Abstract
The majority of mild cognitive impairment (MCI) studies use baseline and one follow-up measurement to determine the clinical course of the disorder. This report of MCI clinical course is based on the a statistical evaluation of multiple neurocognitive tests over a 60 month period in elderly normal and MCI cohorts. The data includes serial informant-based measures (Clinical Dementia Rating [CDR]) and a comprehensive battery of neuropsychological tests analyzed by two different regression methods. Twenty-nine elderly participants entered the study as neurocognitively normal; 26 remained normal, 2 progressed to MCI, and 1 progressed to dementia. Eighty-three participants entered the study as multiple domain MCI cases; 10 became normal, 46 remained MCI, and 27 progressed to dementia. Three of the 27 demented died with full necropsies performed (one case was progressive supranuclear palsy and two confirmed Alzheimer’s disease with severe cerebral amyloid angiopathy (CAA)). Without serial measures, 1 in 8 MCI could be misclassified as “stable MCI” despite reverting to normal. The stable MCI cohorts did not benefit from practice effects though the normal subjects did. Applying Classification and Regression Tree (CART) analysis enabled prediction of the endpoint status of participants from baseline values with 78.6% accuracy. The fluctuating cognitive status of the multiple domain MCI cases implies a remitting pathologic process with elements of recovery consistent with a progressive microvasculopathy such as CAA.
Keywords: Informant-based evaluation, mild cognitive impairment, misdiagnosis, neuropsychological testing, practice effects, serial neurocognitive evaluations
INTRODUCTION
The significance and predictive value of a baseline diagnosis of mild cognitive impairment (MCI) is controversial. Though MCI is defined as “a transitional state between the cognitive changes of normal aging and the earliest features of Alzheimer’s disease (AD)” [1] the natural history of individual cases is known to vary, raising questions regarding the stability of the diagnosis [2, 3]. The National Institutes of Health State-Of-The-Science Conference concluded that “MCI cognitive decline is multicausal, and mild cognitive impairment may not lead to dementias such as Alzheimer’s disease” [4]. Others maintain that MCI is early AD since brain autopsy studies show the pathologic hallmarks of AD [5]. The purpose of this study is to profile the extended longitudinal cognitive status of MCI by serially testing elderly normals and cases classified as multiple domain amnestic MCI [6]. The cognitive status of the cohorts has been correlated with changes in regional brain iron levels and serologic biomarkers and have been reported [7, 8].
PARTICIPANTS AND METHODS
Participants
Our goal was to evaluate the cognitive states of participants approximately every eight months over a 60 month period. (Table 1) Our Normal and MCI cohorts were selected from a pool of 1348 individuals screened to evaluate general health, memory complaints, and willingness to participate over the several year duration of the study with a study partner. The Institutional Review Board of Loma Linda University Medical Center approved the study protocol. Referrals were obtained from local medical practitioners, memory clinics advertised in senior community living centers, health fairs, and advertising media. Prospective participants were screened with an interview, a Mini Mental Status Examination (MMSE) [9] and Logical Memory A I & II subtest from the Weschler Memory Scale-III [10]. Subsequently, 273 people selected from the initial screen had a neuropsychological battery and informant-based Clinical Dementia Rating (CDR). Normal participants had an MMSE = 30, raw scores on Logical Memory II ≥10 for ≥16 years of education, ≥6 for 8 to 15 years of education and ≥4 for ≤7 years of education, CDR Memory Score of zero, Global CDR = 0 and Sum of Boxes ≤1 [1]. The MCI cohort was non-demented but with a complaint of memory loss from the person, family, or physician. Scores for this group were MMSE ranging from 24 to 30, a raw score on Logical Memory II ≤9 for 16 years of education, ≤5 for 8 to 15 years of education and ≤2 for ≤7 years of education, or a CDR Memory Score of 0.5–1.0 with Sum of Boxes <3.5 [1]. All MCI subjects were in the amnestic and multiple domain MCI category.
Table 1.
Participant Evaluations
| MCI Subjects | Normal |
|---|---|
| Average Time Between Follow-up Visits: | Average Time Between Follow-up Visits: |
| 8.20 months (SD, 2.9) | 8.01 months (SD, 2.9) |
| Re-evaluated 1 time: 8 | Re-evaluated 2 times: 2 |
| Re-evaluated 2 times: 11 | Re-evaluated 3 times: 3 |
| Re-evaluated 3 times: 9 | Re-evaluated 4 times: 7 |
| Re-evaluated 4 times: 16 | Re-evaluated 5 times: 4 |
| Re-evaluated 5 times: 11 | Re-evaluated 6 times: 6 |
| Re-evaluated 6 times: 9 | Re-evaluated 7 times: 2 |
| Re-evaluated 7 times: 6 | Re-evaluated 8 times: 1 |
| Re-evaluated 9 times: 2 | |
| Re-evaluated 10 times: 1 |
All CDR evaluations were videotaped by clinicians certified for the test. Additional tests included: North American Adult Reading Test (NAART) to assay intellectual functioning (≥low average range), Word Fluency, Phonemic and Semantic, Wisconsin Card Sorting Test, Trailmaking Test A & B, Boston Naming Test, Short Form, Draw-A-Clock [11], Depressive Features Battery (DFB), Version II, and Geriatric Depression Scale. The NAART and the DFB screen eliminated low intellectual functioning and major depressive subjects. All neuropsychological scoring was conducted by Dr. William Britt. Exclusion criteria consisted of history of dementia, neurological disease, head trauma, depression within the past year, schizophrenia, alcohol or substance abuse, unstable medical conditions (hypertension, diabetes), contraindication to MR study, neuroleptics, chronic anxiolytics, or sedative hypnotics. Individuals with known iron metabolic abnormalities, such as hemochromatosis or anemia were excluded.
After a consensus conference 29 normal and 83 MCI participants were entered into the study. Sixteen MCI participants withdrew from the study over the 5 year duration due to illness, inability to undergo an MRI, loss of transportation/collateral source, loss of interest, or leaving the area. All normals continued in the study.
All of the multiple domain MCI participants had the following characteristics: 1) Memory complaint confirmed objectively (CDR memory = 0.5); 2) Unimpaired activities of daily living; 3) Mild to minimal deficits in other cognitive domains; 4) Abnormal Sum of Boxes (SOB) for memory on the CDR; 5) Global CDR = 0.5; and 6) Non-demented based on consensus clinical judgment. MCI participants that progressed to dementia were defined as having a CDR SOB ≥3.5, neuropsychological tests diagnostic for dementia, and Logical Memory II raw score low to zero.
Statistical methods
Two different statistical models, the slope-intercept and CART (Classification and Regression Tree), were used to analyze cognitive changes over time. Endpoints for study participants are defined as Normal, Stable MCI, or AD.
The first model, the slope-intercept model uses endpoint status, demographic information and number of months in the study to model changes in the neuropsychological test battery for each endpoint group (Normal, MCI, or AD) over time. This model will be fit for each neuropsychological test in the test battery. Algebraically the model is expressed best by the following equation:
where Yijk is a particular test battery response of the ith participant at the jth visit classified as the kth endpoint status (i = 1, 2, …; j = 1, 2, …; k =1, 2, 3). Let (X1,ik, X2,ik) be a dummy variable to indicate the endpoint status of the ith participant, i.e. (X1,ik, X2,ik) = (1, 0) for participants who were classified as Normal at their last visit, (X1,ik, X2,ik) = (0, 1) for MCIs, and (X1,ik, X2,ik) = (0, 0) for ADs. Further, let X3,ik = 1 if the ith participant is male, 0 if female; let X4,ik = (EndpointAge + StartAge)/2; let X5,ik = Number of years of education; and let Tijk = Number of months the ith participant has been in the study at the jth visit. Then, sik and εijk are independent error terms where are the within subject error terms for a given endpoint status and are the overall error terms for a given endpoint status. This covariance structure enables an analysis of the variation between and within the three endpoint groups. With this model it is also easy to compare the slopes of the three endpoint statuses.
The slope of Normal participants over time is given by: γ1 + γ2; the slope of MCI participants over time is given by: γ1 + γ3; and the slope of AD participants over time is given by: γ1. Slopes can be compared to determine if Normals, MCIs, or ADs show practice effects of improvement (i.e., a positive slope) over time, fail to benefit (i.e., a flat slope), or worsen (i.e., a negative slope) on a particular test within the battery (Table 2).
Table 2.
Hypothesis Tests
| Hypotheses | Interpretation |
|---|---|
| H01: Normals Flat ↔ H01: γ1 + γ2 = 0 | If accepted, Normals will show no improvement over time |
| H02: MCIs Flat ↔ H02: γ1 + γ3 = 0 | If accepted, MCIs will show no improvement over time |
| H03: ADs Flat ↔ H03: γ1 = 0 | If accepted, ADs will show no improvement over time |
| H04: Normal Slope = MCI Slope ↔ H04: γ1 + γ2 = γ1 + γ3 ↔ H04 : γ2 = γ3 | If accepted, Normals and MCIs have the same slope |
| H05: MCI Slope = AD Slope ↔ H05: γ1 + γ3 = γ1 ↔ H05: γ3 = 0 | If accepted, MCIs and ADs have the same slope |
| H06: Normal Slope = AD Slope ↔ H06: γ2 + γ2 = γ2 ↔ H06: γ2 = 0 | If accepted, Normals and ADs have the same slope |
| H07: All Slope Equal ↔ H07: γ1 = γ1 + γ2 = γ1 + γ3 | If accepted, then all three endpoints status have the same slope |
Note. The formal tests with respect to model parameters and their interpretations are given above.
In our second model, a post-hoc analysis of putative prognostic factors by CART identifies factors predictive of progression to AD, MCI, or Normal. CART provides a “decision tree” predictive model by repeated mapping of subdivided observations to final endpoint statuses. While in our first model, we treated endpoint status as a covariate to predict outcomes in the neuropsychological test battery, here we use the test battery and demographic information to predict endpoint status.
Within CART, diagnostic information incorporated from successive visits is subdivided at optimal cut-points (node splits) forming a classification tree. Node splits in the decision tree are constructed by searching among variables (participant test scores and demographic information) for those to best differentiate endpoint groups. After the initial split, CART searches for another variable (possibly the same one) to further split the data until all participants are assigned to endpoint status. Results can be summarized in a flow chart that complements standard regression techniques.
Further, additional classification trees can be constructed by incorporating subsequent visit information. For example, Phase 1 incorporates just the information at baseline (one visit), Phase 2 incorporates information from the first two visits by taking the mean of all test scores after two visits, Phase 3 incorporates information from the first three visits by taking the mean of all test scores after three visits, and so on for additional phases 4 and 5.
RESULTS
The demographics of our study cohorts are given in Table 3. The study was initiated with 112 participants, 83 multiple domain amnestic (mda) MCI, and 29 Normal. Of 29 participants classified as normal at baseline, 26 remained normal, 2 progressed to MCI, and 1 progressed to dementia at endpoint (Table 4). Of the 83 participants entering the study as MCI, 10 reverted to normal, 46 remained MCI, and 27 progressed to dementia (Table 4). Three of the 27 demented cases died and had full necropsies (one case was progressive supranuclear palsy and two confirmed AD). MCI participants that progressed to dementia were defined as having a CDR SOB of 3.5, Logical Memory II raw score low to zero, and demented by clinical judgment. MCI participants progressed to dementia with a 15% annual conversion rate over 60 months observation period. The 10 baseline MCIs, who were later re-classified to normal, exhibited normal scores on at least 3 (minimum 3, maximum 7) of their subsequent neuropsychological exams (Table 5).
Table 3.
Demographics of MCI and Control participants at baseline, data reported as n (%) or mean (range) includes sex, age, education and ethnicity
| MCI (n = 83) | Normal (n = 29) | p-value | |
|---|---|---|---|
| Gender: | |||
| Female | 35 (42.2%) | 17 (58.6%) | |
| Male | 48 (57.8%) | 12 (41.4%) | 0.13 |
| Age at enrollment (in years) | 75.8 (54–88) | 72.9 (54–85) | 0.06 |
| Education (in years) | 14.2 (6–20) | 14.6 (9–22) | 0.62 |
| Ethnicity: | |||
| White | 75 (90.4%) | 22 (75.9%) | |
| African American | 2 (2.4%) | 1 (3.5%) | |
| Hispanic | 5 (6.0%) | 4 (13.8%) | |
| American Indian | 1 (1.2%) | 1 (3.5%) | |
| Other | 0 (0%) | 1 (3.5%) | NA |
Table 4.
Baseline status vs. Endpoint status
| Normal ENDPOINT | MCI ENDPOINT | AD ENDPOINT | Total | |
|---|---|---|---|---|
| Normal BASELINE | 26 | 2 | 1 | 29 |
| MCI BASELINE | 10 | 46 | 27 | 83 |
Note. Of the 29 normal at baseline, 26 remained normal, 2 progressed to MCI and 1 to AD. Of the 83 MCI at baseline, 10 reverted to normal, 46 remained MCI and 27 progressed to AD.
Table 5.
CDR History of 10 Baseline MCI participants reverting to Normal status at endpoint
| Participant | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.5 (1.0) | 0 (0) | 0 (0.5) | 0 (0) | 0 (0.5) | 0 (0) | 0.5 (0.5) | 0 (0) |
| 2 | 0.5 (1.0) | 0 (0.5) | 0 (0.5) | 0 (0.5) | 0 (0.5) | 0 (0.5) | 0 (0) | |
| 3 | 0.5 (1.0) | 0.5 (1.0) | 0.5 (0.5) | 0.5 (0.5) | 0 (0) | 0 (0) | 0 (0.5) | |
| 4 | 0.5 (1.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| 5 | 0.5 (0.5) | 0 (0) | 0.5 (1.0) | 0 (0) | 0 (0.5) | 0 (0) | 0 (0) | 0 (0) |
| 6 | 0.5 (1.0) | 0.5 (1.0) | 0 (0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| 7 | 0.5 (1.5) | 0 (0) | 0.5 (1.0)* | 0 (0) | 0 (0) | |||
| 8 | 0.5 (1.0) | 0.5 (1.5) | 0 (0) | 0.5 (0.5) | 0 (0) | 0 (0) | 0.5 (0.5) | |
| 9 | 0.5 (0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0.5) | |||
| 10 | 0.5 (0.5) | 0.5 (1.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0.5) |
Note. Global CDR without parenthesis, () CDR sum of boxes (SoB),
No memory impairment, up to eight visits recorded.
The slope intercept model was fit for the results of each cognitive test over time. The test battery included: Q1: Logical Memory, Q2: Set Shifting (Trails-B), Q3: Word Fluency-Phonemic, Q4: Word Fluency-Semantic, Q5: Confrontational Naming, Q6: Perseverative Errors, Q7: Categories Complete, Q8: Failure to Maintain Set, Q12: Draw-A-Clock, and Q14: CDR Sum of Boxes. Any transformations of the dataset are given in Table 6. MCI participants evaluated less than three times were removed, leaving a sample size of n = 89. Table 6 gives two-tailed p-values for the tests. Bolded values are significant at the < 0.05 confidence level. Table 7 gives slope estimates. We omitted Q9: Learn-to-Learn, Q10: GDI Depression, Q11: NART(IQ), and Q13: Clinical Dementia Rating from the analysis.
Table 6.
Results of hypothesis tests
| Data Type | Transformation | Normals Flat | MCIs Flat | ADs Flat | Normals = MCIs | MCIs = ADs | Normals = ADs | All equal | |
|---|---|---|---|---|---|---|---|---|---|
| Q1 | Raw Score | Square Root | <0.0001 | 0.3825 | 0.0168 | <0.0001 | 0.0195 | <0.0001 | <0.0001 |
| Q2 | T Score | Remove outliers | 0.0018 | 0.2386 | 0.0095 | 0.1366 | 0.1290 | 0.0004 | 0.0003 |
| Q3 | Raw Score | None | 0.0003 | 0.3144 | 0.0080 | 0.0676 | 0.0094 | <0.0001 | 0.0002 |
| Q4 | Raw Score | None | 0.9969 | 0.1436 | 0.0021 | 0.0012 | 0.1946 | 0.0010 | 0.0343 |
| Q5 | Raw Score | None | 0.0982 | 0.1880 | 0.0007 | 0.0220 | 0.0279 | 0.0002 | 0.0004 |
| Q6 | Raw Score | Log | 0.0002 | 0.4350 | 0.4597 | 0.0014 | 0.2819 | 0.0040 | 0.0129 |
| Q7 | Raw Score | None | 0.7247 | 0.4187 | 0.0022 | 0.0148 | 0.0585 | 0.0145 | 0.0465 |
| Q8 | Raw Score | None | 0.5357 | 0.5976 | 0.4503 | 0.4746 | 0.3589 | 0.7522 | 0.6150 |
| Q12 | Raw Score | None | 0.0003 | 0.1302 | <0.0001 | 0.0261 | 0.0238 | 0.0966 | 0.0742 |
| Q14 | Raw Score | None | <0.0001 | 0.3634 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Note: p-values less than 0.05 are bolded. Q1-Logical Memory, Q2- Set Shifting (Trails-B), Q3-Word Fluency-Phonemic, Q4-Word Fluency-Semantic, Q5- Confrontational Naming, Q6-Perseverative Errors, Q7-Categories Complete, Q8- Failure to Maintain Set, Q12- Draw-A-Clock, and Q14-CDR Sum of Boxes.
Table 7.
Regression slope estimates
| Question | Slope (Normal) | Slope (MCI) | Slope (AD) |
|---|---|---|---|
| Q1 | 0.01372 | 0.003547 | −0.01014 |
| Q2 | 0.1232 | −0.08005 | −0.2639 |
| Q3 | 0.09112 | 0.04000 | −0.1062 |
| Q4 | 0.00006 | −0.02847 | −0.06544 |
| Q5 | 0.006012 | −0.01010 | −0.04140 |
| Q6 | −0.00737 | −0.00200 | 0.001873 |
| Q7 | −0.00207 | −0.00623 | −0.02915 |
| Q8 | −0.00403 | 0.004736 | −0.00792 |
| Q12 | −0.01791 | −0.01006 | −0.03266 |
| Q14 | −0.00565 | 0.00420 | 0.09543 |
Note. This table provides only the slope parameter estimates; formal hypothesis tests of slope equivalence are described in Table 4 and the results of those tests are provided in Table 5. Q1-Logical Memory, Q2- Set Shifting (Trails-B), Q3-Word Fluency-Phonemic, Q4-Word Fluency-Semantic, Q5- Confrontational Naming, Q6-Perseverative Errors, Q7-Categories Complete, Q8- Failure to Maintain Set, Q12-Draw-A-Clock, and Q14-CDR Sum of Boxes.
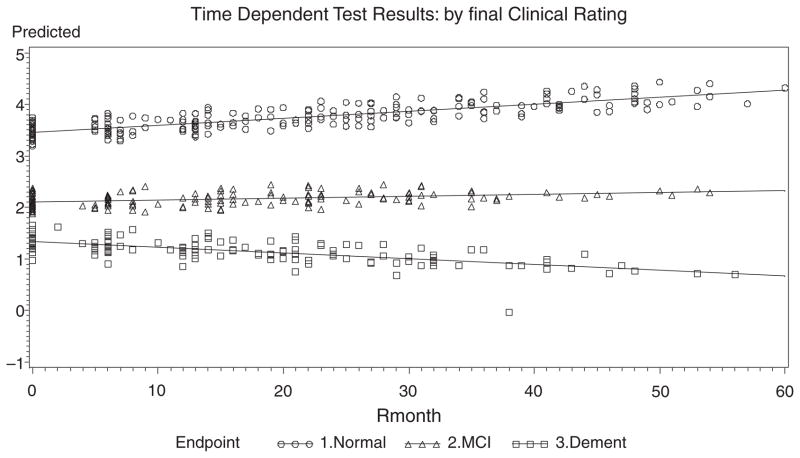
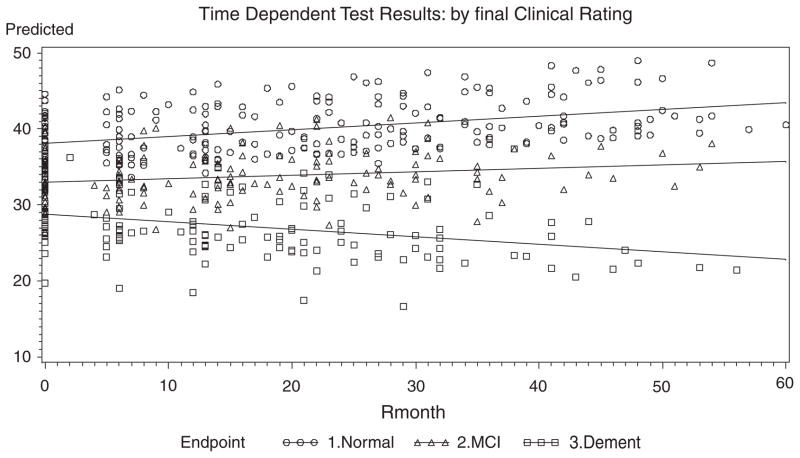
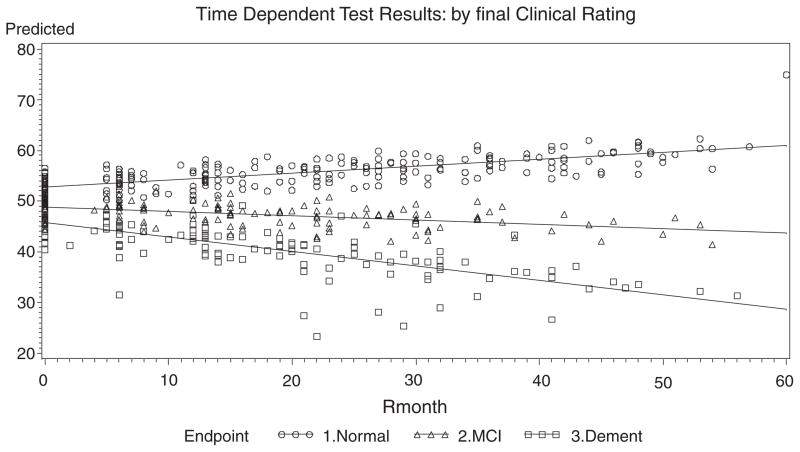
For Q1: Logical Memory II, the slope of the Normals, MCIs, ADs are all different from each other. The positive slope for Normals indicates improvement over time, whereas MCI slopes are flat and ADs decline. The slope patterns are noted for Q2: Set Shifting (Trails-B), and Q3: Word Fluency-Phonemic, displayed in Figs. 1–3. Similar slope patterns occur among Q4: Word Fluency Semantic, Q5: Confrontational Naming and Q7: Categories Complete are also quite similar to each other in that the Normals and MCIs stay somewhat constant over time, whereas the ADs are worsening. For Q6: Perseverative Errors, only the Normal slope is not flat over time. Interestingly, Normals are the only group that declines over time, whereas the ADs and the MCIs remain somewhat constant over time. In Q8: Failure to Maintain Set, all of the slopes are flat and equal, and thus there is neither improvement nor decline for all three endpoints. This measure is probably not useful as a predictor of cognitive status. In Q12: Draw-A-Clock, there is evidence that the Normal and AD slopes are equal and that both decline with time, whereas the MCIs remain constant. In the final measure, Q14: CDR Sum of Boxes, the data shows that the MCIs are flat, whereas ADs increase with time and the Normals decrease slightly over time.
Fig. 1.
Fitted Lines from the Slope-Intercept Model over Time for Q1-Logical Memory II.
Fig. 3.
Fitted Lines from the Slope-Intercept Model over Time for Q3-Word Fluency-Phonemic.
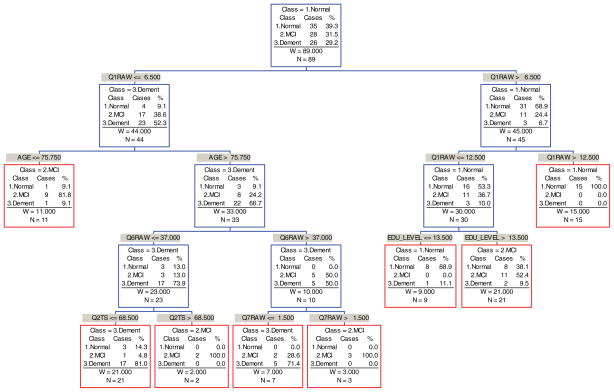
CART analysis conducted on participants who had been tested at least three times (n = 89) determined that the endpoints for 78.6% of participants could be correctly classified after baseline tests (Fig. 4). The relevant predictors in this tree were: Q1: Logical Memory, Q2: Set Shifting (Trails-B), Q6: Perseverative Errors, and Q7: Categories Complete plus the demographic variables Age and Education.
Fig. 4.
Endpoint Classification Tree based on Baseline Evaluation. Note. This tree model was fit using the software CART; equal priors and equal costs were assumed.
Q1: Logical Memory II is the most important initial predictor for splitting the total group into two groups: those with a raw score of (Q1raw> 6.5) are classified as tentatively normal, and those with a raw score of (Q1raw ≤ 6.5) are classified as tentatively demented. For those in the “normal” group, Q1 was again used to differentiate between a mixture of all three groups (Q1raw ≤ 12.5) and endpoint normals (Q1raw> 12.5). At the second split for those in the “demented group,” an age split resulted in an endpoint MCI group (Age ≤ 75.75) with two misclassifications and a tentatively demented group (Age > 75.75). At the third tree split, education level separates participants into endpoint normal with one misclassification (EDU LEVEL ≤ 13.5 years) and endpoint MCI (EDU LEVEL > 13.5 years) with two misclassifications. For those tentatively demented, Q6: Perseverative Errors (WCST)) split the group into a tentatively demented group A (Q6raw ≤ 37) and group B (Q6raw> 37). At the fourth split, Q2: Trails B T-Score split group A into two more groups, an endpoint demented (Q2TS ≤ 68.5) with four misclassifications and an endpoint MCI group (Q2TS > 68.5). Furthermore, an additional fourth split based on the Q7:WCST Categories Completed raw score split the group into endpoint demented (Q7raw ≤ 1.5) with two misclassifications, and another endpoint MCI group (Q7raw> 1.5) with no misclassifications. To summarize, 78.6% of the 89 MCI participants were correctly classified by the CART tree after just the first visit.
Subsequent classification trees were constructed by incorporating additional visit information. For Phase 2, 82% were correctly classified, at Phase 3, 71.9%, at Phase 4, 85.7%, and at Phase 5, 83%. Further visits seem to improve the classification rate from baseline at 78.6% to a high of 85.7% by the 4th visit. However, only 77 of the original 89 participants were evaluated at a 4th visit, so it is difficult to directly compare these classification rates
DISCUSSION
This study has analyzed the clinical course of two elderly cohorts, one normal and the other multiple domain amnestic MCI over 5 years. Our findings are consistent with previous reports of the variable natural history of MCI and fluctuation of cognitive status over time. Of interest is that 10 MCI participants reverted to normal after remaining in MCI status for months (Table 5), observations that support the argument that MCI is not necessarily prodromal AD. Though detailed neuropsychological testing can accurately classify cognitive status, CDR scores can differ significantly over time ranging from 1.5 to zero (see Table 5). Thus Informant-based measures such as the CDR are not immune to within-person fluctuations. Normal participants exposed to Logical Memory I & II, Trailmaking B, Boston Naming Test-Short Version, and Wisconsin Card Sorting Test every eight months benefit from practice effects and improve episodic memory (Logical Memory), set shifting (Trailmaking B), and Word Fluency. MCI subjects and those progressing to dementia do not improve with serial Word Fluency: Phonemic testing. Both normals and MCI remain constant on confrontational naming (BNT) and categories complete (WCST), whereas subjects progressing to AD worsen.
Our finding of different practice effects between normals and MCI subjects is consistent with observations of Darby et al. [12] and Duff et al. [13]. Practice effects can be used as a marker for cognitive status. CART analysis distinguished three endpoint groups at baseline: Logical Memory II, a measure of episodic memory recall is useful in projecting the dementing cohort from normal with the demographic variables of age and education. Age is the most powerful demographic predictor for predicting AD from MCI, with those younger than 75 remaining MCI and those older dementing. Education proves useful in separating normals from MCI for those whose Logical Memory II raw scores are greater than 6 but less than 12.
Though deficits in Word Fluency and Semantic skills are cited as a risk factor for MCI conversion to dementia, in our experience these tests did not have predictive value. For participants in the demented group who are older than age 75, perseverative errors from the WCST surfaced as the third split, which suggests failure to shift set may predict dementia from MCI. This parallels the finding by Woods and Troster [14] that in Parkinson’s patients, perseverative errors on the WCST separated those who later developed dementia from those who did not. Trails B, which measures time involved in set shifting, further terminally separated dementing participants from MCI, suggesting that the executive function task of set shifting/perseveration may be more helpful than expected in predicting which persons will remain MCI versus those who progress to dementia. Another measure from the WCST created a fourth terminal split, separating those with fewer perseverative errors into terminally demented and terminally MCI. Categories Complete is the number of categories (10 consecutive correct matches) to assess executive control. The test is sensitive to age related decline, but is marginally less sensitive than perseverative errors in a previous study [15]. Our study confirmed this observation. Though the fourth visit gave the highest success predictive outcome rate at 85.7%, only 77 of the original 89 participants remained in the study. An outcome predicting greater than 78.6% requires at least four evaluations.
Our study has limitations. Not all of our subjects were followed for five years though the majority of cases had more than 4 detailed neuropsychological evaluations. The outcome of MCI cases that reverted to normal and those who remain MCI would require additional years of follow-up. An important unanswered question is the ultimate fate of these subjects and what factors predispose to reversion to normalcy. This established frequency of reversion to normal will confound early therapeutic intervention strategies.
In summary, detailed serial neurocognitive measurements over a period of 60 months enabled the profiling of cognitive trajectories in both elderly normals and a homogeneous population of multiple domain MCI subjects. 12% of our MCI sample returned to normal, confirming the contention that MCI is not prodromal AD. Normals benefited from practice effects, but those who remained at MCI or demented did not, a failure to improve that may be an indication of MCI. Both regression analysis methods, the slope intercept and CART demonstrate that a combination of 5 test measures and 2 demographic measures at baseline can predict endpoints for the 3 groups with 78.6% accuracy. The variants in neurocognitive measures may account for inaccuracy in endpoint predictions. Without these multiple measures however, 1 in 8 MCI could be misclassified as “stable MCI” when they have actually reverted to normal. In our cohort of MCI subjects we have found that though memory impairment was primary, careful testing detected impairment in other cognitive domains (“multiple domain MCI”). This by far was the universal form of MCI in our study and we did not encounter any cases that effected only a single cognitive domain [16].
One consistent neurocognitive finding was the variance over time in the MCI participants. This changing status may be due to small vessel disease, microbleeds, and neural recovery events that we have documented radiologically in our series. MCI subjects improved in some domains to worsening in others, then changing again either broadly or narrowly. This instability has been reported to be a reflection of whether the study sample is clinical or community based [17]. We feel that the instability is integrated to the microvascular pathology of CAA associated with AD, a clinical observation that warrants further investigation [18].
Fig. 2.
Fitted Lines from the Slope-Intercept Model over Time for Q2-Set Shifting (Trails-B).
Acknowledgments
This research was supported by the National Institutes of Health [grant number AG20948].
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=935).
References
- 1.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR., Jr Mild cognitive impairment: Ten years later. Arch Neurol. 2009;66:1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, Belleville S, Brodaty H, Bennett D, Chertkow H, Cummings JL, de Leon M, Feldman H, Ganguli M, Hampel H, Scheltens P, Tierney MC, Whitehouse P, Winblad B. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 4.Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Jr, Cox NJ, Dunbar-Jacob JM, Granieri EC, Hunt G, McGarry K, Patel D, Potosky AL, Sanders-Bush E, Silberberg D, Trevisan M. National Institutes of Health State-of-the-Science Conference statement: Preventing Alzheimer disease and cognitive decline. Ann Intern Med. 2010;153:176–181. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 5.Morris JC, Storandt M, Miller JP, McKeel DW, Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC. Mild Cognitive Impairment: Aging to Alzheimer’s Disease. Oxford University Press, Inc; New York: 2003. [Google Scholar]
- 7.Kirsch W, McAuley G, Holshouser B, Petersen F, Ayaz M, Vinters HV, Dickson C, Haacke EM, Britt W, III, Larsen J, Kim I, Mueller C, Schrag M, Kido D. Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J Alzheimers Dis. 2009;17:599–609. doi: 10.3233/JAD-2009-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller C, Zhou W, VanMeter A, Heiby M, Magaki S, Ross MM, Espina V, Schrag M, Dickson C, Liotta L, Kirsch WM. The heme degradation pathway as a promising serum biomarker source for the early detection of Alzheimer’s disease. J Alzheimers Dis. 2010;19:1081–1091. doi: 10.3233/JAD-2010-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Wechsler D. The Psychological Corporation; San Antonio TX: 1997. [Google Scholar]
- 11.Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer’s disease by clock drawing. J Am Geriatr Soc. 1989;37:730–734. doi: 10.1111/j.1532-5415.1989.tb02234.x. [DOI] [PubMed] [Google Scholar]
- 12.Darby D, Maruff P, Collie A, McStephen M. Mild cognitive impairment can be detected by multiple assessments in a single day. Neurology. 2002;59:1042–1046. doi: 10.1212/wnl.59.7.1042. [DOI] [PubMed] [Google Scholar]
- 13.Duff K, Beglinger LJ, Schultz SK, Moser DJ, McCaffrey RJ, Haase RF, Westervelt HJ, Langbehn DR, Paulsen JS. Practice effects in the prediction of long-term cognitive outcome in three patient samples: A novel prognostic index. Arch Clin Neuropsychol. 2007;22:15–24. doi: 10.1016/j.acn.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woods SP, Troster AI. Prodromal frontal/executive dysfunction predicts incident dementia in Parkinson’s disease. J Int Neuropsychol Soc. 2003;9:17–24. doi: 10.1017/s1355617703910022. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes MG. Age-related differences in performance on the Wisconsin card sorting test: A meta-analytic review. Psychol Aging. 2004;19:482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC, Ivnik RJ, Boeve BF, Knopman DS, Smith GE, Tangalos EG. Outcome of clinical subtypes of mild cognitive impairment. Neurology. 2004;62:A295. [Google Scholar]
- 17.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: An epidemiologic study. Neurology. 2004;63:115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 18.Maioli F, Coveri M, Pagni P, Chiandetti C, Marchetti C, Ciarrocchi R, Ruggero C, Nativio V, Onesti A, D’Anastasio C, Pedone V. Conversion of mild cognitive impairment to dementia in elderly subjects: A preliminary study in a memory and cognitive disorder unit. Arch Gerontol Geriatr. 2007;44 (Suppl 1):233–241. doi: 10.1016/j.archger.2007.01.032. [DOI] [PubMed] [Google Scholar]