] and ratings of Drug Strength were significantly lower [group effect: F(1, 44) = 4.38, p = .04] in HD women compared to LD women, particularly after 0.75 g/kg alcohol (ps ≤ 0.05). Lastly, alcohol significantly increased Bad Drug Effects [dose effect: F(2, 88) = 18.29, p < .0001] (data not shown), and these ratings were marginally greater in LD women than HD women after 0.75 g/kg alcohol (p = .06).
Figure 4.
Peak Drug Liking and Take Again ratings on the Drug Effects Questionnaire (DEQ) and the Sedation and Stimulant subscale scores on the Biphasic Alcohol Effects Scale (BAES) as a function of group and alcohol dose. See Figure 3 for details.
On the BAES, alcohol significantly increased Sedative scores [dose effect: F(2, 88) = 10.46, p = .0001], although post hoc testing revealed this was the case only after 0.75 g/kg in LD women (p ≤ .05). Conversely, alcohol significantly increased Stimulant scores, but only after 0.75 g/kg in HD women [interaction: F(2, 88) = 4.60, p = .01]. Additionally, alcohol produced a marginal dose-dependent increase in the choice of drug over money on the Multiple Choice Procedure [dose effect: F(2, 88) = 3.47, p = .06], but there were no significant differences in the choice between the groups.
Performance Tasks
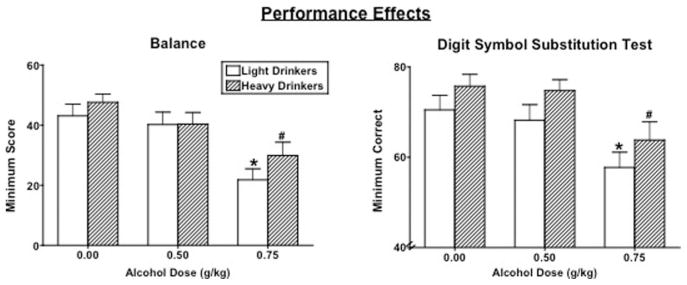
Figure 5 documents peak balance and total arrays correctly completed on the DSST as a function of alcohol dose and group. Performance on the balance task was dose-dependently decreased by alcohol similarly in both groups [dose effect: F(2, 88) = 1.52, p < .0001]. DSST attempts [dose effect: F(2, 88) = 15.29, p < .0001] (data not shown) and correct arrays completed [dose effect: F(2, 88) = 26.65, p < .0001] were also dose-dependently decreased by alcohol to a similar extent in both groups.
Figure 5.
Peak Balance and Digit Symbol Substitution Test (DSST) correct arrays completed as a function of group and alcohol dose. See Figure 3 for details.
Mood Effects
Alcohol significantly and dose-dependently increased State Anxiety scores to a similar extent in both groups [dose effect: F(2, 88) = 6.15, p = .005], but there was no effect of alcohol or any group differences in Beck Depression Scores (all ps > 0.05; data not shown).
On the POMS questionnaire (data not shown), alcohol also significantly increased fatigue scores [dose effect: F(2, 88) = 5.40, p = .006] to the same extent in both groups. However, there were significant interactions of alcohol dose and group on arousal [interaction: F(2, 88) = 3.32, p = .04], confusion [interaction: F(2, 88) = 4.24, p = .02], and, marginally, anxiety [interaction: F(2, 88) = 2.60, p = .09] scores. Specifically, 0.75 g/kg alcohol significantly increased anxiety and confusion scores and decreased arousal scores in LD women, but not HD women (p ≤ .05). There were no significant group or alcohol dose effects on anger, depression, elation, friendly, vigor, or positive mood scores (all ps > 0.05).
Discussion
This is the first study to our knowledge to assess the role of current heavy drinking in the effects of alcohol on various aspects of impulsivity in specifically women in a controlled laboratory study. Although previous laboratory studies have also examined the effects of alcohol on impulsivity, these studies often have included participants with a range of drinking levels (e.g., Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008), mixed gender samples (e.g., Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008; Fillmore & Weafer, 2011; Reynolds, Richards, & de Wit, 2006), and/or only one or two impulsivity performance tasks (e.g., Fillmore & Weafer, 2011; MacKillop, Mattson, MacKillop, Castelda, & Donovick, 2007; Petry, 2001; Rose & Grunsell, 2008). Further, most studies have not excluded, or even assessed, other risk factors that may influence impulsivity and/or alcohol’s effects, such as a family history of alcohol or substance dependence (e.g., Acheson, Richard, Mathias, & Dougherty, 2011; Evans & Levin, 2011; Söderpalm & Söderpalm, 2011), or a history of major trauma (e.g., Bornovalova, Gwadz, Kahler, Aklin, & Lejuez, 2008; Enoch, 2011; Evans, Levin, & Reed, submitted). To this end, the current study focused on well-defined groups of heavy drinking and light drinking women only, used a range of impulsivity tasks, and excluding individuals with a history of trauma and familial alcohol/substance dependence.
The key findings in this study that have not been addressed previously were that HD women were more impulsive on certain tasks than LD women and as hypothesized, alcohol further increased impulsivity, particularly in HD women. Interestingly, overall, response initiation (per the IMT/DMT) and response inhibition (per the GoStop task) were more impaired in HD women than LD women, however alcohol further impaired only response initiation, and only in the HD women. Our results are supported by other studies showing that early onset female drinkers had greater response initiation than late-onset female drinkers (Dougherty, Mathias, Tester, & Marsh, 2004), and “at-risk moderate drinkers” (~13.6 drinks/week) had marginally greater response inhibition than “nonrisk moderate drinkers” (~4.8 drinks/week) (Fillmore & Weafer, 2011). Also similar to our results, response initiation was impaired by alcohol in groups of social drinkers (Dougherty, Marsh, Moeller, Chokshi, & Rosen, 2000; Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008). However, there have been mixed results with respect to alcohol’s effect on response inhibition, with some studies observing an alcohol-induced impairment (Fillmore & Weafer, 2011; Henges & Marczinksi, 2012; Mulvihill, Skilling, & Vogel-Sprott, 1997; Weafer & Fillmore, 2012) and other studies showing no effect of alcohol (Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008; Rose & Duka, 2008), possibly as a result of slight methodological differences between various response inhibition tasks (i.e., cued vs. not cued).
Although alcohol produced an overall increase in delay discounting on the DDT, there were no group differences in DDT performance and there was no effect of group or alcohol dose on risk-taking as per the BART. Results have been inconsistent among studies examining delay discounting (using various tasks) and risk-taking (using the BART) among alcohol users. For example, delay discounting has been shown to predict alcohol use among social drinkers (Christiansen, Cole, Goudie, & Field, 2012) and problem drinkers (Courtney et al., 2011), but not in a group of college students (Fernie, Cole, Goudie, & Field, 2010). In addition, Petry (2001) found that active alcoholics discounted more than abstinent alcoholics or nonalcoholics, but similar to our findings, there were no differences in discounting between hazardous drinkers and social drinkers (MacKillop, Mattson, MacK-illop, Castelda, & Donovick, 2007). In contrast to our results, previous studies found that alcohol did not alter delay discounting in healthy volunteers (Richards, Zhang, Mitchell, & de Wit, 2005), female social drinkers (Reynolds, Richards, & de Wit, 2006), or a mixed gender group of alcohol users (Dougherty, Marsh-Richard, Hatzis, Nouvion, & Mathias, 2008), whereas one study found that intoxicated college students showed lower impulsivity, per the DDT, than sober students (Ortner, MacDonald, & Olmstead, 2003). However, there were multiple methodological differences among the studies, such as delay discounting procedure used, number of time points measured, and level of alcohol use among the groups. With respect to BART performance, risk-taking predicted greater alcohol use in college students (Fernie, Cole, Goudie, & Field, 2010) but not in problem drinkers (Courtney et al., 2011) and, similar to our study, there was no effect of alcohol on BART performance in female social drinkers (Reynolds, Richards, & de Wit, 2006). However, taken together, the variability in the alcohol-related delay discounting and BART studies suggests that the ability of these tasks to pick up subtle drug effects or group differences may be limited or dependent on the specific group of interest (e.g., “social drinkers,” alcoholics, college students).
Overall, these results emphasize the necessity of examining a range of impulsivity and risk-taking measures and focusing on well-defined groups because it appears that drug/alcohol effects, group differences, and the interaction between the two on these tasks can vary. Further, these studies also highlight that response initiation/inhibition appears to be greater in moderate-heavy drinking women across studies, thus these women are likely at a greater risk of developing alcohol use disorders (Flory, Pytte, Hurd, Ferrell, & Manuck, 2011; Marczinski, Combs, & Fillmore, 2007; McKellar, Ilgen, Moos, & Moos, 2008; White et al., 2011) and engaging in other risky/impulsive behaviors such as illicit drug-taking (e.g., Kjome et al., 2010; Liu et al., 2011) and sexual risk-taking (e.g., Black, Serowik, & Rosen, 2009; Lejuez, Simmons, Aklin, Daughters, & Dvir, 2004). This is an important point and should be further explored in future research.
Of additional importance, alcohol increased measures of abuse liability and the positive effects of alcohol more so in HD women than LD women, whereas negative effects of alcohol were increased more in LD women than HD women, similar to our previous study (Evans & Levin, 2004). These findings are clinically relevant since a recent study reported that positive subjective ratings were associated with an increase in future binge drinking and the development of alcohol use disorders in individuals who were heavy binge drinkers (King, de Wit, McNamara, & Cao, 2011). Our study extends these findings by showing that this may be particularly relevant in women.
There were a number of strengths in the current study. Because there is some evidence that the effects of alcohol (Evans & Levin, 2011; Lammers, Mainzer, & Breteler, 1995) and other drugs (Evans & Foltin, 2006; Evans, Haney, & Foltin, 2002; Franklin et al., 2004; Justice & de Wit, 1999; Sofuoglu, Dudish-Poulsen, Nelson, Pentel, & Hatsukami, 1999) vary across the menstrual cycle, all women were tested in the same phase of their menstrual cycle (i.e., follicular). In addition, given the various risks of heavy and/or hazardous drinking that pertain specifically to women (e.g., pregnancy risks, sexual abuse, breast cancer, heart disease; CDC, 2010; NIAAA, 2008), the fact that this study examined the effects of alcohol specifically in women makes this an important addition to the alcohol literature. Lastly, one of the main strengths of this study is that we excluded women who had any Axis I disorders, as well as individuals with first-degree family histories of drug and alcohol abuse or dependence or major trauma histories, all factors that alone can impact the response to alcohol (e.g., Evans & Levin, 2011; Evans, Levin & Reed, submitted) and impulsivity tasks (e.g., Acheson, Richard, Mathias, & Dougherty, 2011; e.g., Enoch, 2011) and would have impeded our ability to make a clear determination of the results. However, this could also be viewed as a limitation because these factors are prevalent in heavy drinkers (e.g., Khoury, Tang, Bradley, Cubells, & Ressler, 2010; Magnus-son et al., 2011; Schuckit & Smith, 2011) and may further exacerbate alcohol-related risks (Hyman et al., 2008; Jenkins et al., 2011; Peters et al., 2012; Söderpalm & Söderpalm, 2011). Another limitation is that we could not make comparisons among the HD group based on their binge drinking behavior because the majority of HD women were binge drinkers based on their prospective self-reported drinking. Additionally, we were unable to address potential sex differences because men were not included.
This study addressed the paucity of research on alcohol-related risks in women by focusing on heavy/hazardous drinking women, a growing population (CDC, 2010; Ceylan-Isik, McBride, & Ren, 2010; Grucza, Norberg, Bucholz, & Bierut, 2008; Keyes, Grant, & Hasin, 2008; SAMHSA, 2011). Interestingly, we have noticed a change in the demographics and drinking patterns among our female research participants over the past few years; although the overall level of drinking in moderate/heavy drinkers in our previous study (Evans & Levin, 2004) was similar to the present study (9 drinks/week vs. 10 drinks/week, respectively), the percentage of binge drinkers has increased (87% vs. 66%, respectively), with a corresponding increase in racial/ethnic diversity (48% White vs. 73% White, respectively). These demographic differences within our own laboratory over time support and confirm the epidemiologic data suggesting a growing prevalence of binge drinking, particularly in women (Balodis, Potenza, & Olmstead, 2009; Keyes, Grant, & Hasin, 2008), and the comparable prevalence of current binge and heavy alcohol use among most racial and ethnic groups (SAMHSA, 2011).
In summary, this study allowed us to determine the effects of heavy drinking alone on impulsivity and alcohol-related abuse liability in women without the potential confound of other risk factors. Other than differences in drinking levels, both of our groups of interest were relatively homogenous (e.g., other risk factors associated with increased impulsivity were excluded, no Axis I disorders, only female participants, all participants run in the same menstrual cycle phase). Although this could have reduced the ability to observe differences between the groups, instead there were robust differences highlighting the influence of heavy drinking on impulsivity and the abuse liability of alcohol. The fact that impulsivity is greater in HD women, that alcohol can further increase impulsivity, and that hazardous alcohol use is increasing in women (e.g., Keyes, Grant, & Hasin, 2008) lays the foundation for a cyclical pattern between impulsiveness and alcohol use, and ultimately, subsequent severe alcohol-related medical and psychological problems. Given the limited research to date on interventions for hazardous or risky drinking behavior (e.g., Foran, Heyman, Slep, & United States Air Force Family Advocacy Research Program, 2011; Palfai, Zisserson, & Saitz, 2011), additional research and intervention techniques for high-risk alcohol-users, particularly women, are essential.
Acknowledgments
We gratefully acknowledge the assistance of the research and clinical staff. Preliminary data from this study were presented at ACNP in December 2009 and CPDD in June 2011. This research was supported by Grants R01 DA009114 (Evans), K24 DA029647 (Levin), and K01 DA022282 (Reed) from the National Institute on Drug Abuse. Dr. Frances R. Levin has received lofexidine medication for a NIDA-funded clinical trial from US World Meds and honoraria for consulting from GW Pharmaceuticals.
References
- Acheson A, Richard DM, Mathias CW, Dougherty DM. Adults with a family history of alcohol related problems are more impulsive on measures of response initiation and response inhibition. Drug and Alcohol Dependence. 2011;117:198–203. doi: 10.1016/j.drugalcdep.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Aragues M, Jurado R, Quinto R, Rubio G. Laboratory paradigms of impulsivity and alcohol and dependence: A review. European Addiction Research. 2011;17:64–71. doi: 10.1159/000321345. [DOI] [PubMed] [Google Scholar]
- Balodis IM, Potenza MN, Olmstead MC. Binge drinking in undergraduates: Relationships with sex, drinking behaviors, impulsivity, and the perceived effects of alcohol. Behavioural Pharmacology. 2009;20:518–526. doi: 10.1097/FBP.0b013e328330c779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and –II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Black RA, Serowik KL, Rosen MI. Associations between impulsivity and high risk sexual behaviors in dually diagnosed outpatients. American Journal of Drug and Alcohol Abuse. 2009;35:325–328. doi: 10.1080/00952990903075034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Gwadz MA, Kahler C, Aklin WM, Lejuez CW. Sensation seeking and risk-taking propensity as mediators in the relationship between childhood abuse and HIV-related risk behavior. Child Abuse & Neglect. 2008;32:99–109. doi: 10.1016/j.chiabu.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: The early trauma inventory. Depression and Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Center for Disease Control (CDC) Fact sheets: Binge drinking. Division of Population Health, National Center for Chronic Disease Prevention and Health Promotion; 2010. Retrieved from http://www.cdc.gov/alcohol/fact-sheets/binge-drinking.htm. [Google Scholar]
- Ceylan-Isik AF, McBride SM, Ren J. Sex difference in alcoholism: Who is at a greater risk for development of alcoholic complication? Life Sciences. 2010;87:133–138. doi: 10.1016/j.lfs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Rosner B, Hankinson SE, Colditz GA, Willett WC. Moderate alcohol consumption during adult life, drinking patterns, and breast cancer risk. The Journal of the American Medical Association. 2011;306:1884–1890. doi: 10.1001/jama.2011.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen P, Cole JC, Goudie AJ, Field M. Components of behavioural impulsivity and automatic cue approach predict unique variance in hazardous drinking. Psychopharmacology. 2012;219:501–510. doi: 10.1007/s00213-011-2396-z. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Arellano R, Barkley-Levenson E, Gálvan A, Poldrack RA, Mackillop J, Ray LA. The relationship between measures of impulsivity and alcohol misuse: An integrative structural equation modeling approach. Alcoholism: Clinical and Experimental Research. 2011 doi: 10.1111/j.1530-0277.2011.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addiction Biology. 2010;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Harper RA, Mathias CW, Moeller FG, Marsh DM. Validation of the immediate and delayed memory tasks in hospitalized adolescents with disruptive behavior disorders. The Psychological Record. 2003a;53:509–532. [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW. Immediate and delayed memory tasks: A computerized behavioral measure of memory, attention, and impulsivity. Behavior Research Methods, Instruments, & Computers. 2002;34:391–398. doi: 10.3758/bf03195467. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Moeller FG, Chokshi RV, Rosen VC. Effects of moderate and high doses of alcohol on attention, impulsivity, discriminability and response bias in immediate and delayed memory task performance. Alcoholism: Clinical and Experimental Research. 2000;24:1702–1711. [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM. Manual. Houston, TX: Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center at Houston; 2003. (IMT/DMT 2.0): a research tool for studying attention, memory, and impulsive behavior. [Google Scholar]
- Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug and Alcohol Dependence. 2008;96:111–120. doi: 10.1016/j.drugalcdep.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM, Jagar AA. Laboratory behavioral measures of impulsivity. Behavioral Research Methods. 2005;37:82–90. doi: 10.3758/bf03206401. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Mathias CW, Marsh DM. Manual. Houston, TX: Neurobehavioral Research Laboratory and Clinic, University of Texas Health Science Center at Houston; 2003b. GoStop impulsivity paradigm (version 1.0) [Google Scholar]
- Dougherty DM, Mathias CW, Tester ML, Marsh DM. Age at first drink relates to behavioral measures of impulsivity: The immediate and delayed memory tasks. Alcoholism: Clinical and Experimental Research. 2004;28:408– 414. doi: 10.1097/01.alc.0000117834.53719.a8. [DOI] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology. 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659– 674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397– 406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Levin FR, Foltin RW, Fischman MW. Mood and performance changes in women with premenstrual dysphoric disorder: Acute effects of alprazolam. Neuropsychopharmacology. 1998;19:499–516. doi: 10.1016/S0893-133X(98)00064-5. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR, Reed SC. Impulsivity and the effects of alcohol in women with childhood trauma compared to control women: A pilot study. Submitted. [Google Scholar]
- Evans SM, Levin FR. Response to alcohol in females with a paternal history of alcoholism. Psychopharmacology. 2003;169:10–20. doi: 10.1007/s00213-003-1474-2. [DOI] [PubMed] [Google Scholar]
- Evans SM, Levin FR. Differential response to alcohol in light and moderate female social drinkers. Behavioural Pharmacology. 2004;15:167–181. [PubMed] [Google Scholar]
- Evans SM, Levin FR. Response to alcohol in women: Role of the menstrual cycle and a family history of alcoholism. Drug and Alcohol Dependence. 2011;114:18–30. doi: 10.1016/j.drugalcdep.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Personality and Individual Differences. 1985;6:613– 619. [Google Scholar]
- Fernie G, Cole JC, Goudie AJ, Field M. Risk-taking but not response inhibition or delay discounting predict alcohol consumption in social drinkers. Drug and Alcohol Dependence. 2010;112:54– 61. doi: 10.1016/j.drugalcdep.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Fillmore MT, Weafer J. Acute tolerance to alcohol in at-risk binge drinkers. Psychology of Addictive Behaviors. 2011 doi: 10.1037/a0026110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM–IV Axis I disorders - Patient Edition (SCID-I/P, version 2.0) Biometrics Research Department; New York, NY: New York State Psychiatric Institute; 1995. [Google Scholar]
- Flory JD, Pytte CL, Hurd Y, Ferrell RE, Manuck SB. Alcohol dependence, disinhibited behavior and variation in the prodynorphin gene. Biological Psychology. 2011;88:51–56. doi: 10.1016/j.biopsycho.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran HM, Heyman RE, Slep AM United States Air Force Family Advocacy. Hazardous drinking and military community functioning: Identifying mediating risk factors. Journal of Consulting and Clinical Psychology. 2011;79:521–532. doi: 10.1037/a0024110. [DOI] [PubMed] [Google Scholar]
- Franklin TR, Napier K, Ehrman R, Garit P, O’Brien CP, Childress AR. Retrospective study: Influence of menstrual cycle on cue-induced cigarette craving. Nicotine and Tobacco Research. 2004;6:171–175. doi: 10.1080/14622200310001656984. [DOI] [PubMed] [Google Scholar]
- Grau E, Ortet G. Personality traits and alcohol consumption in a sample of non-alcoholic women. Personality and Individual Differences. 1999;51:439– 444. [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Experimental and Clinical Psychopharmacology. 1996;4:97–106. [Google Scholar]
- Grucza RA, Norberg K, Bucholz KK, Bierut LJ. Correspondence between secular changes in alcohol dependence and age of drinking onset among women in the United States. Alcoholism: Clinical and Experimental Research. 2008;32:1493–1501. doi: 10.1111/j.1530-0277.2008.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U, Endicott J, Schacht S. Premenstrual syndromes: A new instrument for their assessment. Journal of Psychiatric Treatment and Evaluation. 1982;4:161–164. [Google Scholar]
- Hamilton KR, Ansell EB, Reynolds B, Potenza MN, Sinha R. Self-reported impulsivity, but not behavioral choice or response impulsivity, partially mediates the effect of stress on drinking behavior. Stress. 2012 doi: 10.3109/10253890.2012.67139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand BK, Mukamal KJ, Mittleman MA. Trends in alcohol use among women with and without myocardial infarction in the United States: 1997–2008. Journal of Studies on Alcohol and Drugs. 2011;72:885– 891. doi: 10.15288/jsad.2011.72.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henges AL, Marczinski CA. Impulsivity and alcohol consumption in young social drinkers. Addictive Behaviors. 2012;37:217–220. doi: 10.1016/j.addbeh.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd D. Correlates of heavy drinking and alcohol related problems among men and women in drug treatment programs. Drug and Alcohol Dependence. 1993;32:25–35. doi: 10.1016/0376-8716(93)90019-m. [DOI] [PubMed] [Google Scholar]
- Hinckers AS, Laucht M, Schmidt MH, Mann KF, Schumann G, Schuckit MA, Heinz A. Low level of response to alcohol as associated with serotonin transporter genotype and high alcohol intake in adolescents. Biological Psychiatry. 2006;60:282–287. doi: 10.1016/j.biopsych.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hipwell A, Stepp S, Chung T, Durand V, Keenan K. Growth in alcohol use as a developmental predictor of adolescent girls’ sexual risk-taking. Prevention Science. 2012;13:118–128. doi: 10.1007/s11121-011-0260-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SM, Paliwal P, Chaplin TM, Mazure CM, Rounsaville BJ, Sinha R. Severity of childhood trauma is predictive of cocaine relapse outcomes in women but not men. Drug and Alcohol Dependence. 2008;35:208–216. doi: 10.1016/j.drugalcdep.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KM. Heavy episodic drinking: Determining the predictive utility of five or more drinks. Psychology of Addictive Behavior. 2008;22:68–77. doi: 10.1037/0893-164X.22.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MB, Agrawal A, Lynskey MT, Nelson EC, Madden PA, Bucholz KK, Heath AC. Correlates of alcohol abuse/dependence in early-onset alcohol-using women. The American Journal on Addictions. 2011;20:429– 434. doi: 10.1111/j.1521-0391.2011.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Grant BF, Hasin DS. Evidence for a closing gender gap in alcohol use, abuse, and dependence in the United States population. Drug and Alcohol Dependence. 2008;93:21–29. doi: 10.1016/j.drugalcdep.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ. Substance use, childhood traumatic experience, and Posttraumatic Stress Disorder in an urban civilian population. Depression and Anxiety. 2010;27:1077–1086. doi: 10.1002/da.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, Cao D. Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry. 2011;68:389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby KN, Marakovic NN. Delay-discounting probabilistic rewards: Rates decrease as amounts increase. Psychonomic Bulletin & Review. 1996;3:100–104. doi: 10.3758/BF03210748. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. Journal of Experimental Psychology: General. 1999;128:78– 87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Kjome KL, Lane SD, Schmitz JM, Green C, Ma L, Prasla I, Moeller FG. Relationship between impulsivity and decision making in cocaine dependence. Psychiatry Research. 2010;30:299–304. doi: 10.1016/j.psychres.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers SM, Mainzer DE, Breteler MH. Do alcohol pharmacokinetics in women vary due to the menstrual cycle? Addiction. 1995;90:23–30. doi: 10.1046/j.1360-0443.1995.901235.x. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcoholism: Clinical and Experimental Research. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Brown RA. Evaluation of a behavioral measure of risk taking: The Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75– 84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Simmons BL, Aklin WM, Daughters SB, Dvir S. Risk-taking propensity and risky sexual behavior of individuals in residential substance use treatment. Addictive Behaviors. 2004;29:1643–1647. doi: 10.1016/j.addbeh.2004.02.035. [DOI] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. The American Journal of Drug and Alcohol Abuse. 2011;37:117–122. doi: 10.3109/00952990.2010.543204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Mattson RE, MacKillop EJA, Castelda BA, Donovick PJ. Multidimensional assessment of impulsivity in undergraduate hazardous drinkers and controls. Journal of Studies on Alcohol and Drugs. 2007;68:785–788. doi: 10.15288/jsad.2007.68.785. [DOI] [PubMed] [Google Scholar]
- Magnusson A, Lundholm C, Göransson M, Copeland W, Heilig M, Pedersen NL. Familial influence and childhood trauma in female alcoholism. Psychological Medicine. 2011;29:1–9. doi: 10.1017/S0033291711001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Clifford PR, Stout RL, Davis CM. Moderate drinking in the first year after treatment as a predictor of three-year outcomes. Journal of Studies on Alcohol and Drugs. 2007;68:419– 427. doi: 10.15288/jsad.2007.68.419. [DOI] [PubMed] [Google Scholar]
- Marczinski CA, Combs SW, Fillmore MT. Increased sensitivity to the disinhibiting effects of alcohol in binge drinkers. Psychology of Addictive Behaviors. 2007;21:346–354. doi: 10.1037/0893-164X.21.3.346. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcoholism: Clinical and Experimental Research. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- McKellar J, Ilgen M, Moos BS, Moos R. Predictors of changes in alcohol-related self-efficacy over 16 years. Journal of Substance Abuse and Treatment. 2008;35:148–155. doi: 10.1016/j.jsat.2007.09.003. [DOI] [PubMed] [Google Scholar]
- McLeod D, Griffiths RR, Bigelow GE, Yingling J. An automated version of the digit symbol substitution test (DSST) Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society, Inc. 1982;14:463– 466. [Google Scholar]
- McNair D, Lorr M, Droppleman LF. Profile of mood states. San Diego, CA: Educational and Industrial Testing Services; 1971. [Google Scholar]
- Mulvihill LE, Skilling RA, Vogel-Sprott M. Alcohol and the ability to inhibit behavior in men and women. Journal of Studies on Alcohol and Drugs. 1997;58:600– 605. doi: 10.15288/jsa.1997.58.600. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (U. S.; NIAAA) NIH Publication No 03– 4956. U.S. Department of Health and Human Services; Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2008. Alcohol, a women’s health issue. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (U. S.; NIAAA) NIH publication No 09 –3770. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2009. Rethinking drinking [electronic resource]: Alcohol and your health: Research-based information from the National Institutes of Health, U.S. Department of Health and Human Services. Retrieved from http://rethinkingdrinking.niaaa.nih.gov. [Google Scholar]
- Nees F, Tzschoppe J, Patrick CJ, Vollstädt-Klein S, Steiner S, Poustka L The IMAGEN Consortium. Determinants of early alcohol use in healthy adolescents: The differential contribution of neuroimaging and psychological factors. Neuropsychopharmacology. 2012;37:986–995. doi: 10.1038/npp.2011.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortner CNM, MacDonald TK, Olmstead MC. Alcohol intoxication reduces impulsivity in the delay-discounting paradigm. Alcohol and Alcoholism. 2003;38:151–156. doi: 10.1093/alcalc/agg041. [DOI] [PubMed] [Google Scholar]
- Palfai TP, Zisserson R, Saitz R. Using personalized feedback to reduce alcohol use among hazardous drinking college students: The moderating effect of alcohol-related negative consequences. Addictive Behaviors. 2011;36:539–542. doi: 10.1016/j.addbeh.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Peters EN, Leeman RF, Fucito LM, Toll BA, Corbin WR, O’Malley SS. Co-occurring marijuana use is associated with medication nonadherence and nonplanning impulsivity in young adult heavy drinkers. Addictive Behaviors. 2012;37:420– 426. doi: 10.1016/j.addbeh.2011.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. Journal of Studies on Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology. 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Pollock VE. Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. American Journal of Psychiatry. 1992;149:1534–1538. doi: 10.1176/ajp.149.11.1534. [DOI] [PubMed] [Google Scholar]
- Potenza MN, de Wit H. Control yourself: Alcohol and impulsivity. Alcoholism, Clinical and Experimental Research. 2010;34:1303–1305. doi: 10.1111/j.1530-0277.2010.01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Stappenbeck CA, Fromme K. Collegiate heavy drinking prospectively predicts change in sensation seeking and impulsivity. Journal of Abnormal Psychology. 2011;120:543–556. doi: 10.1037/a0023159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Levin FR, Evans SM. The effects of progesterone pretreatment on the response to oral d-amphetamine in women. Hormones and Behavior. 2010;58:533–543. doi: 10.1016/j.yhbeh.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B, Richards JB, de Wit H. Acute-alcohol effects on the Experiential Discounting Task (EDT) and a question-based measure of delay discounting. Pharmacology, Biochemistry, and Behavior. 2006;83:194–202. doi: 10.1016/j.pbb.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Rice JP, Reich T, Bucholz KK, Neuman RJ, Fishman R, Rochberg N, Begleiter H. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcoholism: Clinical and Experimental Research. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: Effect of alcohol. Journal of the Experimental Analysis of Behavior. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roerecke M, Greenfield TK, Kerr WC, Bondy S, Cohen J, Rehm J. Heavy drinking occasions in relation to ischaemic heart disease mortality– an 11–22 year follow-up of the 1984 and 1995 US National Alcohol Surveys. International Journal of Epidemiology. 2011;40:1401–1410. doi: 10.1093/ije/dyr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AK, Duka T. Effects of alcohol on inhibitory processes. Behavioural Pharmacology. 2008;19:284–291. doi: 10.1097/FBP.0b013e328308f1b2. [DOI] [PubMed] [Google Scholar]
- Rose AK, Grunsell L. The subjective, rather than the disinhibiting, effects of alcohol are related to binge drinking. Alcoholism: Clinical and Experimental Research. 2008;32:1096–1104. doi: 10.1111/j.1530-0277.2008.00672.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. Onset and course of alcoholism over 25 years in middle class men. Drug and Alcohol Dependence. 2011;113:21–28. doi: 10.1016/j.drugalcdep.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;7:492–501. doi: 10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Shin SH, Hong HG, Jeon SM. Personality and alcohol use: The role of impulsivity. Addictive Behaviors. 2012;37:102–107. doi: 10.1016/j.addbeh.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderpalm Gordh AH, Söderpalm B. Healthy subjects with a family history of alcoholism show increased stimulative subjective effects of alcohol. Alcoholism: Clinical and Experimental Research. 2011;35:1426–1434. doi: 10.1111/j.1530-0277.2011.01478.x. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Experimental and Clinical Psychopharmacology. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the State-Trait Anxiety Inventory (“Self-Evaluation Questionnaire”) Palo Alto, CA: Consulting Psychologists Press, Inc; 1970. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) NSDUH Series H-36, HHS Publication No SMA 09 – 4434. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009. Results from the 2008 national survey on drug use and health: Summary of national findings. [Google Scholar]
- Substance Abuse and Mental Health Services Administration (SAMHSA) NSDUH Series H-41, HHS Publication No (SMA) 11– 4658. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. Results from the 2010 national survey on drug use and health: Summary of national findings. [Google Scholar]
- Waldeck TL, Miller SL. Gender and impulsivity differences in licit substance use. Journal of Substance Abuse. 1997;9:269–275. doi: 10.1016/s0899-3289(97)90021-3. [DOI] [PubMed] [Google Scholar]
- Warner LA, White HR, Johnson V. Alcohol initiation experiences and family history of alcoholism as predictors of problem-drinking trajectories. Journal of Studies on Alcohol and Drugs. 2007;68:56– 65. doi: 10.15288/jsad.2007.68.56. [DOI] [PubMed] [Google Scholar]
- Weafer J, Fillmore MT. Comparison of alcohol impairment of behavioral and attentional inhibition. Drug and Alcohol Dependence. 2012 doi: 10.1016/j.drugalcdep.2012.05.010. http://dx.doi.org/10.1016/j.drugalcdep.2012.05.01. [DOI] [PMC free article] [PubMed]
- White HR, Marmorstein NR, Crews FT, Bates ME, Mun EY, Loeber R. Associations between heavy drinking and changes in impulsive behavior among adolescent boys. Alcoholism: Clinical and Experimental Research. 2011;35:295–303. doi: 10.1111/j.1530-0277.2010.01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M, Eysenck S, Eysenck HJ. Sensation seeking in England and America: Cross-cultural, age and sex comparisons. Journal of Consulting and Clinical Psychology. 1978;46:139–149. doi: 10.1037//0022-006x.46.1.139. [DOI] [PubMed] [Google Scholar]