Abstract
Aims
The FDA approved smoking cessation aid varenicline can effectively attenuate nicotine-stimulated dopamine release. Varenicline may also exert important actions on other transmitter systems that also influence nicotine reinforcement or contribute to the drug’s cognitive and affective side effects. In this study, we determined if varenicline, like nicotine, can stimulate presynaptic GABA release.
Main methods
Using whole-cell patch-clamp techniques, we measured GABAAR-mediated asynchronous, spontaneous miniature inhibitory postsynaptic currents (mIPSCs) in acute brain slices from two brain regions important for learning and memory, the hippocampus and basal forebrain.
Key findings
Both varenicline (10 μM) and nicotine (10 μM) applications alone resulted in small but significant increases in amplitude, as well as robustly enhanced frequency of mIPSCs in hippocampal CA1 pyramidal neurons and medial septum /diagonal band (MS/DB) neurons. A unique subpopulation of MS/DB neurons showed decreases in frequency. In the presence of nicotine, varenicline effectively attenuated the expected enhancement of hippocampal mIPSC frequency like a competitive antagonist. However, in the MS/DB, varenicline only partially attenuated nicotine’s effects. Reversing the order of drug application by adding nicotine to varenicline-exposed slices had little effect.
Significance
Varenicline, like nicotine, stimulates presynaptic GABA release, and also exerts a partial agonist action by attenuating nicotine-stimulated release in both the hippocampus and basal forebrain. These effects could potentially affect cognitive functions
Keywords: addiction, dependence, cognitive function, GABA release, mIPSC, basal forebrain, hippocampus
Introduction
Recently, the FDA approved the cytisine derivative varenicline to treat nicotine addiction (Faessel et al., 2010; Rollema et al., 2007b). Like nicotine, varenicline stimulates dopamine release (Rollema et al., 2007b, Pontieri et al., 1996; Quik et al., 2011) but the release is significantly smaller and longer lasting than that of nicotine, thereby diminishing reinforcement and craving of nicotine (Coe et al., 2005; Rollema et al., 2007a,b). Varenicline may also reduce alcohol consumption in humans and animals (McKee et al., 2009; Steensland et al., 2007), and exerts antidepressant-like effects; thus, varenicline may become a widely used drug for the treatment of a number of conditions (McKee et al., 2009; Mineur et al., 2009; Rollema et al., 2009). However, increased suicidality has been reported for varenicline treatment (Kuehn, 2008) emphasizing the need to better understand potential side effects.
The hippocampus and basal forebrain are two brain regions important for learning and memory (Bird and Burgess, 2008; Dwyer et al., 2007; Silvers et al., 2003) where nAChRs are highly expressed on GABAergic neurons (Azam et al., 2003; Son and Winzer-Serhan, 2008). GABA is the major inhibitory neurotransmitter in forebrain structures, and is critical for regulation of network activities (Colom et al., 2005; Manns et al., 2001; Sotty et al., 2003). Nicotine enhances GABA release through activation of presynaptic nicotinic receptors (Maggi et al., 2001; McClure-Begley et al., 2009; Wonnacott, 1997; Yang et al., 1996). Whether varenicline shares this action of nicotine is currently unknown. However, it has been shown that varenicline improves cognitive processes, and this effect could be related to modulation of presynaptic transmitter release including GABA (McKee et al., 2009; Mineur et al., 2009; Rollema et al., 2009, Rollema et al., 2011). We hypothesized that varenicline like nicotine, would also increase GABA transmission in hippocampus and medial septum / diagonal band (MS/DB).
We examined the effects of varenicline on GABAA receptor (GABAAR)-mediated synaptic transmission by measuring asynchronous, spontaneous miniature inhibitory postsynaptic currents (mIPSCs) in the basal forebrain and hippocampus. The mIPSCs are a functional and “real-time” measure of spontaneous presynaptic GABA release which elicits postsynaptic GABAAR mediated currents (Otis and Mody, 1992, Mody et al., 1994), and provide a sensitive and accurate measurement of changes in GABA release in response to drugs in brain slices. Using this approach, our findings show that varenicline like nicotine, enhances the release of GABA in both brain regions. Increased GABAergic synaptic transmission could possibly play a role in varenicline’s improvement of cognitive processes.
Material and methods
Animal Experiments
All animal procedures were performed in accordance with protocols approved by the Texas A&M University Institutional Animal Care and Use Committee, and were consistent with the National Institutes of Health animal care and use policy. All efforts were made to minimize animal suffering and to reduce the number of animals used. Timed pregnant Sprague–Dawley rat dams were obtained from Harlan (Indianapolis, IN), and were housed in an animal care facility at 22–25°C with a 12-h light/dark cycle and ad libitum food and water. Dams were allowed to give birth and both male and female offspring (postnatal day (PD) 14–20) were used for experimentation.
Acute Brain Slice Preparation
On the day electrophysiological recordings were to be made, coronal slices containing hippocampus and MS/DB were prepared using similar techniques to those described previously (DuBois et al., 2006a; Lack et al., 2007). Rats (PD 14–20) were decapitated and brains were rapidly removed and cooled by immersion in 0–4°C artificial cerebral spinal fluid (ACSF) containing (mM): KCl, 2; MgCl2-6H2O, 1; MgSO4-7H2O, 2; CaCl2, 1; NaH2PO4, 1.25; NaHCO3, 26; D-Glucose, 14; Sucrose, 206; Kynurenic Acid, 0.8; bubbled with 95% O2 / 5% CO2; pH 7.4; 290–310 mOsm before blocking and cutting 300 m slices on a Vibra Slice (Campden Instr.) in the same solution. After cutting, slices were transferred to a holding chamber of oxygenated (95 / 5% O2 / CO2) ACSF solution containing (mM): NaCl, 124; KCl, 3; MgSO4, 1.5; CaCl2, 2.4; NaH2PO4, 1.25; D-(+)-glucose, 10; NaHCO3, 26; pH 7.4; 290–310 mOsm, gradually warmed to 32°C and incubated for ~30 min then allowed to cool to room temperature prior to experimentation. Individual slices were transferred to a recording chamber, submerged using a slice anchor (Warner Instruments Corp.) and continuously perfused with the same oxygenated ACSF solution during recordings and drug applications.
Electrophysiology
Conventional whole-cell patch-clamp recording techniques were used as described previously (DuBois et al., 2004; DuBois et al., 2006b; Lack et al., 2007). Briefly, patch pipettes were pulled from glass capillary tubing (KG-33, 1.5 mm, o.d., Garner Glass Co.) on a Brown and Flaming P-97 pipette puller (Sutter Instr.) to resistances of 2–8 MΩ. Pipettes were filled with solution containing (mM): CsCl, 130; EGTA, 10; MgCl2, 2; HEPES, 10; Mg-ATP, 4; GTP, 0.1; pH 7.2 with CsOH; 285–310 mosM. Slices were visualized with Olympus BX50 / 51 WI upright microscopes (40X long working distance water immersion, differential interference contrast, video enhanced optics). Using whole-cell patch-clamp electrophysiology, neurons were voltage clamped at −60 mV and recordings were made of pharmacologically isolated GABA mIPSCs from untreated Sprague-Dawley rats. Tetrodotoxin (TTX, 0.5 μM) was used to block voltage-gated Na+ channels and inhibit action potential-evoked release of neurotransmitter while D,L-2-amino-5-phosphonovaleric acid (AP5, 40 μM), and 6,7-dinitroquinoxaline-2,3-dione (DNQX,10 μM) were used to inhibit glutamate-mediated mEPSCs. GABAAR-mediated mIPSCs were defined by inhibition with bicuculline (30 μM). DL-AP5, bicuculline, DNQX, varenicline and TTX were purchased from Tocris. Kynurenic acid and (−)-Nicotine hydrogen tartrate salt were purchased from Sigma.
The order of nicotine and varenicline application was alternated randomly across brain slices to better understand the pharmacology and partial agonist effects of varenicline. In one subset of experiments, bath solution containing nicotine (10 μM) was initially applied followed by a nicotine (10 μM) plus varenicline (10 μM) bath solution. For a second subset of experiments, the order of drug application was reversed with a varenicline (10 μM) containing bath solution applied first followed by a varenicline (10 μM) plus nicotine (10 μM) bath solution. Test drug concentrations for both varenicline and nicotine were chosen based on saturating concentrations used in previous studies (Kawa, 2007; Lester and Dani, 1995; Mihalak et al., 2006; Rollema et al., 2007a; Wu et al., 2003; Zhu et al., 2005). Drugs were bath applied to brain slices and reached saturation within 2–4 min for cells within ~50–100 μm of the slice surface. Three to five minute voltage-clamp current recordings of GABAAR mIPSCs were then collected and digitized with a Multiclamp 700B amplifier (Axon Inst.), Digidata 1440A interface, and pClamp 10 software (Molecular Devices, LLC). Capacitance (pF) was read from the potentiometer used to zero capacitance transients. Data were low-pass filtered (8 pole Bessel, Frequency Devices) at 1–5 kHz and digitized at 0.5–20 kHz depending on the desired resolution. One randomly sampled neuron per slice was recorded, and multiple random slices from each animal were utilized. Series resistance was continually monitored throughout the experiments. All experiments were carried out at room temperature.
Miniature Postsynaptic Current Data Analysis and Statistics
Off-line analysis of GABAergic mIPSC kinetic parameters was performed using Minianalysis 6.03 (Synaptosoft, Inc.), Prism 5 (GraphPad Software, Inc.), and Microsoft EXCEL as previously described (DuBois et al., 2004; DuBois et al., 2006b; Lack et al., 2007). The mIPSC characteristics of amplitude, frequency (presented as inter-event intervals (IEI)), and decay time constants were determined and compared. Individual currents ≥15 pA could be clearly distinguished above baseline noise in the 3 minute current traces collected from individual neurons. The event frequency was determined from the mean IEI, while event peak amplitude was estimated as the absolute difference between the preceding baseline and maxima of the current. For mIPSC decay analysis, low noise traces and non-overlapping events were used to generate an ensemble average mIPSC by aligning the rising phase, and the 10–90% decay phase for each neuron fitted with a biexponential function: y(t) = A1exp(−t/τ1) + A2exp(−t/τ2) + As, where A1 and A2 are the fraction of the fast and slow decay components, respectively, As is the steady-state current, and τ1 and τ2 are the fast and slow time constants, respectively. Previously we found that mIPSC ensemble decay data for septal neurons under the present conditions gave a significantly better fit with two time constants relative to a fit with a single time constant (DuBois et al., 2004). The means and percent change in response to drug application were established for each recorded neuron, and the results are expressed as mean ± S.E.M. percent change from baseline for each group, with comparisons between groups made using Student’s t-test. Comparisons of cumulative probability distributions were made using the Kolmogorov-Smirnov (K-S) test. Results were considered significant if the two-tailed p values were < 0.05.
Results
Varenicline enhances GABAAR mIPSC activity in hippocampal slices
The activation of presynaptic nAChRs enhances the release of a number of neurotransmitters including GABA (Maggi et al., 2001; McClure-Begley et al., 2009; Yang et al., 1996). To the best of our knowledge, the effects of varenicline on GABAergic synaptic transmission in the hippocampus or MS/DB are unknown. Thus, the impact of varenicline application to brain slice preparations was determined by evaluating spontaneous GABAAR-mediated mIPSCs. GABAergic mIPSC frequency is an index related to functional synapse number and the rate of presynaptic asynchronous transmitter release (Cherubini and Conti, 2001; Dunning et al., 1999; Kohara et al., 2007), while mIPSC amplitude is influenced by factors such as postsynaptic receptor density, synaptic vesicle GABA content, and dendritic cable properties (Cherubini and Conti, 2001; Mody et al., 1994; Nusser et al., 1997; Otis and Mody, 1992). We expected that varenicline, like nicotine, would increase frequency, indicating increased presynaptic GABA release, but would have little to no effect on mIPSC amplitude.
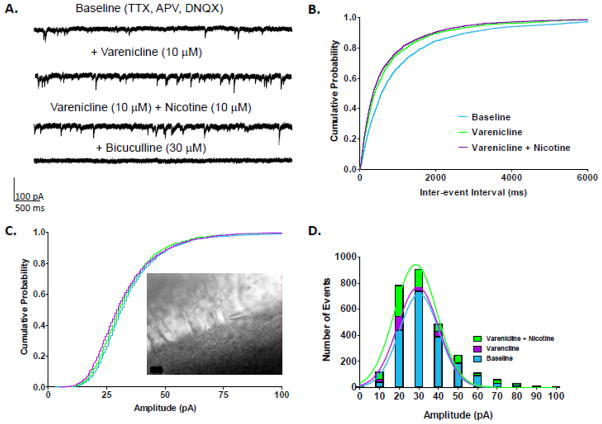
The effects of bath application of drugs to hippocampal slices were recorded in the CA1 pyramidal layer using whole-cell patch-clamp recordings (Fig 1C, inset). Acute bath application of varenicline (10 μM) alone followed by varenciline + nicotine (10 μM) enhanced GABAAR mIPSC frequency in CA1 pyramidal neurons, an effect that was completely blocked by bicuculline which is consistent with GABAAR-mediated mIPSCs (Fig. 1A). The cumulative probability plot demonstrated a significant leftward shift in the IEI, indicative of increased frequency after the application of varenicline alone (K-S test, p<0.001, n = 6 cells) (Fig. 1B). Combined application of varenicline + nicotine further shifted GABAergic mIPSC IEI values slightly but significantly to the left and thus, further increased frequency compared to baseline or to varenicline alone (K-S test: varenicline + nicotine from baseline, p<0.001; and from varenicline alone, p<0.01, n=6 cells) (Fig. 1B). Application of varenicline and varenicline + nicotine to hippocampal slices significantly decreased mean percent of baseline IEI values to 76.2 ± 4.8 % and 69.7 ± 7.7%, respectively (Student’s t-test, baseline vs. varenicline, and vs. varenicline + nicotine, p<0.01) (Table 1). IEI values decreased in 5 out of 6 cells (69.7% nicotine, and 62.2% nicotine + varenicline) with no change in one cell.
Figure 1.
Effects of varenicline and varenicline plus nicotine on mIPSCs in hippocampal CA1 pyramidal neurons. A. Representative traces from a CA1 pyramidal neuron featuring varenicline’s increase in GABA release and nicotine’s further enhancement of GABA release measured as mIPSC frequency. GABAAR mIPSCs were isolated using TTX, APV, and DNQX. B. Cumulative probability plots of varenicline and nicotine’s effects on GABAAR mIPSC IEI from CA1 pyramidal neurons (n = 6 cells; number of events over 3 min: baseline = 1967, varenicline = 2137, varenicline + nicotine = 2763). C. Cumulative probability plot of varenicline and nicotine’s effects on GABAAR mIPSC amplitude from CA1 pyramidal neurons. (inset) Image of a slice from hippocampus featuring CA1 pyramidal neurons with attached patch pipette, scale bar = 10 μm. D. Histogram of mIPSC amplitudes (10 pA bins). Color code: baseline = blue, varenicline = purple, nicotine plus varenicline = green. For clarity, histograms were fit with Gaussian curves expressed as colored lines for the respective drug applications.
Table 1.
Drug-induced changes in mean GABAAR mIPSC inter-event interval
The population mean ± SEM values of GABAAR mIPSC IEI are expressed as % of baseline (100%) after the addition of the drug(s). The percent of baseline in response to drug application was calculated for each individual neuron, and averaged for the number of cells recorded from (n).
| Drugs applied to slices (10 μM) | CA1 Pyramidal neurons | # of cells (n) | MS/DB neurons | # of cells (n) |
|---|---|---|---|---|
| Mean IEI (% of baseline) | Mean IEI (% of baseline) | |||
| Varenicline | 76.2 ± 4.8%* Decreased: 5/6 (75.5%) |
6 | 128.1 ± 21.0% Decreased: 6/13 (70.3%) Increased: 5/13 (205.4%) |
13 |
| Varenicline + Nicotine | 69.7 ± 7.7%* Decreased: 5/6 (62.2%) |
6 | 107.4 ± 22.0% Decreased: 6/11 (64.0%) Increased: 3/11 (195.3%) |
11 |
| Nicotine | 80.4 ± 7.4%* Decreased: 6/10 (69.2%) |
10 | 74.8 ± 13.3% Decreased: 6/9 (52.8%) |
9 |
| Nicotine + Varenicline | 84.3 ± 7.0%* Decreased: 6/10 (73.0%) |
10 | 88.4 ± 11.8% Decreased: 3/9 (49.2) Increased: 3/9 (117.5%) |
9 |
indicates significance (p>0.05, Student’s t-test). The number of cells responding with a decrease in IEI (increased frequency) or an increase in IEI (decrease in frequency) and their % of baseline values are listed underneath the mean population change. A change of less than ±10% from baseline was considered as no change.
The effects of varenicline and varenicline + nicotine on mIPSC amplitude in hippocampal slices were small. Based on the cumulative probability distributions, varenicline had no significant effect on mIPSC amplitude in CA1 pyramidal neurons (K-S test, p>0.05) (Fig. 1C). Varenicline slightly increased the number of events with amplitudes of 20 to 40 pA (Fig. 1D), which is likely a result of the increase in mIPSC frequency and thus, the number of events. When nicotine was subsequently applied along with varenicline, there was a marginal but significant leftward shift in the cumulative probability plots for mIPSC event amplitudes relative to baseline or varenicline alone (K-S test from baseline, p<0.001; varenicline alone, p<0.01). Again, the increase in events appeared to be predominantly due to more events in the 20 to 40 pA range. Consistent with the findings from the K-S tests showing very little change, the mean values for mIPSC amplitude were not significantly different from baseline for varenicline alone or varenicline + nicotine (Student’s t-test, p>0.05).
Varenicline enhances GABAAR mIPSC activity in MS/DB slices
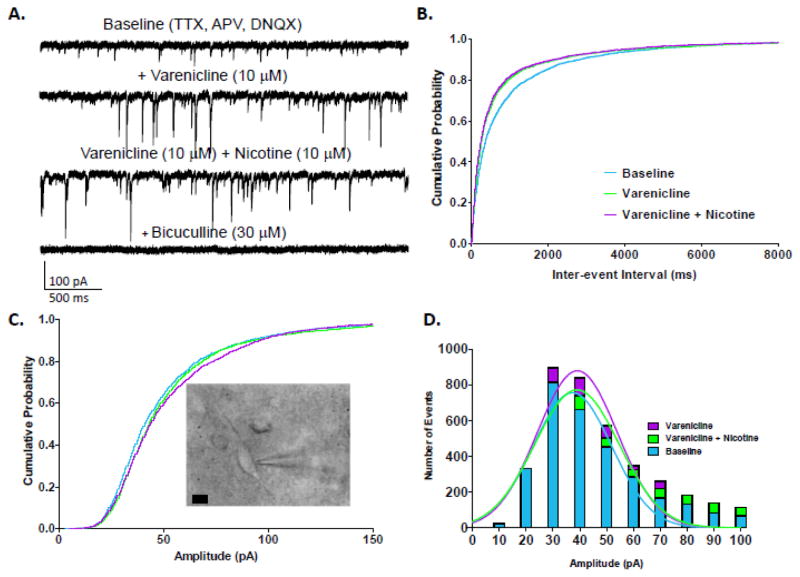
The effects of bath application of drugs to septal slices were recorded in neurons located in the MS/DB (Fig 2C, inset). In whole-cell recordings of MS/DB neurons, varenicline alone, when applied first, followed by varenicline + nicotine, enhanced GABAAR mIPSC frequency which was blocked by bicuculline (Fig. 2A). Varenicline shifted IEI values to the left (K-S test, varenicline vs. baseline, p<0.001) (Fig. 2B). When varenicline + nicotine were then applied together, a very small but significant further decrease in IEI from varenicline alone was observed (K-S test, varenicline + nicotine vs. baseline, p<0.001, vs. varenicline alone, p<0.01).
Figure 2.
Effects of varenicline and varenicline plus nicotine on mIPSCs in MS/DB neurons.A. Representative traces from a MS/DB pyramidal neuron featuring varenicline’s increase in GABA release and nicotine’s further enhancement of GABA release measured as mIPSC frequency. B. Cumulative probability plots of varenicline and nicotine’s effects on GABAAR mIPSC IEI from MS/DB neurons (n = 11–13 cells; number of events over 3 min: baseline = 3267, varenicline = 3864, varenicline + nicotine = 3659). C. Cumulative probability plots of varenicline and nicotine’s effects on GABAAR mIPSC amplitude from MS/DB neurons. (inset) Image of a basal forebrain slice featuring a bi-polar medial septum neuron with attached patch pipette, scale bar = 10 μm. D. Histograms and Gaussian curves of mIPSC amplitudes (10 pA bins). Color code: baseline = blue, varenicline = purple, nicotine plus varenicline = green.
Cumulative probability plots demonstrated a significant change in mIPSC amplitude in the presence of varenicline alone (K-S test from baseline, p<0.001) (Fig. 2C). Co-application of varenicline + nicotine shifted the probability plots towards larger amplitudes, and thus, significantly enhanced GABAAR mIPSC amplitude in cells of the MS/DB slices (K-S test from varenicline + nicotine vs. baseline, p<0.001, vs. varenicline alone, p<0.05). However, mean mIPSC amplitude values were unaffected in response to varenicline alone or varenicline + nicotine application (Table 1). The shift towards larger amplitudes shown by cumulative probability distribution analysis could be partially due to an increase in the number of events in the 30 to 60 pA range for varenicline and varenicline + nicotine, compared to baseline (Fig. 2D).
MS/DB neurons showed either pronounced increased or decreased frequency responses to varenicline unlike hippocampal neurons which predominantly exhibited an increase in frequency (i.e. smaller IEI). Because of the variability in response to varenicline and varenciline + nicotine, the average mean IEI change across all cells resulted in a non-significant increase to 128.1 ± 21.0 % and 107.7 ± 22.0 % of baseline, respectively, and thus, no change in the overall population mean frequency for MS/DB neurons (Table 1). However, evaluation of neurons with more than 10% change in frequency resulted in one population (6 out of 13 cells) where varenicline application decreased IEI values, and in another population (5 out of 13 cells) where varenicline substantially increased IEI values, with mean percent decrease in IEI compared to baseline of 70.3% (Student’s t-test comparison, p<0.05) and mean percent increase in IEI compared to baseline of 205.4% (Student’s t-test comparison, p<0.01), respectively. The addition of nicotine to varenicline only marginally changed the mean percent change from baseline IEI values in these two cell population resulting in changes of 64.0%, and 195.3%, respectively.
Nicotine enhances GABAAR mIPSC activity in hippocampal slices
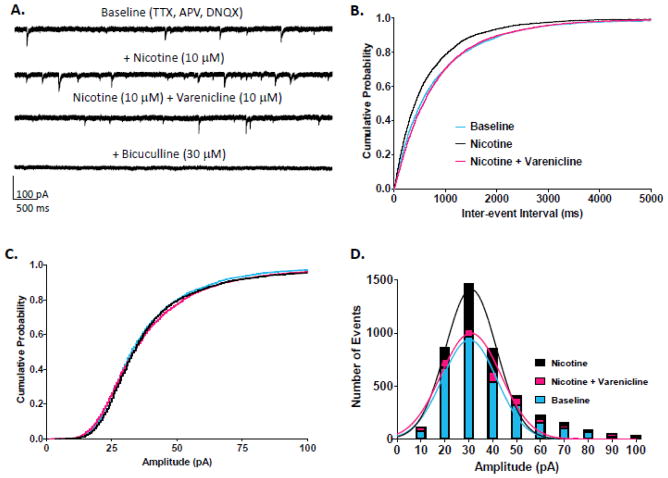
Next, we determined the effects of nicotine alone followed by co-application of nicotine + varenicline in CA1 pyramidal neurons. Application of nicotine (10 μM) and nicotine + varenicline (10 μM) to hippocampal slices increased the occurrence of spontaneous inhibitory events, which were completely blocked by bicuculline (30 μM) (Fig 3A). Cumulative probability plots for IEI indicated that application of nicotine resulted in a significant and robust leftward shift for IEI for GABAAR mIPSCs in CA1 pyramidal neurons (K-S test, p<0.001; n = 10 cells) (Fig 3B). The addition of varenicline to nicotine significantly reduced frequency, which almost returned to baseline levels (K-S test varenicline + nicotine from nicotine alone, p<0.001). However, although the change was small, IEI values for the combined varenicline + nicotine treatment were still significantly different from baseline (p<0.01).
Figure 3.
Effects of nicotine and nicotine plus varenicline on mIPSCs in hippocampal CA1 pyramidal neurons. A. Representative traces from a CA1 pyramidal neuron featuring nicotine’s increase in GABA release and varenicline’s subsequent reduction in GABA release measured as mIPSC frequency. B. Cumulative probability plots of nicotine and varenicline’s effects on GABAAR mIPSC IEI from CA1 pyramidal neurons (n = 10 cells; number of events over 3 min: baseline = 3069, nicotine = 4430, nicotine + varenicline = 3584). C. Cumulative probability plots of nicotine and varenicline’s effects on GABAAR mIPSC amplitude from CA1 pyramidal neurons. D. Histograms and Gaussian curves of mIPSC amplitudes (10 pA bins). Color code: baseline (blue), nicotine (black), nicotine plus varenicline (red).
Cumulative probability plots for amplitude indicated that nicotine resulted in an increase in GABAAR mIPSC amplitude in CA1 pyramidal neurons as well, indicated by a small but significant rightward shift for amplitude (K-S test, p<0.01) (Fig 3C). Most changes in mIPSC amplitude with nicotine were seen as an increase in the number of mIPCS events with amplitudes ranging from 20 to 50 pA (Fig 3D). When varenicline was co-applied with nicotine, mIPSC amplitude was significantly decreased compared to nicotine alone, returning to near baseline levels (K-S test from baseline, p>0.05, from nicotine alone p<0.01). However, small increases in the number of events were still apparent in the 20 to 50 pA range when varenicline was present (Fig 3D).
Mean values for percent change from baseline demonstrated that nicotine and nicotine + varenicline decreased mean IEI values to 80.4 ± 7.4% and 84.3 ± 7.0 of baseline, respectively (Student’s t-test, p<0.05) (Table 1). Nicotine applied alone resulted in decreased IEI in 6 out of 10 and no change (± 7% of baseline) in 4 cells. The addition of varenicline had the same effect (6 out of 10), although, in a few cells, a switch from “no change” to “decreased” (two cells) or vise versa (two cells) was observed. An increase in IEI was not detected with any drug treatment. In contrast, application of nicotine or nicotine + varenicline did not change percent mean GABAergic mIPSC amplitude values (nicotine: 100.82%, nicotine + varenicline: 104.76%; Student’s t-test, p>0.05).
Nicotine enhances GABAAR mIPSC activity in MS/DB slices
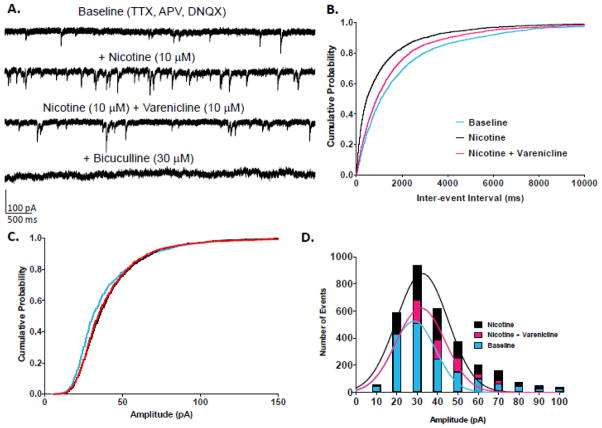
Application of nicotine and nicotine + varenicline increased the number of spontaneous inhibitory events, which were completely blocked by bicuculline (Fig 4A). When nicotine was applied to slices, the cumulative probability plot for IEI demonstrated a significant leftward shift (K-S test, p<0.001; n = 9 cells) (Fig 4B). The addition of varenicline significantly increased IEI, and thus, decreased mIPSC frequency, compared to nicotine alone but did not completely block the action of nicotine (K-S test from baseline, p<0.001, from nicotine alone, p<0.001).
Figure 4.
Effects of nicotine and nicotine plus varenicline on mIPSCs in MS/DB neurons. A.Representative traces from a MS/DB neuron featuring nicotine’s increase in GABA release and varenicline’s subsequent reduction in GABA release measured as mIPSC frequency. B. Cumulative probability plots of nicotine and varenicline’s effects on GABAAR mIPSC IEI from MS/DB neurons (n = 9 cells; number of events over 3 min: baseline = 1688, nicotine = 3155, nicotine + varenicline = 2129). C. Cumulative probability plot of nicotine and varenicline’s effects on GABAAR mIPSC amplitude from MS/DB neurons. D. Histograms and Gaussian curves of mIPSC amplitudes (10 pA bins). Color code: baseline = blue, nicotine = black, nicotine plus varenicline = red.
Cumulative probability plots for amplitude demonstrated a significant difference from baseline after nicotine application (K-S test, p<0.001; n = 9 cells) (Fig 4C). Nicotine especially enhanced the number of events with amplitudes of 20 pA and higher, consistent with an increase in frequency (Fig 4D). When varenicline was added in addition to nicotine, the enhancement of mIPSC amplitude was significantly smaller compared to nicotine alone, but amplitude values for nicotine + varenicline were still significantly different from baseline (K-S test nicotine + varenicline vs. baseline, p<0.001, vs. nicotine alone, p>0.05). Residual increases in the number of mIPCS were most apparent where amplitudes ranged from approximately 30 to 60 pA.
Application of nicotine and nicotine + varenicline to MS/DB slices decreased mean IEI values to 74.8 ± 13.3% and 88.4 ± 11.8% of baseline, respectively (Table 1). However, mean percent of baseline mIPSC IEI values only trended toward significance (Student’s t-test, baseline vs. nicotine alone, p=0.08). Nicotine application to MS/DB slices decreased mean IEI values in 6 out of 9 cells (52.8%) when compared to baseline IEI values, and no change was detected in three cells. After addition of varenicline to nicotine, only 3 out of 9 cells exhibited decreased IEI (49.2%), but now 3 out of 9 cells exhibited an increase in mean IEI (117.5%). Nicotine increased mean values of amplitude in 5 out of 9 cells, and resulted in no change in 4 cells, causing a slight overall increase in mean percent amplitude of 114.6 % over baseline (Student’s t-test, baseline vs. nicotine, p=0.046). The addition of varenicline to nicotine resulted in percent mean amplitude of 118.8% with 5 out of 9 cells exhibiting an increase greater than 10% (Student’s t-test, baseline vs. nicotine plus varenicline, p=0.068).
Effects of varenicline and nicotine on decay kinetics in hippocampal and MS/DB slices
The analysis of GABAAR-mediated mIPSC decay kinetics of τ1 and τ2 values for nicotine or varenicline application alone or in combination, revealed little effect on either τ1 or τ2 values. Only varenicline applied alone to CA1 pyramidal neurons resulted in significantly enhanced GABAAR mIPSC raw τ2 values above baseline levels (Student’s t-test comparison, τ2 (ms); baseline = 36.8 ± 3.8, varenicline = 50.1 ± 3.8; p<0.05). All other combinations of τ values were not significantly different.
Discussion
In this study, we demonstrated that varenicline, similar to nicotine, stimulated GABA release and enhanced GABAergic synaptic transmission both in CA1 pyramidal and MS/DB neurons, in an action potential-independent manner. These results, extend findings from previous studies with the partial nicotinic agonist varenicline which demonstrated regulation of presynaptic dopamine release which is important for smoking cessation (Coe et al., 2005; Faessel et al., 2010; Rollema et al., 2007a; Rollema et al., 2007b). Both varenicline and nicotine increased mIPSC frequency, a measure of presynaptic GABA release, when bath applied to hippocampal and MS/DB slices, suggesting localized presynaptic nAChR activation on GABAergic terminals.
Effects of varenicline and nicotine on mIPSCs in the hippocampus
In this study, we focused on action potential-independent, spontaneous postsynaptic mIPSCs as a measure of presynaptic GABA release. The results confirmed that varenicline or nicotine both significantly increase the frequency of postsynaptic GABAAR-mediated mIPSCs in CA1 pyramidal cells, consistent with the interpretation that activation of presynaptic nAChRs enhances vesicular GABA release from inhibitory terminals in CA1 pyramidal cell layer. The mean increase in frequency was similar for both drugs, suggesting that varenicline was acting like an agonist, similar to nicotine, when applied alone. Interestingly, the addition of nicotine to varenicline did not have any further effect on varenicline-induced responses which is consistent with the fact that varenicline has a higher affinity than nicotine for nAChRs (Coe et al., 2005; Ortiz et al., 2012; Rollema et al., 2007a). Thus, at equimolar concentrations, nicotine should not out compete and displace binding of varenicline to neuronal nAChRs, and therefore, leaves responses to varenicline unchanged. In contrast, the nicotine-induced increase in mIPSC frequency returned to near baseline levels after adding varenicline, significantly reducing nicotine’s effects. In this case, varenicline acted like an antagonist blocking nicotine’s action on nAChR-facilitated GABA release. Thus, in the hippocampus, varenicline seems to act like an agonist when used alone but like an antagonist of nicotine depending on the order in which the drugs were applied to the slices. It is not immediately clear why varenicline would effectively antagonize nicotine’s effect at presynaptic nAChRs on GABAergic terminals but also have significant agonistic properties when tested alone. Most likely, nicotine and varenicline are acting on heteromeric nAChRs which have been strongly implicated in presynaptic GABA release (McClure-Begley et al., 2009). These heteromeric nAChRs can undergo conformational changes resulting in receptor desensitization (Wu and Lukas, 2011). Perhaps the co-application of nicotine plus varenicline favored the desensitized state.
In addition to increasing the frequency of mIPSCs, the very sensitive K-S test analysis indicated that varenicline and nicotine also caused small increases in amplitude, although, mean values were unaffected. When the distribution pattern of mIPSC amplitudes was compared in the hippocampus, it was evident that varenicline and nicotine mostly induced an increase in the number of mIPSCs with smaller amplitudes which is consistent with an increase in the number of mIPSC events, and probably does not reflect changes occurring on the postsynaptic side.
Effects of varenicline and nicotine on mIPSCs in the medial septum / diagonal band
In this study we show that both nicotine and varenicline enhance presynaptic GABA release in neurons located in the MS/DB. The majority of neurons responded with an increase in mIPSCs frequency and a small increase in amplitude, after bath application of varenicline or nicotine. This is consistent with the conclusion that most neurons in the MS/DB are innervated by GABAergic neurons that also express presynaptic nAChRs which facilitate presynaptic GABA release. However, the nAChR-mediated GABA release in the MS/DB is less well characterized than in hippocampus. Several studies using electrophysiological methods demonstrated fast somatic nicotinic responses on GABAergic neurons located in the MS/DB (Thinschmidt et al., 2005, Henderson et al., 2005; Wu et al., 2003). Other studies have demonstrated functional presynaptic nAChRs on GABAergic terminals in MS/DB; and their activation resulted in increased GABA release (Yang et al., 1996). Our results extend these findings by demonstrating a direct action of nicotine and varenicline on presynaptic nAChRs located on GABA-releasing terminals in the absence of action potentials.
In the basal forebrain, varenicline significantly increased mIPSC frequency acting like a nicotinic agonist, and the addition of nicotine had little effect on varenicline-induced responses similar to the findings in the hippocampus. However, in contrast to the hippocampus where varenicline mostly increased neuronal frequency, in the basal forebrain, varenicline decreased IEI, and thus increased mIPSC frequency, in 6 out of 13 neurons, but in a significant number of neurons (5 out of 13), varenicline application resulted in a drastic decrease in frequency (Table 1). In contrast, when nicotine was applied to basal forebrain neurons, 6 out of 9 cells responded with an increase in frequency, and in the remaining neurons, nicotine caused no change. This difference in response profile between varenicline and nicotine might reflect their interaction with different nAChRs subtypes located on presynaptic GABAergic terminals that innervate neurons in the MS/DB (Henderson et al., 2005). As in the hippocampus, nicotine application significantly increased frequency of mIPSCs in neurons of the MS/DB. However, in contrast to the hippocampus, the addition of varenicline reduced nicotine’s effect but the response remained significantly above baseline, which would be expected for a partial agonist with higher affinity for nAChRs than nicotine (Coe et al., 2005; Rollema et al., 2007a). Thus, varenicline differentially affected nicotine-stimulated increases in mIPSC frequency; antagonizing the effects of nicotine in hippocampal pyramidal neurons, but only partially antagonizing nicotine’s effects in basal forebrain neurons.
Conclusion
To date, most of the studies on varenicline have focused on its effects on DA release in the mesolimbic system, due to its effective use as a smoking cessation aid (Coe et al., 2005; Reperant et al., 2010). However, varenicline taken for smoking cessation will also assert its effects on other brain areas and affect other neurotransmitter systems that express pre- and postsynaptic nAChRs. As demonstrated in this study, varenicline increases GABA release in basal forebrain and hippocampus, thus, acting also in other brain areas and neurotransmitter systems. The functional profile of varenicline seems to be more complex than that of nicotine. Based on our results, varenicline could have agonistic and antagonistic properties on transmitter release in cortical structures, which could also change release of other endogenous transmitters such as acetylcholine and glutamate, and therefore, alter mood and cognitive performance.
Acknowledgments
Supported in by AA012386 (GDF), and a Research Development and Enhancement Grant from Texas A&M Health Science Center (UWS).
Footnotes
Conflict of interest:
The authors declare that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–55. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–74. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Azam L, Winzer-Serhan U, Leslie FM. Co-expression of alpha7 and beta2 nicotinic acetylcholine receptor subunit mRNAs within rat brain cholinergic neurons. Neuroscience. 2003;119:965–77. doi: 10.1016/s0306-4522(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Matthews P, Deacon RM, Rawlins JN. Medial septal lesions mimic effects of both selective dorsal and ventral hippocampal lesions. Behav Neurosci. 2004a;118:1033–41. doi: 10.1037/0735-7044.118.5.1033. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004b;28:273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–94. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Decker MW, Gamboa LP, Izquierdo I, McGaugh JL. Muscimol injections in the medial septum impair spatial learning. Brain Res. 1990;522:227–34. doi: 10.1016/0006-8993(90)91465-s. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Conti F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001;24:155–62. doi: 10.1016/s0166-2236(00)01724-0. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Napier TC. Antagonism of GABAergic transmission within the septum disrupts working/episodic memory in the rat. Neuroscience. 1992;47:833–41. doi: 10.1016/0306-4522(92)90033-x. [DOI] [PubMed] [Google Scholar]
- Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rollema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, 3rd, O’Neill BT. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48:3474–7. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- Colom LV, Castaneda MT, Reyna T, Hernandez S, Garrido-Sanabria E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse. 2005;58:151–64. doi: 10.1002/syn.20184. [DOI] [PubMed] [Google Scholar]
- DuBois DW, Parrish AR, Trzeciakowski JP, Frye GD. Binge ethanol exposure delays development of GABAergic miniature postsynaptic currents in septal neurons. Brain Res Dev Brain Res. 2004;152:199–212. doi: 10.1016/j.devbrainres.2004.06.017. [DOI] [PubMed] [Google Scholar]
- DuBois DW, Perlegas A, Floyd DW, Weiner JL, McCool BA. Distinct functional characteristics of the lateral/basolateral amygdala GABAergic system in C57BL/6J and DBA/2J mice. J Pharmacol Exp Ther. 2006a;318:629–40. doi: 10.1124/jpet.105.100552. [DOI] [PubMed] [Google Scholar]
- DuBois DW, Trzeciakowski JP, Parrish AR, Frye GD. GABAergic miniature postsynaptic currents in septal neurons show differential allosteric sensitivity after binge-like ethanol exposure. Brain Res. 2006b;1089:101–15. doi: 10.1016/j.brainres.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Dunning DD, Hoover CL, Soltesz I, Smith MA, O’Dowd DK. GABA(A) receptor-mediated miniature postsynaptic currents and alpha-subunit expression in developing cortical neurons. J Neurophysiol. 1999;82:3286–97. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- Dwyer TA, Servatius RJ, Pang KC. Noncholinergic lesions of the medial septum impair sequential learning of different spatial locations. J Neurosci. 2007;27:299–303. doi: 10.1523/JNEUROSCI.4189-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faessel HM, Obach RS, Rollema H, Ravva P, Williams KE, Burstein AH. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49:799–816. doi: 10.2165/11537850-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fenster CP, Beckman ML, Parker JC, Sheffield EB, Whitworth TL, Quick MW, Lester RA. Regulation of alpha4beta2 nicotinic receptor desensitization by calcium and protein kinase C. Mol Pharmacol. 1999a;55:432–43. [PubMed] [Google Scholar]
- Fenster CP, Hicks JH, Beckman ML, Covernton PJ, Quick MW, Lester RA. Desensitization of nicotinic receptors in the central nervous system. Ann N Y Acad Sci. 1999b;868:620–3. doi: 10.1111/j.1749-6632.1999.tb11335.x. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–43. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Garza A, Huang LZ, Son JH, Winzer-Serhan UH. Expression of nicotinic acetylcholine receptors and subunit messenger RNAs in the enteric nervous system of the neonatal rat. Neuroscience. 2009;158:1521–9. doi: 10.1016/j.neuroscience.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Nusser Z, Rancz EA, Freund TF, Mody I. Cell type- and synapse-specific variability in synaptic GABAA receptor occupancy. Eur J Neurosci. 2000;12:810–8. doi: 10.1046/j.1460-9568.2000.00964.x. [DOI] [PubMed] [Google Scholar]
- Henderson Z, Boros A, Janzso G, Westwood AJ, Monyer H, Halasy K. Somato-dendritic nicotinic receptor responses recorded in vitro from the medial septal diagonal band complex of the rodent. J Physiol. 2005;562:165–82. doi: 10.1113/jphysiol.2004.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993;60:9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Jayakar SS, Margiotta JF. Abelson family tyrosine kinases regulate the function of nicotinic acetylcholine receptors and nicotinic synapses on autonomic neurons. Mol Pharmacol. 2011;80:97–109. doi: 10.1124/mol.111.071308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen AA, Frolund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005;48:4705–45. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol. 1997;504(Pt 3):603–10. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa K. Inhibitory synaptic transmission in area postrema neurons of the rat showing robust presynaptic facilitation mediated by nicotinic ACh receptors. Brain Res. 2007;1130:83–94. doi: 10.1016/j.brainres.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Kohara K, Yasuda H, Huang Y, Adachi N, Sohya K, Tsumoto T. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27:7234–44. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. FDA warns of adverse events linked to smoking cessation drug and antiepileptics. JAMA. 2008;299:1121–2. doi: 10.1001/jama.299.10.1121. [DOI] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–96. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RA, Dani JA. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. J Neurophysiol. 1995;74:195–206. doi: 10.1152/jn.1995.74.1.195. [DOI] [PubMed] [Google Scholar]
- Levin ED, Simon BB. Nicotinic acetylcholine involvement in cognitive function in animals. Psychopharmacology (Berl) 1998;138:217–30. doi: 10.1007/s002130050667. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–40. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–39. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, Hu G, Chang Y, Lukas RJ, Wu J. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29:918–29. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Sher E, Cherubini E. Regulation of GABA release by nicotinic acetylcholine receptors in the neonatal rat hippocampus. J Physiol. 2001;536:89–100. doi: 10.1111/j.1469-7793.2001.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns ID, Mainville L, Jones BE. Evidence for glutamate, in addition to acetylcholine and GABA, neurotransmitter synthesis in basal forebrain neurons projecting to the entorhinal cortex. Neuroscience. 2001;107:249–63. doi: 10.1016/s0306-4522(01)00302-5. [DOI] [PubMed] [Google Scholar]
- McClure-Begley TD, King NM, Collins AC, Stitzel JA, Wehner JM, Butt CM. Acetylcholine-stimulated [3H]GABA release from mouse brain synaptosomes is modulated by alpha4beta2 and alpha4alpha5beta2 nicotinic receptor subtypes. Mol Pharmacol. 2009;75:918–26. doi: 10.1124/mol.108.052274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison EL, O’Malley SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–90. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–5. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Mineur YS, Eibl C, Young G, Kochevar C, Papke RL, Gundisch D, Picciotto MR. Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Ther. 2009;329:377–86. doi: 10.1124/jpet.108.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 2011;70:20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–25. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Newhouse P, Singh A, Potter A. Nicotine and nicotinic receptor involvement in neuropsychiatric disorders. Curr Top Med Chem. 2004;4:267–82. doi: 10.2174/1568026043451401. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Cull-Candy S, Farrant M. Differences in synaptic GABA(A) receptor number underlie variation in GABA mini amplitude. Neuron. 1997;19:697–709. doi: 10.1016/s0896-6273(00)80382-7. [DOI] [PubMed] [Google Scholar]
- Ortiz NC, O’Neill HC, Marks MJ, Grady SR. Varenicline Blocks beta2*-nAChR-Mediated Response and Activates beta4*-nAChR-Mediated Responses in Mice In Vivo. Nicotine Tob Res. 2012 doi: 10.1093/ntr/ntr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992;49:13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61:75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Brunzell DH, Caldarone BJ. Effect of nicotine and nicotinic receptors on anxiety and depression. Neuroreport. 2002;13:1097–106. doi: 10.1097/00001756-200207020-00006. [DOI] [PubMed] [Google Scholar]
- Poincheval-Fuhrman S, Sara SJ. Chronic nicotine ingestion improves radial arm maze performance in rats. Behav Pharmacol. 1993;4:535–539. [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–7. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Quik M, Perez XA, Grady SR. Role of alpha6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders. Biochem Pharmacol. 2011;82:873–82. doi: 10.1016/j.bcp.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reperant C, Pons S, Dufour E, Rollema H, Gardier AM, Maskos U. Effect of the alpha4beta2* nicotinic acetylcholine receptor partial agonist varenicline on dopamine release in beta2 knock-out mice with selective re-expression of the beta2 subunit in the ventral tegmental area. Neuropharmacology. 2010;58:346–50. doi: 10.1016/j.neuropharm.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, 3rd, Williams KE. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007a;52:985–94. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci. 2007b;28:316–25. doi: 10.1016/j.tips.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, McLean S, Freeman J, Williams KE. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol. 2009;78:813–24. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Rollema H, Wilson GG, Lee TC, Folgering JH, Flik G. Effect of co-administration of varenicline and antidepressants on extracellular monoamine concentrations in rat prefrontal cortex. Neurochem Int. 2011;58:78–84. doi: 10.1016/j.neuint.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Berry RB, White AM, Matthews DB. Impairments in spatial learning and memory: ethanol, allopregnanolone, and the hippocampus. Brain Res Brain Res Rev. 2003;43:275–84. doi: 10.1016/j.brainresrev.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Skok VI. Nicotinic acetylcholine receptors in autonomic ganglia. Auton Neurosci. 2002;97:1–11. doi: 10.1016/s1566-0702(01)00386-1. [DOI] [PubMed] [Google Scholar]
- Son JH, Winzer-Serhan UH. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs in rat hippocampal GABAergic interneurons. J Comp Neurol. 2008;511:286–99. doi: 10.1002/cne.21828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol. 2003;551:927–43. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taly A, Corringer PJ, Guedin D, Lestage P, Changeux JP. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–50. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- Thinschmidt JS, Frazier CJ, King MA, Meyer EM, Papke RL. Medial septal/diagonal band cells express multiple functional nicotinic receptor subtypes that are correlated with firing frequency. Neurosci Lett. 2005;389:163–8. doi: 10.1016/j.neulet.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Turner JR, Castellano LM, Blendy JA. Parallel anxiolytic-like effects and upregulation of neuronal nicotinic acetylcholine receptors following chronic nicotine and varenicline. Nicotine Tob Res. 2011;13:41–6. doi: 10.1093/ntr/ntq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GB. Structural answers and persistent questions about how nicotinic receptors work. Front Biosci. 2008;13:5479–510. doi: 10.2741/3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–8. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Wu J, Lukas RJ. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem Pharmacol. 2011;82:800–7. doi: 10.1016/j.bcp.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Hajszan T, Leranth C, Alreja M. Nicotine recruits a local glutamatergic circuit to excite septohippocampal GABAergic neurons. Eur J Neurosci. 2003;18:1155–68. doi: 10.1046/j.1460-9568.2003.02847.x. [DOI] [PubMed] [Google Scholar]
- Yang X, Criswell HE, Breese GR. Nicotine-induced inhibition in medial septum involves activation of presynaptic nicotinic cholinergic receptors on gamma-aminobutyric acid-containing neurons. J Pharmacol Exp Ther. 1996;276:482–9. [PubMed] [Google Scholar]
- Zhu PJ, Stewart RR, McIntosh JM, Weight FF. Activation of nicotinic acetylcholine receptors increases the frequency of spontaneous GABAergic IPSCs in rat basolateral amygdala neurons. J Neurophysiol. 2005;94:3081–91. doi: 10.1152/jn.00974.2004. [DOI] [PubMed] [Google Scholar]