Abstract
Because the toxicological effects of mercury (Hg) are more serious in the developing central nervous system of children than adults, there are growing concerns about prenatal and early childhood Hg exposure. This study examined postnatal methylmercury (MeHg) exposure and cognition and behavior in 780 children enrolled in the Treatment of Lead (Pb) - exposed Children clinical trial (TLC) with 396 children allocated to the succimer and 384 to the placebo groups. Mercury exposure was determined from analyses of blood drawn 1 week before randomization and 1 week after treatment began when succimer had its maximal effect on blood Pb (PbB). The baseline MeHg concentrations were 0.54 μg/L and 0.52 μg/L and post-treatment concentrations were 0.51 μg/L and 0.48 μg/L for placebo and succimer groups respectively. Because the baseline characteristics in the two groups were balanced and because succimer had little effect on MeHg concentration and no effect on the cognitive or behavioral test scores, the groups were combined in the analysis of MeHg and neurodevelopment. The children's IQ and neurobehavioral performance were tested at age 2, 5 and 7 years. We saw weak, non-significant but consistently positive associations between blood MeHg and IQ test scores in stratified, spline regression and generalized linear model data analyses. The behavioral problem scores were constant or decreased slightly with increasing MeHg concentration. Additional adjustment for PbB levels in multivariable models did not alter the conclusion for MeHg and IQ scores, but did confirm that concurrent PbB was strongly associated with IQ and behavior in TLC children. The effects of MeHg on neurodevelopmental indices did not substantially differ by PbB strata. We conclude that at the present background postnatal MeHg exposure levels of US children, adverse effects on children's IQ and behavior are not detectable.
Keywords: methylmercury, lead, children, neuropsychological tests, postnatal exposure
1. Introduction
Mercury (Hg) is ubiquitous in the global environment and derives from both natural sources and human enterprise. Because the toxicological effects of this heavy metal are more serious in the developing central nervous system of children than adults, the greatest concern is about prenatal and early childhood mercury exposure[1]. There are three forms of Hg that differ toxicologically: elemental Hg, organic Hg [mainly methylmercury (MeHg)], and inorganic Hg. Children are exposed to elemental Hg by inhaling the vapor from the elemental liquid or “quicksilver”, folk medications, or dental amalgam; to inorganic Hg from old teething powders containing calomel, from certain religious rituals, and from metabolic oxidation of the elemental form. High elemental and inorganic Hg exposures produce acrodynia, proteinuria, hypertension, mood disturbances, cognitive declines, urticaria, insomnia and a painful rash[2]. High prenatal exposure to MeHg produces a cerebral palsy-like illness accompanied by limb deformities, severe mental retardation and sensory handicaps in children. There have been two major outbreaks, one in Japan from contaminated fish[3], the other in Iraq [4] from consumption of seed wheat grain preserved with MeHg.
In 2000, the U.S. National Research Council (NRC) concluded that chronic low-dose prenatal MeHg exposure from maternal consumption of fish (> 0.1 ug/kg/day after accounting for uncertainty factors) may be associated with poorer performance on neurobehavioral tests, particularly those measuring attention, fine-motor function, language, visual-spatial abilities, and verbal memory [5]. This conclusion was supported mainly by results from studies in the Faroe Islands [6] and New Zealand [7] although a study conducted in the Seychelles did not confirm these observations [8].
An integrative analysis of epidemiologic data from these 3 large scale cohort studies estimated the dose-response relationship of prenatal MeHg exposure and IQ in children. It concluded that, despite some inconsistencies, IQ was a useful end point for estimating cognitive effects of prenatal MeHg exposure[9]. In the United States, several Federal agencies have examined data on MeHg exposure and its consequences, and have attempted to establish recommendations for safe exposures in children and pregnant women in the general population[5,10,11]. However, most existing studies are of children with relatively high prenatal MeHg exposure owing to high volume consumption of finfish and/or sea mammals, and the results cannot be easily extrapolated to lower-level prenatal as well as postnatal exposure conditions.
Concerns over early low level postnatal exposure to organic Hg from vaccines have been raised in recent years. For decades, vaccines such as pertussis, diptheria, tetanus, and influenza were preserved with thimerosal, an antimicrobial preparation composed of 50% ethylmercury. Assuming that the toxicity of ethylmercury is similar to MeHg, the exposure from a single vaccination would exceed Federal guidelines for that day and adherence to the usual pediatric vaccination schedule would deliver up to 188 ug by six months of age [12]. However, ethyl mercury is believed to be eliminated more quickly than MeHg, resulting in lower concentrations in central nervous system tissues [13]. Nevertheless, it has been speculated that the use of this preservative may have contributed to the “epidemic” number of cases of Autism Spectrum Disorder (ASD) observed in the United States and other developed countries [14]. However, the results of several large scale epidemiological studies have not supported this association [15].
Because we conducted a large trial of the treatment of pediatric Pb poisoning that included detailed, prospective measures of cognition and behavior, and because we had blood samples available for further analysis, we were well positioned to study presumably low-level postnatal Hg exposure and neurodevelopment. Thus we chose to assess the effects of Hg exposure at around age 2 years with cognitive and behavioral tests scores at baseline and up to 5 years later.
2. Material and methods
2.1. Sample and study design
The data in this study came from the Treatment of Lead-exposed Children trial (TLC), a 4-site, placebo-controlled, randomized clinical trial. The TLC study was conducted between September 1994 and June 2003 in Philadelphia, PA; Newark, NJ; Cincinnati, OH; and Baltimore, MD. It was approved by the institutional review boards at the clinical sites, data coordinating center, the CDC, and the National Institute of Environmental Health Sciences.
The TLC study design is published elsewhere[16]. Briefly, we accepted referral of children who were 12 to 33 months of age, had blood Pb levels between 20 and 44 μg/dL and could be tested in English or Spanish. Children who had confirmed venous PbB concentrations between 20 and 44 μg/dL and lived in cleanable housing had a second visit to confirm PbB levels. A total of 780 children entered the randomization phase, with 396 children allocated to succimer and 384 allocated to placebo.
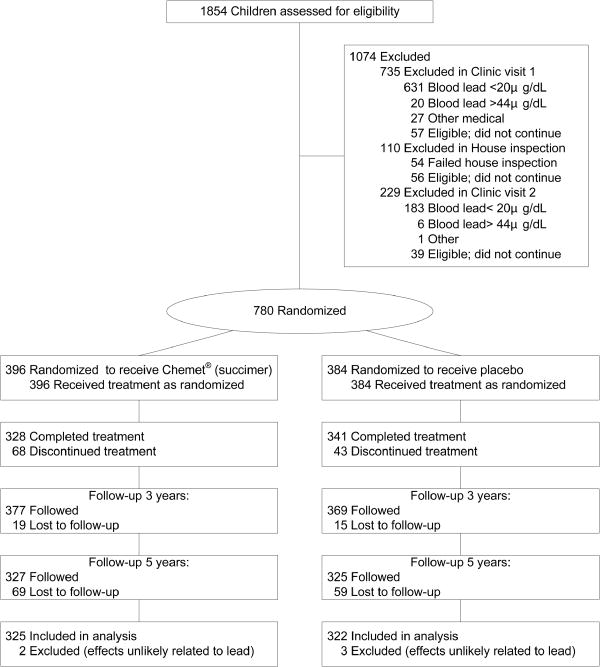
In the last wave of follow-up, a total of 128 subjects were lost: 59 in the placebo group and 69 in the active drug group. Three subjects in the placebo group and 2 subjects in the succimer group were excluded from the final analysis because of conditions affecting mental or neuromotor status that are unlikely to be related to their exposure to Pb or mercury (autism spectrum disorder, epilepsy, retinal degeneration.) The participant flow of TLC study is shown in Figure 1.
Figure 1. Flow of patients through the TLC clinical trial.
* Children who discontinued treatment and participated in the study through 7 years were included in the intent-to-treat analysis
2.2. IQ, cognitive, neuropsychological and behavioral tests
Before treatment began, we administered the Bayley Scales of Infant Development-II (BSID-II), the most widely used measure of infant intelligence[17]. The Mental Development Index (MDI) of the BSID-II is statistically analogous to an IQ score. After treatment, two waves of cognitive, behavioral, psychological and developmental assessment were administered at about age 5 and 7 years. Of all children who participated in the trial, 745 had caregiver's IQ measured with a short form of the Wechsler Adult Intelligence Scale-Revised [18]. The administration of behavioral questionnaires to parents was completed by trained examiners reading questions aloud and providing clarification of items as necessary; All TLC examiners were blind to caregiver's IQ levels and their children's blood Hg and PbB concentrations. Table 1 presents TLC neurodevelopmental assessments by age of follow-up.
Table 1. TLC Neuropsychological and behavioral test instruments.
| Neuropsychological and behavioral domains | Instruments (scale) |
|---|---|
| Age 5 | |
| Cognition, learning, and memory | |
| Intellectual attainment | Wechsler Preschool and Primary Scales of Intelligence-Revised (WPPSI-R) (full-scale IQ) [19] |
| Attention/Executive functions | Developmental Neuropsychological Assessment (NEPSY) (attention/executive function subscale)[20] |
| Behavior | |
| Behavioral conduct | Conners' Parent Rating Scale-Revised (CPRS- R) (externalizing problems)[21] |
| Age 7 | |
| Cognition, learning, and memory | |
| Intellectual attainment | Wechsler Intelligence Scale for Children-III(WISC-III) (full-scale IQ) [22] |
| Attention/Executive functions | NEPSY (attention/executive function subscale)[20] |
| Conners' Continuous Performance Test(CPT) (d prime)[23] | |
| Verbal learning and memory | California Verbal Learning Test for Children(CVLTC) (list A memory and learning slope) [24] |
| Reading | Woodcock Language Proficiency Battery- Revised (WLPB-R) (broad reading score) [25] |
| Behavior | |
| Behavioral conduct | Behavioral Assessment System for Children- Parent Rating Scale (BASC-PRS) (externalizing problems)[26] |
| Behavioral and academic conduct | Behavioral Assessment System for Children- Teacher Rating Scale (BASC-TRS) (adaptive skills, externalizing problems, school problems)[26] |
| Neuromotor | |
| Neurological | Neurological Examination for Subtle Signs(NESS) (rapid sequential movements time) [27] |
| Motor speed | CPT (hit reaction time)[23] |
2.3. Blood mercury measurement
The Division of Laboratory Sciences at the National Center for Environmental Health of the Centers for Disease Control and Prevention analyzed the blood sample drawn about 1 week before randomization, and the sample drawn 1 week after treatment began (this sample showed the largest estimated mean difference between treatment groups in PbB level). Whole blood specimens were analyzed for Hg concentration. We measured total Hg in all samples. Since the lab's experience was that inorganic Hg was not detected in samples with less than 1 μg/L total Hg, we measured inorganic Hg only when the total Hg concentration was ≥ 1 μg/L, and in post-treatment samples with < 1 μg/L if the baseline sample had been measured. In the US, 80%-95% of Hg in blood is MeHg, while Hg in urine consists mostly of inorganic Hg [28,29]. These known distinctions assist in interpreting blood Hg levels in study subjects as well as patients in clinical studies [30]. Thus, we calculated blood MeHg as total Hg minus inorganic Hg, and assumed that all Hg was MeHg in samples with total Hg < 1 μg/L or with undetectable inorganic Hg.
Specimens were analyzed using automated cold vapor atomic absorption spectrophotometry. The detection limit was 0.33 μg/L for total Hg and 0.35 μg/L for inorganic Hg. Throughout this study, all results of Hg given in μg/L can be converted to nmol/L by multiplying a conversion factor of 4.99.
2.4. Statistical analysis
Because more than 15% of the total Hg levels and more than 60% of inorganic Hg levels were below the limit of detection (LOD), we treated them with techniques for heavily left-censored data. In summary analyses, instead of simply replacing the non-detectable observations with ½ LOD, in the dataset, the non-detectable values were read as being between zero and LOD (the “censoring interval”), then a maximum likelihood estimation (MLE) method was used to estimate mean, percentiles and variance. Censored data analysis results indicated that concentrations of total Hg, MeHg and inorganic Hg were lognormally distributed and logarithmic transformation of these dependent variables was used to conform to the requirement of a normal distribution. Censored data analyses were done with Minitab 15.1 software (Minitab Inc, State College, PA). For geometric mean (GM) estimation, however, we used the method of replacing non-detectable values with ½ LOD.
We examined associations between two year blood MeHg, prospectively measured PbB and IQ or behavioral indices at 2, 5, and 7 years. For censored data, the non-parametric Kendall's tau-b correlation coefficient (ktau-b) was used, which makes no assumption of linearity between variables and accommodates multiply-censored data [31]. We examined the MeHg and IQ and behavior associations using scatter plot and cubic smoothing splines (which provided a fitted curve constructed by piecewise polynomials) with SPLUS 8.0 software (Insightful Corp, Seattle, WA).
Because spline regressions showed an approximately linear relation, we used a general linear model (GLM) for examining the MeHg effect. Based on the MeHg literature [6,7,8,32] and our previous work with these data [33,34], a priori covariates included exact age at IQ measurement, treatment group (not included in baseline model), caregiver's IQ, clinical center, single parent (yes or no), language (English or Spanish), race (non-Hispanic black or others), gender, parent's employment (neither working or either working), parent's education (<12 years, 12 years or >12years) and concurrent PbB level. Treatment per se was not associated with mercury, IQ or behavior scores in GLM analysis, and additional adjustment for treatment group did not markedly change the result; thus, the groups were combined in subsequent analyses. The same set of covariates was included for the regression models for 2 year blood MeHg and outcomes at baseline, 5 years and 7 years of age.
We analyzed the results for organic and inorganic Hg separately. All models were examined for statistical outliers and influential points. Each model was run first with outliers, then without outliers, and the results were compared. All of the results were essentially the same with or without outliers. The regression analyses for all measures were also repeated without influential points to determine whether the original results were dependent on such points. The results without influential points were consistent with the original analysis. Correlation and GLM analyses were done by Stata 10.0 software (Stata Corp, College Station, TX) and SAS 9.13 software (SAS Institute, Inc., Cary, NC).
3. Results
3.1. Patient characteristics
There were no differences between treatment and placebo groups in age, gender, race and socioeconomic status (parent's education, employment and life style). Overall, the children in the study were predominantly from low socioeconomic status families. The children were mostly of African-American heritage, spoke English, with a single parent. Girls accounted for 44% of children, 40% of children had parents with <12 years of education and more than half of children had neither parent employed. These and other baseline characteristics were balanced in the two groups (Table 2). A comparison of the baseline demographic characteristics and blood lead concentrations between children dropped or excluded in the last wave of TLC follow-up revealed few differences. Those not included were slightly younger (23 v. 25 months), and shorter in stature (84cm, v. 86cm) at baseline.
Table 2. Demographic characteristics and blood Hg levels of TLC children at baseline and follow-ups.
| Characteristic | Placebo group (N=384) | Succimer Group (N=396) |
|---|---|---|
| Clinic center-no. (%) | ||
| Baltimore | 104(27.1) | 109(27.5) |
| Newark | 102(26.6) | 106(26.8) |
| Philadelphia | 83(21.6) | 82(20.7) |
| Cincinnati | 95(24.7) | 99(25.0) |
| Ethnic group or race-no.(%) | ||
| White | 42(10.9) | 46(11.6) |
| African American or Black | 292(76.1) | 310(78.3) |
| Other | 50(13.0) | 40(10.1) |
| Female sex-no.(%) | 166(43.2) | 179(45.2) |
| English-speaking-no.(%) | 364(94.8) | 377(95.2) |
| Parent's education <12 yrs -no.(%) | 155(40.4) | 161(40.7) |
| Neither parent working-no.(%) | 220(57.3) | 234(59.4) |
| Living with single parent-no.(%) | 279(73.4) | 282(72.3) |
| Baseline blood Hg-μg/L | ||
| Total* | 0.56(0.52, 0.61)(N=374) | 0.54(0.50, 0.59)(N=393) |
| Organic* | 0.54(0.50, 0.58)(N=328) | 0.52(0.48, 0.56)(N=342) |
| Inorganic† | 0.28 (0.23, 0.33)(N=67) | 0.25(0.21, 0.28)(N=76) |
| Post-treatment blood Hg-μg/L | ||
| Total* | 0.55(0.52, 0.59)(N=379) | 0.52(0.49, 0.55)(N=389) |
| Organic* | 0.51(0.49, 0.54)(N=325) | 0.48(0.45, 0.51)(N=335) |
| Inorganic | 0.28(0.24, 0.33)(N=71) | 0.30(0.25, 0.36)(N=72) |
| Age-yrs | ||
| Baseline | 2.0±0.5‡ | 2.0±0.5 |
| At Hg measurement | 2.1±0.5(N=337) | 2.1±0.5(N=345) |
| At MDI measurement | 2.0±0.5(N=382) | 2.0±0.5(N=394) |
| At 5 yr IQ measurement | 5.0±0.5(N=360) | 5.0±0.5(N=370) |
| At 7 yr IQ measurement | 7.1±0.2(N=323) | 7.1±0.2(N=324) |
| IQ score | ||
| MDI (analogous to 2 yr IQ) | 81.5±13.7(N=375) | 82.8±13.8(N=390) |
| 5 yr IQ | 80.7±13.1(N=358) | 80.6±13.4(N=369) |
| 7 yr IQ | 86.5±13.4(N=321) | 86.9±13.2(N=323) |
| Blood Pb -μg/dL | ||
| Baseline | 25.9±4.8 | 26.5±5.4 |
| 5 yr | 11.93±4.9(N=360) | 12.2±5.5(N=371) |
| 7 yr∇ | 8.0±4.1(N=312) | 8.0±4.0(N=310) |
Geometric mean and 95% confidence interval, adjusted by age, gender, race and center.
Unadjusted value, only include children with total Hg>1.0 μg/L.
Plus-minus values are means±standard deviation.
One child with a blood Pb concentration of 50.8 μg/dL was excluded from the analysis.
3.2. Blood mercury concentrations
Total Hg was detected and quantified in 657 (85%) baseline samples and in 623 (80%) post-treatment samples. Inorganic Hg was detected and quantified in 42 (29%) baseline samples and in 57 (40%) post-treatment samples. The higher percentage of detectable levels in the post-treatment samples arises from the requirement that any sample with a inorganic Hg detected in the baseline sample would also have a measurement done of the post-treatment sample, whether it was above 1 μg/L or not (that accounts for 9% of post-treatment detectable rate). Because more than 60% values were censored, we only estimate unadjusted inorganic Hg concentration for children with total Hg>1.0 μg/L. The baseline and post-treatment inorganic Hg concentrations were quite similar between placebo and succimer groups. There were no differences between placebo and succimer groups for total Hg, inorganic Hg and MeHg concentrations of baseline or post-treatment (Table 2). All adjusted geometric means in Table 2 were adjusted for age, gender, race and center and based on complete cases. Thus, the number of children included depended upon the choice of exposure biomarker and covariates.
Almost all Hg in the blood (>80%) was MeHg, as was consistent with previous studies.[6, 35, 36]. Because the inorganic Hg analyses were not sufficiently powered due to the small proportion of children in this sample with detectable values, the subsequent analyses of effects of mercury exposure on IQ and behavior were conducted with MeHg.
3.3. IQ, neuropsychological and behavioral tests
The mean (±SD) of children's IQ for placebo vs. succimer groups at 2 years, 5 years and 7 years of age were 82±14 vs. 83±14, 81±13 vs.81±13 and 87±13 vs. 87±13 respectively. These scores did not differ by treatment, and the succimer and placebo groups were combined to study the effect of blood Hg concentrations on neuropsychological and behavioral test scores. The cognitive and behavioral test scores (mean±SD) at 2, 5 and 7 years are shown in Table 3. These scores were found to be correlated with one another but not with baseline blood MeHg .
Table 3. Mean and standard deviation of blood MeHg concentrations, IQ and behavioral outcomes and correlations between baseline blood MeHg concentration and IQ and behavioral outcomes at specific ages.
| Age | Variables | n | Median (Q1, Q3) or Mean ±SD | Correlation with baseline blood MeHg (ktau-b) | Correlation with concurrent IQ (Pearson r) |
|---|---|---|---|---|---|
| 2 | Pretreatment baseline blood MeHg concentration, μg/L | 767 | 0.6(0.4,0.8) | -0.04† | |
| Post-treatment blood MeHg concentration, μg/L | 767 | 0.5(0.4,0.8) | 0.58* | 0.01† | |
| MDI | 765 | 82.2±13.7 | -0.04 | ||
| 5 | IQ | 727 | 80.7±13.3 | -0.01 | |
| CPRS-R | |||||
| Oppositional index | 721 | 58±14 | <-0.01 | -0.14* | |
| Hyperactivity index | 721 | 64±14 | <0.01 | -0.14* | |
| ADHD index | 721 | 60±13 | 0.01 | -0.23* | |
| Behavioral index | 721 | 60±12 | 0.01 | -0.20* | |
| 7 | IQ | 644 | 87.1±13 | <0.01 | |
| BASC-TRS | |||||
| Adaptive skills | 531 | 46±10 | -0.04 | 0.39* | |
| Behavioral symptoms index | 541 | 54±12 | 0.04 | -0.28* | |
| Externalizing problems | 540 | 55±13 | 0.03 | -0.18* | |
| Internalizing problems | 542 | 52±10 | 0.01 | -0.17* | |
| School problems | 542 | 56±12 | 0.03 | -0.48* | |
| BASC-PRS | |||||
| Adaptive skills | 648 | 46±11 | 0.03 | 0.33* | |
| Behavioral symptoms index | 648 | 56±15 | 0.02 | -0.21* | |
| Externalizing problems | 648 | 58±15 | 0.03 | -0.28* | |
| Internalizing problems | 648 | 50±12 | 0.03 | -0.06 |
p<0.05
ktau-b
3.4. MeHg and IQ
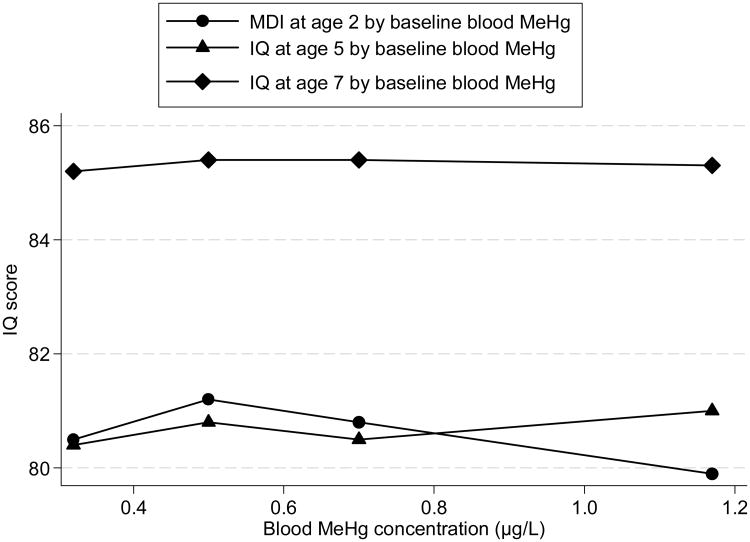
First, we stratified the children by quartiles of baseline MeHg levels. Figure 2 displays the mean IQ at age 2 (baseline), 5 and 7 years by quartiles of blood MeHg levels. Although MDI slightly decreased with increasing baseline MeHg, there is no consistent pattern for the IQ test scores at age 5 and 7 years by baseline MeHg levels.
Figure 2.
IQ test scores by baseline blood MeHg level. Each data point shows the mean IQ test scores (adjusted for treatment group, exact age at IQ measurement, caregivers' IQ, clinical center, single parent, language, race, gender, parents' occupation, education and concurrent PbB) of children assessed at age 2, 5 and 7 years, grouped by quartiles of baseline blood MeHg concentration. The abscissa of each point is the median of each blood MeHg concentration category.
We then plotted 2 year blood MeHg and IQ (at age 2, 5 and 7 years) and conducted spline regression analysis. At baseline, MDI did not change with MeHg, but at ages 5 and 7, IQ tended to increase with increasing MeHg concentration in the unadjusted data (after omitting two extreme blood MeHg values). Based on the approximately linear relationship, we conducted GLM analyses of MeHg and IQ at age 2 (baseline), 5 and 7 years by adjusting for a variety of covariates. The results are shown in Table 4. Although in none of the GLM models at 3 age points was the coefficient of MeHg statistically significant, it was consistently positive at all ages. The positive association between blood MeHg and IQ test scores remained constant in stratified data analysis and spline regression models. In GLM models, caregiver's IQ and concurrent PbB level were significant at all ages as expected. Language, race and gender were significant but only at some ages.
Table 4. GLM regression coefficients of MeHg and covariates on IQ at baseline and follow-up.
| Independent variables | MDI | IQ at 5 yrs | IQ at 7 yrs | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| coefficient | 95% CI | coefficient | 95% CI | coefficient | 95% CI | |
| Pretreatment baseline MeHg (μg/L, ln) | 0.3 | (-1.3, 1.9) | 0.8 | (-0.7, 2.3) | 0.5 | (-1.0, 2.1) |
| Treatment group | -0.5 | (-2.3, 1.4) | 0.3 | (-1.7, 2.2) | ||
| Exact age at IQ measurement (in year) | -8.5* | (-10.6, -6.3) | -3.0* | (-4.9,-1.1) | -2.3 | (-8.7, 4.1) |
| Caregiver's IQ | 0.2* | (0.1, 0.3) | 0.3* | (0.2, 0.4) | 0.4* | (0.3, 0.5) |
| Center | ||||||
| Baltimore | Reference | |||||
| Newark | -0.4 | (-3.2, 2.4) | -5.6* | (-8.2, -2.9) | 1.5 | (-1.7, 4.6) |
| Philadelphia | -1.2 | (-4.0, 1.7) | 0.8 | (-1.9, 3.5) | 6.1* | (2.8, 9.4) |
| Cincinnati | -2.7 | (-5.5, 0.2) | -4.7* | (-7.4, -2.0) | -1.4 | (-4.6, 1.9) |
| Living with single parent | ||||||
| No | Reference | |||||
| Yes | 1.4 | (-1.2, 4.0) | 0.1 | (-2.4, 2.6) | 0.7 | (-1.8, 3.3) |
| Language | ||||||
| English | Reference | |||||
| Spanish | -2.5 | (-7.8, 2.9) | -2.1 | (-7.1, 2.9) | -6.7* | (-11.5, 1.8) |
| Race | ||||||
| Black | Reference | |||||
| Non-black | 0.4 | (-2.5, 3.2) | 3.0* | (0.2, 5.7) | 4.8* | (2.0, 7.7) |
| Gender | ||||||
| Boy | Reference | |||||
| Girl | 2.0* | (0.1, 4.0) | -0.6 | (-2.5, 1.2) | -1.0 | (-2.9, 1.0) |
| Neither parent working | ||||||
| Yes | Reference | |||||
| No | 1.1 | (-1.2, 3.5) | 0.9 | (-1.3, 3.1) | 1.8 | (-0.5, 4.2) |
| Parent's education | ||||||
| =12 yr | Reference | |||||
| < 12 yr | -0.6 | (-2.9, 1.6) | -2.2* | (-4.4, -0.1) | -1.4 | (-3.7, 0.8) |
| >12 yr | -1.9 | (-4.8, 1.0) | -1.5 | (-4.3, 1.3) | 1.3 | (-1.6, 4.2) |
| Concurrent Pb level (μg/dl) | -0.3* | (-0.5, -0.1) | -0.4* | (-0.5, -0.2) | -0.4* | (-0.6, -0.3) |
p<0.05
3.5. MeHg and neuropsychological and behavioral test scores
The neuropsychological and behavioral test scores were not significantly associated with MeHg concentration (Table 3). Although not statistically significant, four behavioral problem scores at age 5 years and four teacher-rated behavior problems scores at age 7 years decreased (meaning more optimal) with increasing MeHg (after omitting two extreme MeHg values).
The scores of neuropsychological and behavioral tests at age 5 and age 7 stratified by baseline MeHg quartiles are shown in Table 5. Not all children completed all tests and so the numbers vary slightly. The differences between MeHg categories were compared by a Bonferroni method with the lowest MeHg concentration category as reference group. For the most part, there were no differences between MeHg categories among these scores. However, increasing blood MeHg categories were associated with a shallower learning slope on CVLTC (the slope of the increase in learning a list words over 5 trials) and slightly higher (i.e., less optimal) parent-reported behavioral symptoms index and externalizing behavior problems T scores on the BASC.
Table 5. Neuropsychological and behavioral test scores at age 5 and age 7 by baseline MeHg level.
| Neuropsychological and behavioral test | MeHg<Q1 | MeHg≥Q1,<Median | MeHg≥Median, <Q3 | MeHg≥Q3 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| N | Mean±SD | n | Mean±SD | n | Mean±SD | n | Mean±SD | |
| Age 5 | ||||||||
| NEPSY | ||||||||
| Attention and executive | 158 | 89±17 | 161 | 88±19 | 181 | 85±18 | 147 | 87±19 |
| Language | 160 | 85±15 | 151 | 84±14 | 173 | 83±15 | 145 | 83±14 |
| Sensorimotor | 161 | 87±15 | 163 | 84±15 | 185 | 86±16 | 151 | 84±16 |
| Visuospatial | 165 | 91±13 | 167 | 90±12 | 190 | 89±12 | 154 | 90±12 |
| Memory | 160 | 89±14 | 162 | 87±16 | 185 | 88±16 | 153 | 88±14 |
| CPRS-R | ||||||||
| Oppositional index | 176 | 58±14 | 175 | 58±13 | 198 | 58±15 | 160 | 57±14 |
| Hyperactivity index | 176 | 63±14 | 175 | 64±14 | 198 | 64±14 | 160 | 63±13 |
| ADHD index | 176 | 59±13 | 175 | 60±13 | 198 | 60±13 | 160 | 60±13 |
| Behavioral index | 176 | 60±12 | 175 | 61±12 | 198 | 61±12 | 160 | 60±12 |
| Age 7 | ||||||||
| NEPSY | ||||||||
| Attention and executive | 145 | 86±17 | 138 | 87±17 | 161 | 87±17 | 138 | 87±17 |
| CPT d-Prime | 138 | 56±11 | 134 | 57±8 | 154 | 55±10 | 135 | 55±10 |
| CVLTC | ||||||||
| List A memory | 154 | 46±12 | 154 | 44±11 | 179 | 43±12 | 148 | 42±11 |
| Learning slope | 154 | -0.2±1.1 | 154 | -0.5±1.1* | 179 | -0.6±1.1* | 148 | -0.4±1.1 |
| WLPB-R | ||||||||
| Broad reading score | 146 | 95±19 | 139 | 94±17 | 164 | 94±19 | 140 | 93±18 |
| CPT Hit response time | 138 | 41±14 | 134 | 44±14 | 154 | 44±12 | 135 | 41±12 |
| NESS | ||||||||
| Sequential movement time | 140 | 0.9±1.3 | 128 | 1.1±1.3 | 152 | 0.9±1.2 | 135 | 1.1±1.3 |
| BASC-TRS | ||||||||
| Adaptive skills | 125 | 48±10 | 124 | 46±10 | 148 | 46±9 | 126 | 45±9 |
| Behavioral Symptoms Index | 127 | 53±11 | 127 | 55±12 | 153 | 55±11 | 126 | 55±12 |
| Externalizing problems | 127 | 54±13 | 127 | 56±13 | 151 | 55±13 | 127 | 56±14 |
| Internalizing problems | 128 | 52±9 | 127 | 52±11 | 153 | 53±10 | 126 | 53±11 |
| School problems | 127 | 55±12 | 127 | 56±12 | 153 | 57±12 | 127 | 57±12 |
| BASC-PRS | ||||||||
| Adaptive skills | 155 | 46±10 | 154 | 45±11 | 179 | 46±11 | 149 | 46±11 |
| Behavioral Symptoms Index | 155 | 54±14 | 154 | 57±17* | 179 | 56±15 | 149 | 55±15 |
| Externalizing problems | 155 | 56±15 | 154 | 60±17* | 179 | 59±15 | 149 | 57±14 |
| Internalizing problems | 155 | 49±11 | 154 | 51±13 | 179 | 51±12 | 149 | 50±12 |
p<0.05
3.6. Relation to PbB
PbB concentrations in TLC children were part of the eligibility criteria and, thus, have a restricted spread and may have potential effects on the range of scores on IQ, neuropsychological and behavioral tests. The mean concentrations of PbB at baseline, 5 years of age and 7 years of age were 26.2 μg/dL, 12.0 μg/dL and 8.0 μg/dL respectively. As we have previously reported, the associations between lower IQ and concurrent PbB were significant [33]. Behavioral problems also tended to increase with increasing PbB concentration at both ages 5 and 7 in the unadjusted data. [34].
Baseline PbB level was not correlated with baseline blood MeHG level (Ktau-b=0.02, p=0.79), indicating that Hg exposure was independent of Pb exposure. For the test scores that were previously found to be associated with PbB,, regression analyses were repeated with and without and with PbB as an independent variable. Although the regression coefficients for MeHg adjusted for PbB are different from those not adjusted, in both conditions, all three of the MeHg regression coefficients (at baseline, 5 years and 7 years of age) were not significant. However, concurrent PbB regression coefficients were significant in all models (Table 4). Additional adjustment for PbB level in multivariable models did not alter the conclusion of a negligible association between MeHg and IQ, but confirm a strong association between concurrent PbB concentration and IQ.
We also tested the blood MeHg by concurrent PbB interaction on IQ at 2, 5 and 7 years, including the same variables in the model previously presented in Table 4. The interaction was statistically significant only for MDI at 2 years. We also conducted an analysis of blood MeHg and IQ stratified by baseline PbB concentration (PbB ≤ 24.9 ug/dL, PbB > 24.9 ug/dL). In this analysis, higher blood MeHg was significantly associated with higher MDI scores at 2 years in the higher PbB stratum. Nearly identical results were obtained when stratified by concurrent PbB concentration. Similar stratified analyses were also conducted for behavioral outcomes, including the BASC-TRS and BASC-PRS subscales. MeHg was not significantly associated with any of these behavioral outcomes within any baseline or concurrent PbB strata.
4. Discussion
This study presents results on prospectively studied neuropsychological performance in children with low but typical postnatal exposure to MeHg in the US. MeHg accounted for more than 80% of blood total Hg in our sample. This percentage of blood MeHg was consistent with the results from the National Health and Nutrition Examination Survey (NHANES) [28], the Faroe Islands study [6] and the Hong Kong study [35]. In those studies, greater than 90% of blood total Hg was estimated to be MeHg, and as the total Hg concentrations increase, the fraction of MeHg increases. Because MeHg exposure is of the greatest public health concern, our analyses were limited to the relationship between MeHg and child neurodevelopment.
After adjusting for effects of PbB and potential confounders, there were few and largely inconsistent associations between blood MeHg and tests of cognition or behavior; the contribution of MeHg to the regression models was very small (<1% explained variance). These findings were consistent at all three ages and in continuous and categorical analysis that take into account different combinations of covariates and multiple testing. The few significant blood MeHg associations observed in our study were limited to higher 2 year Bayley MDI scores for children within the higher baseline PbB stratum, and, at 7 years, a slightly shallower learning curve on a test of verbal memory and slightly more optimal scores on parental ratings of their children's behavior.
As postnatal blood MeHg concentrations increased, IQ tended to increase and behavioral problems tended to decrease, although in most instances these associations were statistically non-significant. However, this counter-intuitive finding is consistent with results from the Seychelles study. In that study, four of the 6 measures showed better scores in the highest MeHg groups compared with lower groups for both prenatal and postnatal exposure [8]. In the Peru study, there also was no increase in the frequency of neurodevelopmental abnormalities associated with MeHg exposure in early childhood.[36] A possible mechanism for these findings is that exposure to MeHg comes mainly from ocean fish, which has other components such as selenium and amino acids that may buffer mercury's potential toxicity. In addition, ocean fish provide important nutrients, such as omega-3-fatty acids, which may improve brain performance to such an extent that any adverse effects from low levels of Hg are not apparent or mitigated[37,38].
Compared with studies that found adverse effects for child development with prenatal and/or postnatal MeHg exposure, the MeHg concentrations in our study were much lower. In those studies that measured MeHg concentration in cord blood or children's blood samples, MeHg concentrations were from 0.88 μg/L to 22.9 μg/L, which were 1.5 to 40 times higher than those in this study [38,39]. In the studies with higher levels of Hg exposure there were inverse associations between prenatal MeHg exposure and cognitive, psychological and scholastic test results. But, as was the case in our study, the Seychelles study did not find adverse neurobehavioral outcomes associated with either prenatal or postnatal MeHg exposure [8].
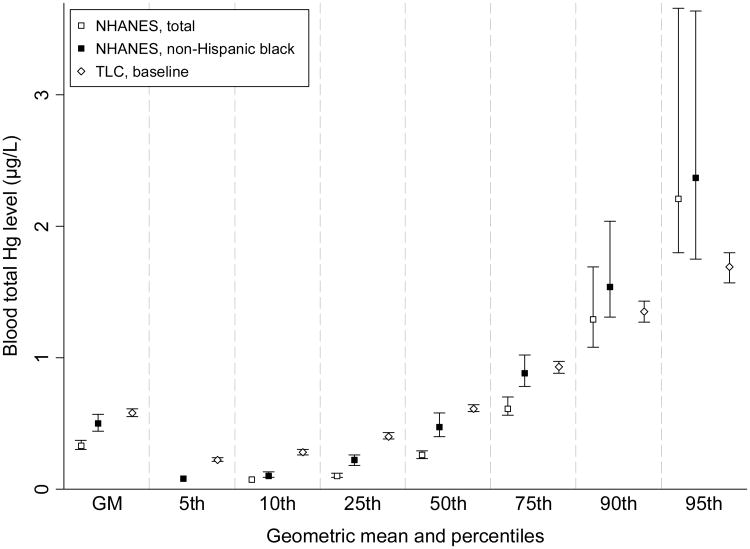
According to the NRC's report on prenatal exposure to MeHg, the likelihood of subnormal scores on neurodevelopment tests increased as cord blood MeHg concentrations increased from levels as low as 5 μg/L. The children we studied had much lower Hg level, about 10% of NRC's reference dose. Compared with the data from NHANES [28], the Hg level in our children was a little higher (Figure 3). But it should be considered that NHANES reported mean blood Hg levels were significantly higher for African-Americans (non-Hispanic Black, 0.50 μg/L) than others (0.29-0.35 μg/L) [40]. Most children in our studies are non-Hispanic Black (77%), and the Hg levels (0.58 μg/L) were close to the NHANES non-Hispanic Black level (Figure 3).
Figure 3. Comparison of blood total Hg concentration between NHANES and TLC data.
The major strengths of the present study include the relatively large sample size and the degree of testing and quality control that went into the measurement of both blood Hg and neuropsychological outcomes. The longitudinal nature of these data is a requirement for this kind of analysis, and the TLC study enjoyed remarkably high retention rates for a longitudinal investigation.
Study limitations include as-yet unmeasured mercury levels at 5 and 7 years of age. However, it is not clear that Hg at these low levels would affect cognition or behavior at school age. In our data, blood MeHg at age 2 years was not associated with deficits in neuropsychological outcomes. However, further research is needed to better understand the relationships between developmental exposure to MeHg in the womb, postnatally and neurodevelopment. We also did not have biomarkers of prenatal Hg exposure. While this makes it impossible for this study to address the issue of exposure in utero, most positive studies on this topic have recruited cohorts that obtain a significant proportion of their dietary protein from finfish or sea mammals (6, 8). It is unlikely that this was the case among the cohort of urban, inner-city families involved in TLC.
It could be argued that relatively high Pb exposure in TLC children may have masked possible adverse effects from low level Hg exposure. However, regression analyses that included a PbB by MeHg interaction term or stratified by baseline and concurrent PbB concentrations did not lend support to this as a factor in our results.
It has been widely speculated that exposure to organic Hg in utero and especially in vaccines may play a role in the etiology of Autism Spectrum Disorders (ASD) We did not study ASD, and our study did not attempt to designate children as being at risk for ASD . However, we can note that the Hg levels in the few untestable children in TLC, which likely included some with autistic disorders, were no different from those of the rest of the children. The baseline MeHg concentrations of IQ tested and untested subjects were 0.59 μg/L vs. 0.58 μg/L (p>0.95).
5. Conclusions
In summary, although our study can not determine a threshold above which postnatal Hg or MeHg exposure has detectable neurological and developmental consequences in children, at least we can conclude that at the present postnatal MeHg exposure level of U.S. children, adverse effects on children's cognition and behavior were not detectable, since they did not appear in a longitudinal study among children living in poverty in the U.S.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Footnotes
The authors have indicated they have no financial relationship relevant to this article to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration clinicaltrials.gov Identifier: NCT00342849
References
- 1.Stokstad E. Environment. Uncertain science underlies new mercury standards. Science. 2004;303:34. doi: 10.1126/science.303.5654.34. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson TW, Magos L, Myers GJ. The toxicology of mercury--current exposures and clinical manifestations. N Engl J Med. 2003;349:1731–1737. doi: 10.1056/NEJMra022471. [DOI] [PubMed] [Google Scholar]
- 3.Harada M. Congenital Minamata disease: intrauterine methylmercury poisoning. Teratology. 1978;18:285–288. doi: 10.1002/tera.1420180216. [DOI] [PubMed] [Google Scholar]
- 4.Amin-Zaki L, Elhassani S, Maheed MA, Clarkson TW, Doherty RA, Greenwood MR, Giovanoli-Jakubczak T. Perinatal methylmercury poisoning in Iraq. Am J Dis Child. 1976;130:1070–1076. doi: 10.1001/archpedi.1976.02120110032004. [DOI] [PubMed] [Google Scholar]
- 5.National Research Council (NRC) Toxicological Effects of Methylmercury. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 6.Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sorensen N, Dahl R, Jorgensen PJ. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997;19:417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- 7.Kjellstrom T, Kennedy P, Wallis P, Mantell C. Physical and Mental Development of Children with Prenatal Exposure to Mercury from Fish Stage 2: Interviews and Psychological Tests at Age 6. InSolna, Sweden: National Swedish Environmental Protection Board; 1989. [Google Scholar]
- 8.Davidson PW, Myers GJ, Cox C, Axtell C, Shamlaye C, Sloane-Reeves J, Cernichiari E, Needham L, Choi A, Wang Y, Berlin M, Clarkson TW. Effects of prenatal and postnatal methylmercury exposure from fish consumption on neurodevelopment: outcomes at 66 months of age in the Seychelles Child Development Study. JAMA. 1998;280:701–707. doi: 10.1001/jama.280.8.701. [DOI] [PubMed] [Google Scholar]
- 9.Axelrad DA, Bellinger DC, Ryan LM, Woodruff TJ. Dose-response relationship of prenatal mercury exposure and IQ: an integrative analysis of epidemiologic data. Environ Health Perspect. 2007;115:609–615. doi: 10.1289/ehp.9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schober SE, Sinks TH, Jones RL, Bolger PM, McDowell M, Osterloh J, Garrett ES, Canady RA, Dillon CF, Sun Y, Joseph CB, Mahaffey KR. Blood mercury levels in US children and women of childbearing age, 1999-2000. JAMA. 2003;289:1667–1674. doi: 10.1001/jama.289.13.1667. [DOI] [PubMed] [Google Scholar]
- 11.Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological profile for mercury. U.S. Department of Health and Human Services PHS; Atlanta, GA: 1999. [Google Scholar]
- 12.Goldman LR, Shannon MW. Technical report: Mercury in the Environment: Implications for pediatricians. Pediatrics. 2001;108:197–205. doi: 10.1542/peds.108.1.197. [DOI] [PubMed] [Google Scholar]
- 13.Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposed to methylmercury or vaccines containing thimerosal. Environ Health Perspect. 2005;113:1015–1021. doi: 10.1289/ehp.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard S, Enayarti A, Redwood L, Roger H, Binstock T. Autism, a novel form of mercury poisoning. Med Hypotheses. 2001;56:462–471. doi: 10.1054/mehy.2000.1281. [DOI] [PubMed] [Google Scholar]
- 15.Rutter M. Incidence of autism spectrum disorders: Changes over time and their meaning. Acta Pediatrica. 2005;94:2–15. doi: 10.1111/j.1651-2227.2005.tb01779.x. [DOI] [PubMed] [Google Scholar]
- 16.Treatment of Lead-exposed Children Trial Group. The Treatment of Lead-exposed Children (TLC) trial: design and recruitment for a study of the effect of oral chelation on growth and development in toddlers. Paediatr Perinat Epidemiol. 1998;12:313–333. doi: 10.1046/j.1365-3016.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 17.Bayley N. Bayley Scales of Infant Development: Manual. second. The Psychological Corporation; San Antonio, TX: 1993. [Google Scholar]
- 18.Silverstein AB. Two- and four-subtest short forms of the WAIS-R: a closer look at validity and reliability. J Clin Psychol. 1985;41:95–97. doi: 10.1002/1097-4679(198501)41:1<95::aid-jclp2270410116>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Wechsler D. The Wechsler Preschool and Primary Scales of Intelligence– Revised. The Psychological Corporation; San Antonio, TX: 1989. [Google Scholar]
- 20.Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment: Manual. The Psychological Corporation; San Antonio, TX: 1998. [Google Scholar]
- 21.Conners CK Conners Rating Scales. Technical Manual. Multi-Health Systems; North Tonawanda, NY: 1997. [Google Scholar]
- 22.Wechsler D. Wechsler Intelligence Scales for Children–III. The Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- 23.Conners CK. Conners Continuous Performance Test. Multi-Health Systems; North Tonawanda, NY: 1995. [Google Scholar]
- 24.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test–Children's Version. The Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- 25.Woodcock RW. Woodcock Language Proficiency Battery–Revised. Riverside Publishing; Itasca, IL: 1991. [Google Scholar]
- 26.Reynolds CR, Kamphaus RW. Behavior Assessment System for Children. American Guidance Service; Circle Pines, MN: 1992. [Google Scholar]
- 27.Denckla MB. Revised neurological examination for subtle signs (1985) Psychopharmacol Bull. 1985;21:773–800. [PubMed] [Google Scholar]
- 28.Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. Am J Epidemiol. 2008;167:1171–1181. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers of Disease Control and Prevention. Third National Report on Human Exposure ot Environmental Chemicals. United States Department of Health and Human Services; 2005. NCEH Pub. No. 05-0570. [Google Scholar]
- 31.Helsel DR Nondetects And Data Analysis. Statistics for Censored Environmental Data. John Wiley & Sons; Hoboken, NJ: 2004. [Google Scholar]
- 32.van Wijngaarden E, Beck C, Shamlaye CF, Cernichiari E, Davidson PW, Myers GJ, Clarkson TW. Benchmark concentrations for methyl mercury obtained from the 9-year follow-up of the Seychelles Child Development Study. Neurotoxicology. 2006;26:702–709. doi: 10.1016/j.neuro.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Chen A, Cai B, Dietrich KN, Radcliffe J, Rogan WJ. Lead exposure, IQ, and behavior in urban 5- to 7-year-olds: does lead affect behavior only by lowering IQ? Pediatrics. 2007;119:650–658. doi: 10.1542/peds.2006-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen A, Dietrich KN, Ware JH, Radcliffe J, Rogan WJ. IQ and blood lead from 2 to 7 years of age: are the effects in older children the residual of high blood lead concentrations in 2-year-olds? Environ Health Perspect. 2005;113:597–601. doi: 10.1289/ehp.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fok TF, Lam HS, Ng PC, Yip AS, Sin NC, Chan IH, Gu GJ, So HK, Wong EM, Lam CW. Fetal methylmercury exposure as measured by cord blood mercury concentrations in a mother-infant cohort in Hong Kong. Environ Int. 2007;33:84–92. doi: 10.1016/j.envint.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Marsh DO, Turner MD, Smith JC, Allen P, Richdale N. Fetal methylmercury study in a Peruvian fish-eating population. Neurotoxicology. 1995;16:717–726. [PubMed] [Google Scholar]
- 37.Ronchetti R, Zuurbier M, Jesenak M, Koppe JG, Ahmed UF, Ceccatelli S, Villa MP. Children's health and mercury exposure. Acta Paediatr Suppl. 2006;95:36–44. doi: 10.1080/08035250600886157. [DOI] [PubMed] [Google Scholar]
- 38.Jedrychowski W, Jankowski J, Flak E, Skarupa A, Mroz E, Sochacka-Tatara E, Lisowska-Miszczyk I, Szpanowska-Wohn A, Rauh V, Skolicki Z, Kaim I, I, Perera F. Effects of prenatal exposure to mercury on cognitive and psychomotor function in one-year-old infants: epidemiologic cohort study in Poland. Ann Epidemiol. 2006;16:439–447. doi: 10.1016/j.annepidem.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 39.Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol. 2006;28:536–547. doi: 10.1016/j.ntt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Blood mercury levels in young children and childbearing-aged women--United States, 1999-2002. MMWR Morb Mortal Wkly Rep. 2004;53:1018–1020. [PubMed] [Google Scholar]