Abstract
Activation of human blood plasma coagulation by contact with hydrophilic or hydrophobic surfaces (procoagulants) is dominated by kallikrein (Kal)-mediated activation of the blood zymogen FXII (Hageman Factor). Mathematical modeling of prekallikrein (PK)-deficient platelet-poor plasma (dPKPPP) and PK-reconstituted dPKPPP (RdPKPPP) coagulation shows that autoactivation of FXII ( ) produces no more than about 25% of the total FXIIa produced by the intrinsic pathway. Autoactivation and reciprocal-activation increase in the same proportion with procoagulant surface energy (water-wettability), while total amount of FXIIa produced per-unit-area procoagulant remains roughly constant for any particular procoagulant. These results suggest that procoagulant surfaces initiate the intrinsic cascade by producing a bolus of FXIIa in proportion to surface energy or surface area but play no additional role in subsequent molecular events in the cascade. Results further suggest that reciprocal-activation occurs in proportion to the amount of FXIIa produced by the initiating autoactivation step.
Keywords: coagulation, blood compatibility, Hageman factor, plasma proteins
INTRODUCTION
Development of truly blood-compatible materials remains a largely unrealized objective of biomaterial surface science 1–3. Coagulation and thrombosis persist as major risks to patient health associated with the use of blood-contacting medical devices 4,5. An improved understanding of the molecular basis of hemocompatibility is essential to the prospective engineering of advanced cardiovascular biomaterials.
The intrinsic cascade of blood plasma coagulation is a complex process linking a series of limited proteolytic conversions of zymogens to active enzymes 6. This enzyme cascade acts as a biochemical amplifier that converts a procoagulation stimulus to thrombin, a potent enzyme that both hydrolyses fibrinogen to fibrin and activates FXIII, ultimately resulting in the formation of a cross-linked fibrin mesh that is generally referred to as a fibrin clot.
Molecular interactions at the blood-material interface involved in the activation of the intrinsic cascade, often referred to as contact activation, have been extensively investigated. These results have been compiled into a mechanistic sequence of molecular events at the blood-material interface (see 7–9 and references therein) that describes initiation of material-induced blood coagulation. According to this traditional biochemical theory of contact activation, negatively-charged surfaces are efficient activators of the coagulation cascade because these materials can bind to one-or-more proteins of the so-called contact activation complex. This binding induces zymogen-enzyme conversions through poorly-understood surface-catalyzed biochemistry. The contact activation complex is believed to involve four proteins; coagulation factor XII (FXII, Hageman factor), prekallikrein (PK, Fletcher factor), high-molecular weight kininogen (HMWK, Fitzgerald factor), and coagulation factor XI (FXI, Plasma Thromboplastin Antecedent) 10 that somehow preferentially adsorb to procoagulant surfaces in the presence of overwhelming concentrations of other blood proteins such as albumin. It is thought that FXIIa can be produced by at least three different biochemical reactions. Autoactivation produces FXIIa by contact of FXII with an activating surface ( ), presumably due to a “conformational change” upon binding of FXII to the surface 11,12. Binding of FXII to a negatively-charged surface is also believed to render it susceptible to cleavage by kallikrein (Kal) presumably generated by FXIIa-mediated hydrolysis of PK 13. This mutual activation of PK and FXII is known as reciprocal-activation, the second known reaction route to FXIIa. Finally, autohydrolysis or self amplification produces FXIIa by the action of FXIIa on FXII (FXIIa + FXII → 2FXIIa) 14–16.
Interestingly, while each of these three biochemical reactions can be observed in defined solutions containing one-or-more components of the contact activation complex, apparently all three reactions do not occur in plasma to the same extent. For example, Zhuo and Vogler report that autohydrolysis is a facile reaction in buffer solutions of FXIIa but that autohydrolysis is not a significant reaction in plasma 16. This observation is consistent with mathematical-modeling of the intrinsic cascade predicting that autohydrolysis of FXII was not a significant source of FXIIa following activation with either exogenous FXIIa or solid procoagulants 17. Zhuo and Vogler speculated that autohydrolysis is not significant in plasma because PK and FXI are preferred substrates for FXIIa in plasma which are not available in neat-buffer solutions of FXII. Other investigations have reported similar observations, demonstrating that hydrolysis of a zymogen by its activated enzymatic form is negligible compared to cleavage by other preferred enzymes. Notably among these reports, Tans et al demonstrated using rigorous enzyme-kinetics measurements that, although Kal may autohydrolyze PK in neat solution, the rate constant for this reaction is about three orders of magnitude smaller than that for the cleavage of PK by FXIIa 18. Also, Dunn et al made measurements of the time course of generation of activated FXII products to show that the rate of activation of FXII by Kal was much faster than the rate of autohydrolysis 19. Thus, it seems that the contribution of autohydrolysis overall FXIIa yield in plasma can be considered negligible.
However, it is not at all clear which of the remaining two known chemical reactions that produce FXIIa is most important in contact activation of plasma coagulation, as the proportions for these two (or possibly more) reactions that contribute to overall FXIIa yield are not known. And it is not clear how these proportions may depend on the surface chemistry of procoagulant surfaces. This lack of clarity is an important impediment to the prospective design of cardiovascular biomaterials with improved hemocompatibility; for if the elementary chemical reactions at a procoagulant surface producing the first activated enzyme of an enzyme-amplifier cascade are unknown, then evidenced-based surface engineering routes to minimizing or eliminating contact activation of plasma coagulation cannot be proposed. Further complicating a full understanding of contact activation, it has been shown recently that FXII activation is not, in fact, specific for anionic/hydrophilic surfaces as purported by the traditional theory of contact activation 20. Rather, apparent specificity for hydrophilic surfaces is actually due to a relative diminution of the FXII→FXIIa yield at hydrophobic surfaces immersed in plasma 20. This finding discounts the specific-binding argument that is central to traditional theory and instead focuses our attention to biochemical details of how FXIIa is actually produced at procoagulant surfaces with different hydrophilicity. Toward this objective, we report herein semi-quantitative determination of the contributions of different FXII activation pathways to the total FXIIa enzyme produced by blood plasma contact with three model surfaces spanning the full range of observable water-wettability (surface energy). Results indicate that reciprocal-activation is the principal biochemical pathway to FXIIa. Autoactivation and reciprocal-activation seem to increase in the same proportion as procoagulant surface energy (water-wettability) is varied. For any particular procoagulant material, the total amount of FXIIa produced per-unit-surface area remains roughly constant. However, the amount of FXIIa produced per unit area is different for different materials.
MATERIALS AND METHODS
Plasma and Proteins
Frozen PK-deficient plasma (anticoagulated with 3.8 % sodium citrate) from a human donor with a congenital deficiency was obtained commercially from George King Biomedical (Overland Park, KS) and stored at −80°C. Prior to use, the plasma was thawed in a water-bath at 37°C for ~ 40 min and centrifuged at 500 × g for 10 min at 35°C to remove remnant platelets. This was designated as PK-deficient platelet-poor plasma (dPKPPP). For this work, a single lot of dPKPPP obtained from a single donor was used.
Human FXIIa (80 kDa) and human PK (86 kDa) were obtained from Enzyme Research Laboratories (South Bend, IN). Upon receipt, the proteins were thawed in a 37°C water-bath, distributed into small aliquots without dilution and stored at −80°C. Prior to use, proteins were thawed in a water-bath at 37°C and adjusted to desired concentration by serial dilution of stock solutions with phosphate buffered saline (PBS, pH 7.4, Sigma-Aldrich, St. Louis, MO) based on supplier-provided values. Single lots of FXIIa and PK were used throughout this study, with supplier-provided activity values of 73 PEU/mg and 28 PEU/mg, respectively. FXIIa concentration is reported in molar units assuming 100% protein activity as input into a previously-published enzyme-kinetic model.
Surface Preparation of solid procoagulants
Surfaces with controlled chemistry and water-wettability were prepared by surface-treatment of borosilicate glass particles. The procedures were detailed previously 17,21 and are briefly outlined here for the purpose of this paper. Glass particles of diameter 425–600 μm (nominal diameter = 500 μm, Sigma-Aldrich, St. Louis, MO) were cleaned by sonication in chloroform for at least 15 min and subsequently cleaned in a glow-discharge chamber at 100W power for 30 min. Resulting clean-glass particles were either used as-prepared or following silanization with 3-aminopropyltriethoxysilane (APS) or n-octadecyltrichlorosilane (OTS, both silanes obtained from Gelest Inc., Morrisville, PA). Glow-discharge cleaned particles were incubated for 5 min in a solution of 2% (v/v) APS in ethanol in a glass petri dish. The APS solution was aspirated and the particles were rinsed in fresh chloroform by sonication in a glass beaker for 15 min followed by air-drying overnight. For OTS modification, glow-discharge cleaned particles were incubated for 60 min in a solution of 5% (v/v) OTS in chloroform. After washing in chloroform the particles were annealed overnight in a vacuum-oven at 110°C. Water-wettability for each surface was measured on 12 mm diameter glass coverslip witness samples (VWR). Contact angles on the resultant surfaces were measured by the horizontal sessile drop method using a Krüss goniometer with 18 MΩ water (Millipore simplicity unit) as the probe liquid.
In vitro Coagulation Assay
An in vitro coagulation assay was used to measure the response of plasma to a dose of exogenous enzyme or solid procoagulants 17,21,22. The coagulation response was measured in terms of a coagulation time (CT), where CT was defined as the time required from activation of the intrinsic pathway of the coagulation cascade by a dose of enzyme and/or solid procoagulant to the appearance of a visible clot. Clot was detected visually and the onset of clotting clearly identified by a sharp change in plasma from fluid- to gel-phase. Prior experience with the assay has shown that while the absolute values of CT may be affected by variations in plasma batches, trends of measured coagulation responses for different procoagulants were consistent across different batches of plasma. Therefore, all PPP used for this work was prepared by reconstituting a single batch of dPKPPP with PK at physiological concentration, and was designated as RdPKPPP.
Briefly, 0.5 mL of dPKPPP was mixed with 0.1 mL of 0.1 M calcium chloride (CaCl2, Sigma-Aldrich) and a known dose of procoagulant (either surface-treated particles or soluble enzyme) in a 5 mL polystyrene vial (12 mm × 75 mm, VWR). For measurements performed in RdPKPPP, PK was added at 20 μg/mL, assuming a nominal physiological [PK] = 40 μg/mL 10,23 and accounting for the 50% dilution of plasma in the assay. The volume was adjusted by adding PBS to obtain a 1 mL solution resulting in a 1:1 dilution of plasma in buffer. Assay vials were capped with parafilm and continuously rotated at 8 rpm on a hematology mixer until the plasma underwent a sharp change from liquid to a gel-like state, at which time the corresponding CT was recorded. CT measurements for different doses of exogenous FXIIa or solid procoagulants area were measured in dPKPPP and RdPKPPP with n ≥ 3 for each dose.
Mathematical Analysis
Quantitative analysis of enzyme production in this work utilized a previously-published mathematical model of the intrinsic cascade of coagulation 17. The model was based on the premise that material-induced blood-coagulation may be modeled as a two-step process. In the first step, surface contact generates FXIIa from the endogenous plasma FXII, which is then processed in the second step by a “gray box” containing all the intermediate reactions to ultimately generate the output fibrin clot (see Guo et. al.17 for detailed description). The values of the parameters of the gray box were obtained by statistical non-linear least-squares fit to FXIIa-titration data in PPP using the commercial SIGMAPLOT® 8 software (Systat Software Inc., Point Richmond, CA). For each solid procoagulant the mathematical model was then solved for the “catalytic efficiency”, the ability of a material to produce FXIIa from endogenous FXII. Note that all data is presented using units of μM and m2/mL for enzyme concentration and added procoagulant area, respectively, consistent with units used for the mathematical model 17.
RESULTS
The objective of this work was to determine the amounts of FXIIa generated via the autoactivation and reciprocal-activation pathways at surfaces spanning a wide-range of surface water-wettability. Experiments performed may be broadly grouped into two categories: (i) measurement of the coagulation response to exogenous FXIIa in PK-deficient and reconstituted PK-deficient plasma (dPKPPP and RdPKPPP, respectively) or (ii) coagulation response to three solid procoagulants with different surface water-wettability in both dPKPPP and RdPKPPP. Mathematical modeling was used in both cases to calculate the amount of FXIIa generated.
FXIIa Titration of dPKPPP and RdPKPPP
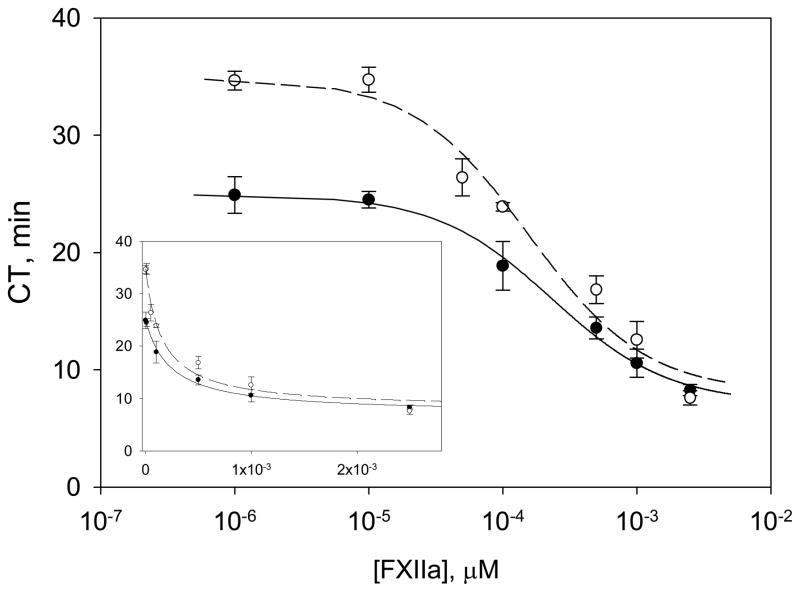
Figure 1 plots observed CT measured in response to exogenous FXIIa enzyme doses to dPKPPP and RdPKPPP. Note that CT is a sharper function of FXIIa concentration for RdPKPPP compared to dPKPPP, especially at the intermediate FXIIa concentrations studied. Both plasmas showed nearly-constant responses at low and high enzyme concentrations. Mathematical analysis of coagulation quantified the parameters a, b and c that describe the propagation of the exogenous FXIIa dose through the cascade. Table 1 compiles parameters obtained by statistical best fit of the model discussed in Methods to experimental data of Figure 1. Estimated error fell between 10–25% which was deemed adequate for the limited purpose of comparing activation in different plasmas with test surfaces. Lines through the data of Figure 1 represent the statistical best fit to the mathematical model. It is important to note that the coagulation response induced by the surface of the polystyrene vial and other possible sources such as remnant platelets and FXIIa initially present in the system are incorporated into the gray box model and accounted for by the parameters b and c, in particular.
Figure 1.
FXIIa titration in PK-deficient PPP (dPKPPP) (open circles, o), and reconstituted dPKPPP (RdPKPPP) (solid circles, ●) showing experimental coagulation time (CT) values vs. concentration of exogenous soluble FXIIa. The smooth curve shows the best fit of a mathematical model to the data for soluble FXIIa titration. Inset presents data using linear scale for FXIIa concentration. (All data is shown as mean ± S.D. from at least three individual measurements).
Table 1.
Parameters describing the gray box processing of FXIIa
| Plasma type | a (min) | b × 10−3 (μM.min) | c × 10−4 (μM) |
|---|---|---|---|
| dPKPPP | 8.1 ± 2.0 | 5.4 ± 1.9 | 1.6 ± 0.6 |
| RdPKPPP | 7.1 ± 0.8 | 5.8 ± 1.2 | 2.3 ± 0.5 |
Values are displayed as mean ± S.D.
Surface Area Titration of Solid Procoagulants
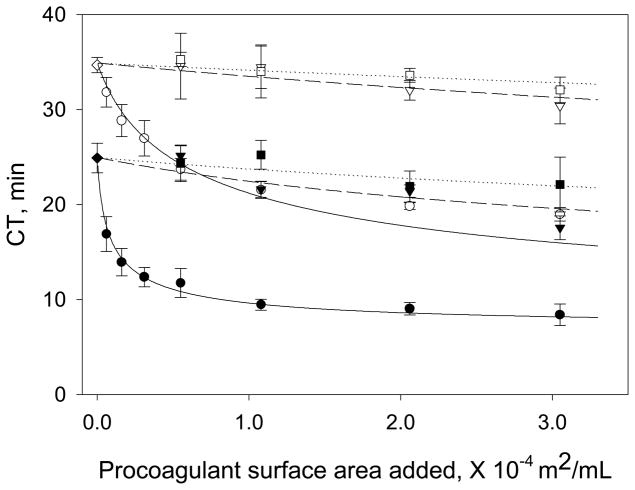
Glow-discharge cleaned and silane-treated glass particles were used as solid procoagulants spanning the full range of surface water-wettability. Measured contact angles (listed in Table 2 as mean and standard deviation) were in good agreement with the expected trend of increasing water-wettability for the OTS, APS, and glass surfaces, respectively, and are completely consistent with our previous results. Figure 2 collects surface-area-titration (SAT) plots for each of the three test surfaces in dPKPPP and RdPKPPP, showing CT values measured in response to variable surface area of solid procoagulant. Utilizing the corresponding gray box parameters (a, b and c) generated from FXIIa titration (Figure 1 and Table 1), mathematical modeling of material-induced coagulation was used to solve for K1, a characteristic parameter that measures the “catalytic efficiency” of the test procoagulant in the corresponding plasma type17. Table 2 lists the mean K1 values for each solid procoagulant/plasma combination along with estimated uncertainty. Error in K1 increased with decreasing procoagulant hydrophilicity, ranging from about 35% uncertainty in glass K1 to 70% uncertainty in OTS, reflecting diminishing procoagulant activity in the order glass >APS> OTS (uncertainty increases with decreasing procoagulant activity). As such, K1 values compiled in Table 1 were interpreted as best estimates. However, lines drawn through the data of Figure 2 calculated from the model using the mean K1 values listed in Table 2 were observed to adequately represent trends in the experimental data for all test surfaces, including OTS for which decrease in coagulation time with surface area was barely discernable.
Table 2.
K1 values for the solid procoagulants
| Procoagulant Surface | Sessile Drop Contact Angle | Plasma type | K1 (μM/min) |
|---|---|---|---|
| Glass | <5° | dPKPPP | (7.7 ± 2.7) × 10−2 |
| RdPKPPP | (1.5 ± 0.4) × 100 | ||
| APS | 63° ± 3° | dPKPPP | (2.6 ± 1.1) × 10−3 |
| RdPKPPP | (1.7 ± 1.4) × 10−2 | ||
| OTS | 108° ± 3° | dPKPPP | (1.3 ± 0.9) × 10−3 |
| RdPKPPP | (7.0 ± 5.3) × 10−3 |
Values are displayed as mean ± S.D.
Figure 2.
Surface area titration (SAT) in dPKPPP and RdPKPPP showing experimental CT vs. added surface area of three different procoagulant materials. Data for glass-particles (circles, ●, o), APS particles (triangles, ▼, ▽) and OTS particles (squares, ■, □) are shown, where solid and open symbols represent measurements in RdPKPPP and dPKPPP, respectively. The smooth lines represent CT values calculated from the model using the mean K1 values listed in Table 2. (All data expressed as mean ± S.D. from at least three individual measurements).
Calculation of FXIIa generation
The total amounts of FXIIa generated by each solid procoagulant were calculated using the nominal K1 values listed in Table 2. Although autohydrolysis of FXII by FXIIa may be a significant reaction in neat protein solutions, it has been observed to be insignificant in plasma16,17. Therefore, autohydrolysis may be considered as a negligible contributor to FXII activation in material-induced plasma coagulation. Thus, the calculated yield of FXIIa in RdPKPPP (equivalent to PPP) represented the total amount of enzyme produced by both the autoactivation and reciprocal-activation pathways, while enzyme produced in response to procoagulants in dPKPPP corresponded to the amount of FXIIa generated via autoactivation alone (reciprocal-activation cannot occur in the absence of PK). The difference between these two quantities was therefore the amount of FXIIa produced by reciprocal-activation at a given surface area of solid procoagulant.
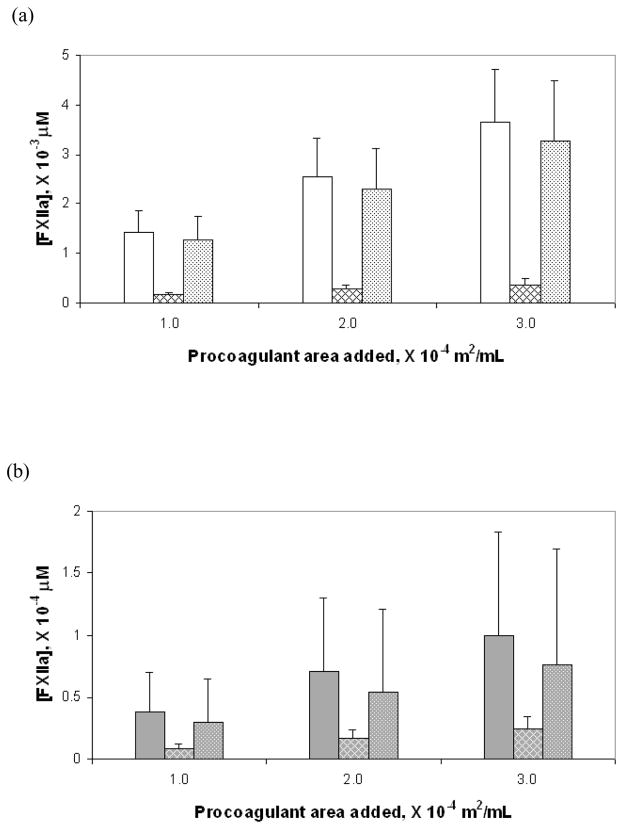
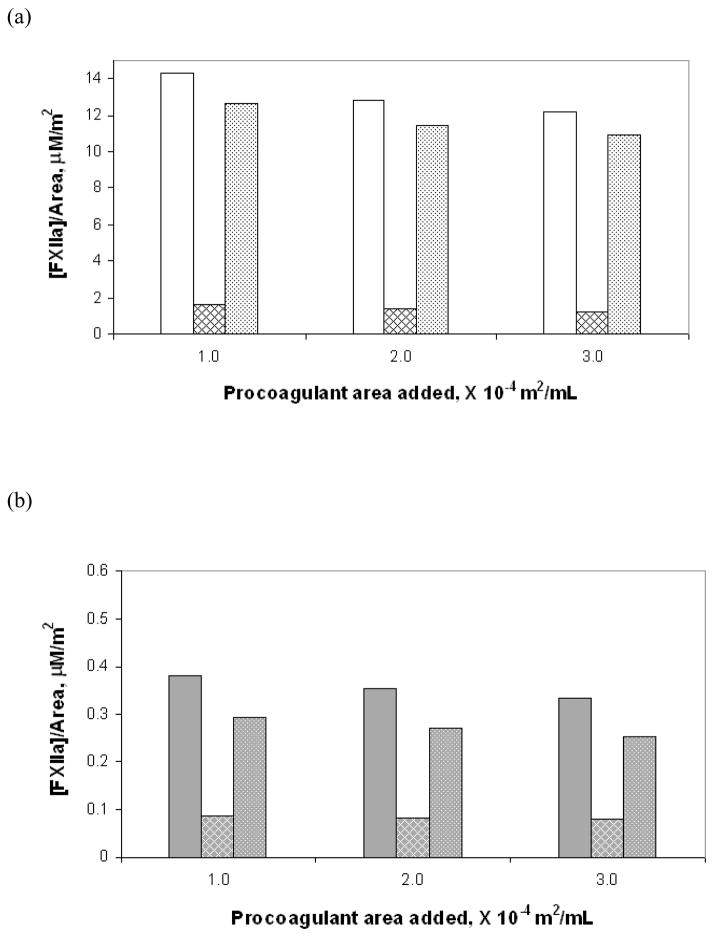
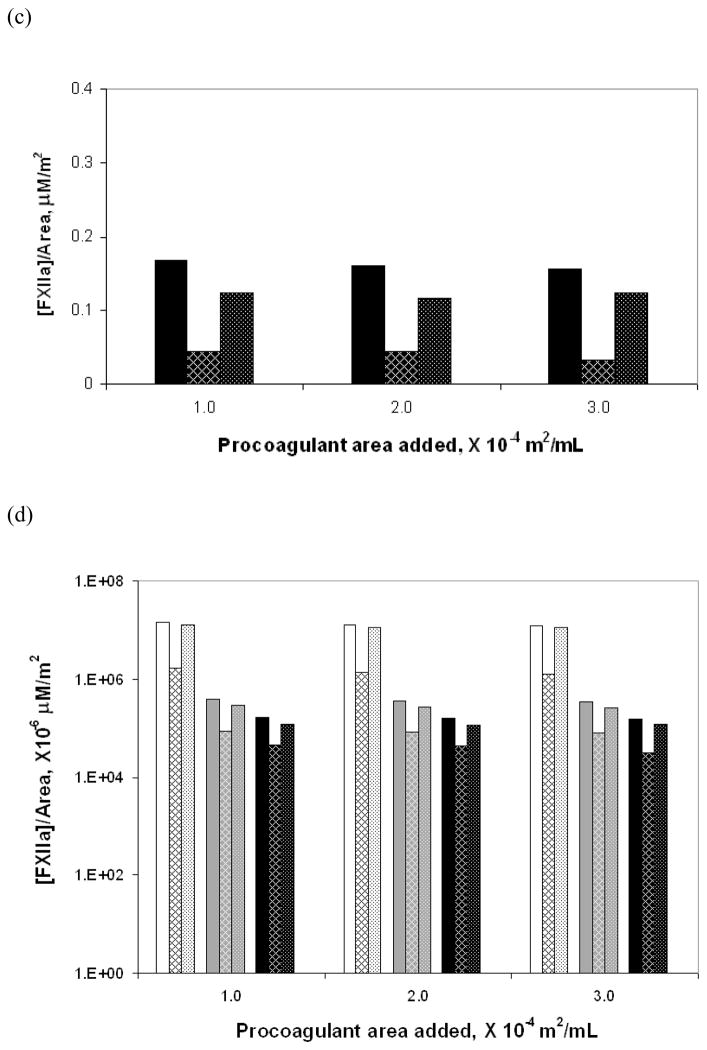
Figure 3a–c compares in bar-graph form the total amounts of FXIIa generated for the three test surfaces at three different surface area values as well as the amounts generated individually by the autoactivation and reciprocal-activation pathways. Note the vastly different scales on the y-axis for the three different materials. Figure 3d is compilation of data from Figures 3a–c presented as a semi-log plot in order to more clearly illustrate the trends in enzyme production due to change in water-wettability of the activating surface. Error bars represent the total uncertainty in calculated values based on rigorous propagation of experimental error. Differences in the amounts of FXIIa generated by autoactivation and reciprocal-activation were not statistically significant, as can be readily ascertained by inspection of Figure 3d. Nevertheless, nominal values were found to be of interpretive value as further elaborated in the Discussion section. Figure 4 plots the nominal amount of enzyme produced under each condition, normalized to the procoagulant surface area using the same illustrative scheme as Figure 3. Note the declining relationship between enzyme production in total and along each of the pathways with decreasing water-wettability of the activating surface, consistent with catalytic efficiency scaling exponentially with the surface water-wettability24.
Figure 3.
FXIIa produced in total and via the autoactivation and reciprocal-activation pathways at three surface area values for the each of the three solid test procoagulants: (a) glass, (b) APS and (c) OTS. The bars show in groups of three at each surface area the total amount of enzyme (solid bar), enzyme produced by autoactivation (hatched bar) and enzyme produced by reciprocal-activation (dotted bar). (d) is a compilation of values from the different surface presented using a semi-log plot for ease of comparison among the procoagulants showing glass (white background), APS (gray background) and OTS (black background) from left to right at each surface area.
Figure 4.
FXIIa produced per unit surface area in total and via the individual pathways at three surface area values for each of the three solid test procoagulants: (a) glass, (b) APS and (c) OTS. The bars show in groups of three at each surface area the total amount of enzyme (solid bar), enzyme produced by autoactivation (hatched bar) and enzyme produced by reciprocal-activation (dotted bar). (d) is a compilation of values from the different surface presented using a semi-log plot for ease of comparison among the procoagulants showing glass (white background), APS (gray background) and OTS (black background) from left to right at each surface area.
DISCUSSION
Prospective surface engineering of cardiovascular biomaterials with improved hemocompatibility requires a secure understanding of the biochemical reactions leading to contact activation of blood plasma. Recent investigations show that the traditional mechanism of contact activation requires substantial revision to account for new experimental findings. In particular, the discovery that FXII autoactivation is not, in fact, specific to hydrophilic surfaces20 refocuses attention on the surface-catalyzed biochemical reactions which generate the FXIIa that triggers the intrinsic cascade of plasma coagulation.
Results summarized herein corroborate previous work showing that the efficiency of contact activation of whole plasma scales exponentially with surface energy (water wettability) of blood-contacting materials (procoagulants) 17,21 and confirms the expectation that increasing activation is linked to increased production of FXIIa. Furthermore, we show that FXIIa predominately (75%) arises from kallikrein-mediated reciprocal activation, with the remaining 25% most probably due to autoactivation ( ) since autohydrolysis (FXIIa + FXII → 2FXIIa) is an inconsequential reaction in plasma. The outcome of our efforts to quantify differences in pathway utilization (measured by the ratio of reciprocal activation to autoactivation) at test surfaces spanning the full range of observable wettability was not statistically significant because the exponential-like relationship between plasma activation and surface energy causes the slight procoagulation by hydrophobic materials to be very difficult to precisely measure with techniques applied herein. Nevertheless, we contend that nominal values obtained from mathematical modeling of experimental data are of value because these results provide some insights into how the intrinsic pathway actually works in whole plasma where no such other source of information is currently available. We infer from this analysis that pathway utilization does not change as a function of procoagulant surface energy and that activation at hydrophobic surfaces is not fundamentally different from activation at hydrophilic surfaces. Efficiency of plasma activation by hydrophobic surfaces is much lower because autoactivation, which apparently feeds reciprocal activation, is substantially attenuated in the presence of plasma proteins{Zhuo, 2007 #31}. This interpretation is completely consistent with the observation that FXII activation in neat buffer solutions does not depend on procoagulant surface energy{Zhuo, 2006 #6}.
Modeling material-induced coagulation
We used a previously-published model 17 of material-induced coagulation. This model construes activation to consist of two major steps. The first step is activation of plasma FXII by either autoactivation (in both plasma types) or reciprocal-activation (in RdPKPPP alone) in response to interactions with procoagulant materials. The second step is processing this newly-formed FXIIa through a ‘gray box’ of linked enzyme reactions leading to formation of a fibrin clot.
The parameters a, b and c that describe the processing of FXIIa were obtained by fitting the model to FXIIa titration experiments performed in dPKPPP and RdPKPPP. Use of depleted and reconstituted plasma sources allowed us to correct for background activation (due to inevitable activation by vials in which experiments were carried out) and thereby account for differences in the processing of FXIIa in the presence and absence of PK.
Calculation of FXIIa generation
The K1 values listed in Table 2 indicate that catalytic efficiency of test surfaces increases sharply with increasing water-wettability in both dPKPPP and RdPKPPP, consistent with trends previously observed in whole plasma 17,21 mentioned above When the amount of FXIIa generated is very small, the measured coagulation response is only marginally faster than the background response. As a result, statistical significance of differences between materials is low. But the importance of inefficient activation at hydrophobic materials in development of cardiovascular biomaterials should not be underestimated because it suggests that chronic exposure to relatively hemocompatible materials will eventually lead to coagulation, perhaps accounting for formation of thrombus on all known biomaterials. Bearing in mind experimental uncertainties, our results suggest that the amount of FXIIa produced per-unit-area for a given procoagulant material is constant and the relative proportion of pathway activation is constant among materials. This interpretation implies that the activating surface does not play a significant role in propagating reactions of the activation complex, as envisioned in the traditional mechanism of contact activation, beyond catalyzing production of a bolus of FXIIa in proportion to surface area and wettability. Otherwise, if an activating surface behaved in allosteric-like manner, increasing FXIIa per-unit-area would be anticipated. All taken together, evidence at hand corroborates our previous findings 17,21 and leads us to the conclusion that autoactivation is the only significant biochemical reaction occurring at an activating surface and that reciprocal activation is not surface mediated.
CONCLUSIONS
The amount of FXIIa produced in plasma by autoactivation and reciprocal-activation routes were calculated by mathematical-modeling of plasma coagulation induced by contact with procoagulant surfaces spanning the full range of observable hydrophilicity. Results of this study corroborate and extend previous studies, together suggesting that:
Total FXIIa yield scales with increasing surface area of a particular solid procoagulant, so that the normalized amount of enzyme produced per-unit-area procoagulant is constant.
For any given procoagulant surface area, the total amount of FXIIa generated increases with increasing surface water-wettability.
Kal-mediated reciprocal-activation pathway is the principal contributor to FXIIa generation at all procoagulant test surfaces studied (75%) and the amount of enzyme generated by this pathway scales with procoagulant hydrophilicity.
Autoactivation primes Kal-mediated reciprocal-activation by producing approximately 25% of the total FXIIa yield of the intrinsic pathway. Autoactivation scales with procoagulant hydrophilicity which, in combination with finding (3), accounts for (1) above.
Autoactivation is the only significant biochemical reaction occurring at an activating surface and that reciprocal activation is not surface mediated.
These results provoke a revision of the standard understanding of hemocompatibility and restatement of biochemical mechanism of blood-plasma activation. Better understanding of activation mechanisms may lead to improved strategies for the surface engineering of cardiovascular biomaterials.
Table 3.
Estimated fraction of enzyme generation via reciprocal-activation.
| Surface | Sessile Drop Contact Angle | [reciprocal-FXIIa] / [total-FXIIa] (%) |
|---|---|---|
| Glass | <5° | ~ 89 |
| APS | 63° ± 3° | ~ 76 |
| OTS | 108° ± 3° | ~ 72 |
Acknowledgments
The authors gratefully acknowledge financial support for this work provided by the National Institutes of Health (RO1 HL69965).
Footnotes
A Contribution from the Hematology at Biomaterial Interfaces Research Group
References
- 1.Andrade JD, Coleman DL, Didisheim P, Hanson SR, Mason R, Merrill E. Blood-materials interaction - 20 years of frustation. Transactions of the American Society of Artificial Internal Organs. 1981;27:659–662. [PubMed] [Google Scholar]
- 2.Ratner BD. The blood compatibility catastrophe. Journal of Biomedical Materials Research. 1993;27(3):283–7. doi: 10.1002/jbm.820270302. [DOI] [PubMed] [Google Scholar]
- 3.Ratner BD. Blood compatibility- a perspective. Journal of Biomaterials Science: Polymer edition. 2000;11(11):1107–1119. doi: 10.1163/156856200744219. [DOI] [PubMed] [Google Scholar]
- 4.Galloway AC, Anderson RV, Grossi EA, Spencer FC, Colvin SB. Acquired heart diseases. In: Schwartz SI, editor. Principles of Surgery. New York: McGraw Hill; 1999. pp. 845–908. [Google Scholar]
- 5.McBride LR, Naunheim KS, Fiore AC, Moroney DA, Swartz MT. Clinical Experience With 111 Thoratec Ventricular Assist Devices. Annals of Thoracic Surgery. 1999;67:1233–9. doi: 10.1016/s0003-4975(99)00246-5. [DOI] [PubMed] [Google Scholar]
- 6.Colman RW, Clowes AW, George JN, Hirsh J, Marder VJ. Overview of Hemostasis. In: Colman RW, Hirsh J, Marder VJ, Clowes AW, George JN, editors. Hemostasis and Thrombosis- Basic Principles and Clinical Practice. Philadelphia: Lippincott William and William; 2001. pp. 103–122. [Google Scholar]
- 7.Davie EW, Fujikawa K, Kurachi K, Kisiel W. The role of serine proteases in the blood coagulation cascade. Advances in Enzymology & Related Areas of Molecular Biology. 1979;48:277–318. doi: 10.1002/9780470122938.ch6. [DOI] [PubMed] [Google Scholar]
- 8.Kaplan AP. Initiation of the intrinsic coagulation and fibrinolytic pathways of man: the role of surfaces, Hageman factor, prekallikrein, high molecular weight kininogen, and factor XI. Progress in Hemostasis and Thrombosis. 1978;4:127–175. [PubMed] [Google Scholar]
- 9.Griffin JH, Cochrane CG. Recent advances in the understanding of contact activation reactions. Seminars in Thrombosis and and Hemostasis. 1979;5(4):254–273. doi: 10.1055/s-0028-1087158. [DOI] [PubMed] [Google Scholar]
- 10.Williams DF. Blood physiology and biochemistry: hemostasis and thrombosis. In: Williams DF, editor. Blood Compatibility. Boca Raton, Florida: CRC Press; 1987. pp. 5–35. [Google Scholar]
- 11.Samuel M, Pixley RA, Villanueva MA, Colman RW, Villanueva GB. Human factor XII (Hageman factor) autoactivation by dextran sulfate. Circular dichroism, fluorescence, and ultraviolet difference spectroscopic studies. Journal of Biological Chemistry. 1992;267(27):19691–19697. [PubMed] [Google Scholar]
- 12.Vroman L. Effects of hydrophobic surfaces upon blood coagulation. Thrombosis et Diathesis Haemorrhagica. 1964;10:455–493. [PubMed] [Google Scholar]
- 13.Griffin JH. Role of surface in surface-dependent activation of Hageman factor (blood coagulation Factor XII) Proceedings of the National Academy of Sciences USA. 1978;75(4):1998–2002. doi: 10.1073/pnas.75.4.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiggins RC, Cochrane CG. The autoactivation of rabbit Hageman factor. Journal of Experimental Medicine. 1979;150(5):1122–33. doi: 10.1084/jem.150.5.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller G, Silverberg M, Kaplan AP. Autoactivatability of human Hageman factor (factor XII) Biochemical & Biophysical Research Communications. 1980;92(3):803–10. doi: 10.1016/0006-291x(80)90774-3. [DOI] [PubMed] [Google Scholar]
- 16.Zhuo R, Vogler EA. Practical application of a chromogenic FXIIa assay. Biomaterials. 2006;27:4840–4845. doi: 10.1016/j.biomaterials.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Guo Z, Bussard KM, Chatterjee K, Miller R, Vogler EA, Siedlecki CA. Mathematical modeling of material-induced blood plasma coagulation. Biomaterials. 2006;27:796–806. doi: 10.1016/j.biomaterials.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 18.Tans G, Rosing J, Berrettini M, Lammle B, Griffin JH. Autoactivation of human plasma prekallikrein. Journal of Biological Chemistry. 1987;262(23):11308–14. [PubMed] [Google Scholar]
- 19.Dunn JT, Silverberg M, Kaplan AP. The cleavage and formation of activated human Hageman factor by autodigestion and by kallikrein. Journal of Biological Chemistry. 1982;257(4):1779–1784. [PubMed] [Google Scholar]
- 20.Zhuo R, Siedlecki CA, Vogler EA. Autoactivation of blood factor XII at hydrophilic and hydrophobic surfaces. Biomaterials. 2006;27(24):4325–4332. doi: 10.1016/j.biomaterials.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Chatterjee K, Vogler EA, Siedlecki CA. Procoagulant activity of surface-immobilized Hageman factor. Biomaterials. 2006;27(33):5643–5650. doi: 10.1016/j.biomaterials.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Zhuo R, Miller R, Bussard KM, Siedlecki CA, Vogler EA. Procoagulant stimulus processing by the intrinsic pathway of blood plasma coagulation. Biomaterials. 2005;26(16):2965–2973. doi: 10.1016/j.biomaterials.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Dobrovolsky AB, Titaeva EV. The fibrinolysis system: regulation of activity and physiologic functions of its main components. Biochemistry-Russia. 2002;67(1):99–108. doi: 10.1023/a:1013960416302. [DOI] [PubMed] [Google Scholar]
- 24.Vogler EA, Graper JC, Harper GR, Sugg HW, Lander LM, Brittain WJ. Contact activation of the plasma coagulation cascade I. Procoagulant surface chemistry and energy. Journal of Biomedical Materials Research. 1995;29:1005–1016. doi: 10.1002/jbm.820290813. [DOI] [PubMed] [Google Scholar]