Abstract
Objectives
Compare Clostridium difficile infection (CDI) rates using a traditional definition [i.e. diagnosed > 48 hours after admission, healthcare-onset CDI (HO/CDI)] versus expanded definitions, including both HO/CDI cases and community-onset CDI cases diagnosed ≤ 48 hours from admission who were hospitalized in the previous 30 or 60 days [healthcare facility-associated (HCFA)-30 and HCFA-60]. Determine if differences exist between patients with CDI onset in the community versus healthcare setting.
Design
Prospective cohort
Setting
Tertiary acute-care facility.
Patients
Medicine patients diagnosed with CDI from 1/1/04 through 12/31/05.
Methods
CDI cases were classified as HO/CDI, HCFA-30, and/or HCFA-60. Patient demographics and medication exposures were obtained. The CDI incidence per the definitions, CDI rate variability, patient demographics, and medication exposures were compared.
Results
The HO/CDI rate (1.6 cases/1000 patient days) was significantly lower than the HCFA-30 (2.4) and the HCFA-60 (2.6) rates (p<0.01, both). There was good correlation between the HO/CDI rate and both the HCFA-30 and HCFA-60 rates (correlation=0.69 and 0.70, p<0.01 both). There were no months where the CDI rate was > 3 SD from the mean. Patients with community-onset CDI were less likely to have received a fourth-generation cephalosporin (p=0.02) or IV vancomycin (p=0.01) while hospitalized.
Conclusions
Expanded definitions identify more patients with CDI. There is good correlation between traditional and expanded CDI definitions; therefore it is unclear if expanded surveillance is necessary to identify an abnormal change in CDI rates. Cases that met the expanded definitions were less like to have fourth-generation cephalosporin and vancomycin exposure.
MeSH Terms: Clostridium difficile, surveillance, hospitals
Introduction
Recommendations for Clostridium difficile infection (CDI) surveillance definitions have been published recently (1). At a minimum, it is recommended healthcare facilities track all cases of CDI that occur >48 hours after admission to the healthcare facility [healthcare-onset CDI (HO/CDI)]. It is also recommended to track CDI cases with onset ≤ 48 hours after admission to the hospital if they had been admitted to a healthcare facility within the previous 30 days [community-onset, healthcare facility associated (HCFA) CDI] if resources are available. This recommendation is based on the observation that many patients develop CDI soon after discharge from a healthcare facility (1–4). Presumably C. difficile was acquired in the healthcare facility, but symptomatic CDI did not develop until after discharge. Although identifying patients with HO/CDI is relatively simple, determining HCFA CDI requires chart review for all patients with CDI onset ≤ 48 hours from admission. Whether it is necessary or beneficial to track HCFA CDI in addition to HO/CDI to accomplish the primary reason for conducting CDI surveillance, identification of a CDI outbreak, is not known. It is also unclear if there are differences between patients with HO/CDI and patients with HCFA CDI. We performed a prospective cohort study of all patients with CDI admitted to three medicine floors at our hospital to determine: 1) the impact tracking HCFA CDI, in addition to HO/CDI, has on perceived CDI incidence; 2) if tracking HCFA CDI might enhance the ability to detect an abnormal increase in CDI; and 3) if patients with HCFA CDI differ from patients with HO/CDI.
Methods
All patients admitted to one of three medicine wards with diarrhea and a positive test for C. difficile toxin A or B (C. difficile toxin A/B II EIA, TECHLAB, Inc, Blacksburg, VA) from January 1, 2004 through December 31, 2005 were prospectively identified. CDI cases were classified as healthcare-onset (HO) or community-onset healthcare facility associated (HCFA), according to published surveillance definitions (1). HO cases were defined as patients with positive toxin assays > 48 hours after hospital admission. Community-onset HCFA cases were defined as patients with positive toxin assays ≤ 48 hours after hospital admission, provided that diagnosis occurred within 60 days after the last discharge from one of the study wards and there were no other inpatient healthcare exposure from the time of discharge to readmission. CDI cases who did not meet one of these definitions or who had an episode of CDI in the previous 8 weeks were excluded (1). The following three CDI rates were calculated: HO/CDI rates included only HO cases, HCFA-30 rates included HO cases as well as all community-onset HCFA cases discharged in the previous 30 days, and HCFA-60 rates included HO cases as well as all community-onset HCFA cases discharged in the previous 60 days. For patients who had community-onset HCFA CDI, the case of CDI was attributed to the day of discharge from their last hospitalization.
The hospital medical informatics database was queried for patient demographics and risk factors. Comorbidites were classified according to the Deyo adaptation of the Charlson Comorbidity Index (5;6). Medication exposures were collected dichotomously (exposed or not exposed). Exposure to all antibiotics, H2 antagonists and proton pump inhibitors were gathered from date of admission to date of symptom onset for HO/CDI. All exposures from the last hospitalization were collected for patients with community onset HCFA CDI.
The CDI incidence per the different definitions, patient demographics, and medication exposures were compared with Chi-square. Cross correlation coefficients (ρ) were calculated to assess the monthly correlation between CDI definitions over time. Run control chart methodology was used to determine if an abnormal increase, defined as a rate > 3 standard deviations from the mean, was identified by any of the definitions. Analyses were completed using Epi Info 3.01 (Centers for Disease Control and Prevention, Atlanta, GA) and SPSS v14.0 (SPSS Inc, Chicago IL). This study was approved by the Washington University School of Medicine Human Research Protection Office.
Results
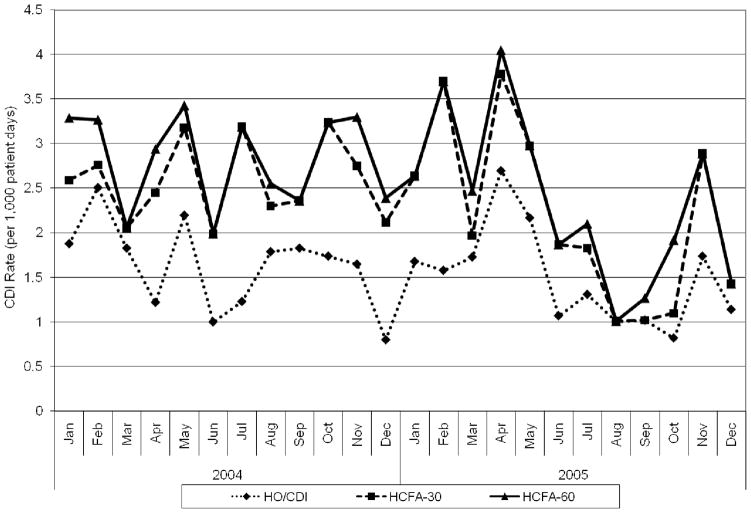
Overall, 247 patients with CDI were included in this study. Of these, 148 were classified as HO/HCFA, 225 as HCFA-30, and 247 as HCFA-60 CDI. The HO/HCFA CDI rate (1.6 cases/1,000 patient-days) was significantly lower than the CO/HFCA-30 rate (2.4/1,000 patient-days, p < 0.01) and the HCFA-60 rate (2.6/1,000 patient-days, p < 0.01). The HCFA-30 rate was not significantly different from the HCFA-60 rate (p = 0.31). (Table 1, Figure 1).
Table 1.
CDI rates by definition
| Study Ward | Healthcare onset | HCFA-30 | p-value (HO vs. HCFA-30) | HCFA-60 | p-value (HO vs. HCFA-60) | p-value (HCFA-30 vs. HCFA-60) | |||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Rate | Cases | Rate | Cases | Rate | ||||
| A | 57 | 1.8 | 75 | 2.4 | 0.12 | 84 | 2.7 | 0.02 | 0.48 |
| B | 54 | 1.7 | 87 | 2.7 | <0.01 | 97 | 3.0 | <0.01 | 0.46 |
| C | 37 | 1.3 | 63 | 2.1 | <0.01 | 66 | 2.2 | <0.01 | 0.79 |
| Total | 148 | 1.6 | 225 | 2.4 | <0.01 | 247 | 2.6 | <0.01 | 0.31 |
All rates calculated as cases per 1,000 patient days
Figure 1.
Clostridium difficile infection (CDI) rates by definition over time
There was good correlation between the HO/HCFA CDI rate variability and the HCFA-30 rate (correlation = 0.69, p < 0.01, Table 2) over time. There was also good correlation between the HO/HCFA and the HCFA-60 rates (correlation = 0.70, p < 0.01). There was excellent correlation between the HCFA-30 and the HCFA-60 rates (correlation = 0.95, p < 0.01). Since the HCFA-60 CDI rate was not significantly different from the HCFA-30 rate and the two rates varied together almost 100%, run control charts were created only for the HO/HCFA and HCFA-30 rates. There were no abnormal increases in the CDI rate for either definition (Figure 1).
Table 2.
Correlations of CDI rate definitions
| Study ward | Healthcare onset vs. HCFA-30 | Healthcare onset vs. HCFA-60 | HCFA-30 vs. HCFA-60 | |||
|---|---|---|---|---|---|---|
| Partial Correlation | P value | Partial Correlation | P value | Partial Correlation | P value | |
| A | 0.79 | <0.01 | 0.82 | <0.01 | 0.95 | <0.01 |
| B | 0.73 | <0.01 | 0.72 | <0.01 | 0.97 | <0.01 |
| C | 0.62 | <0.01 | 0.63 | <0.01 | 0.99 | <0.01 |
| Total | 0.69 | <0.01 | 0.70 | <0.01 | 0.95 | <0.01 |
The 148 patients with HO/CDI were compared to the 99 patients with community-onset HCFA CDI to determine if there were differences in the patient populations (Table 3). There were no significant differences between patients with HO/CDI and community-onset HCFA CDI in regards to age, gender, and comorbidities. There was a trend toward more white patients developing HO/CDI than community-onset HCFA CDI (53% vs. 41%, p = 0.08). There were no differences in exposure to any antibiotic, fluoroquinolones, carbapenems, clindamycin, H2 antagonists, or proton pump inhibitors. HO/CDI cases were more likely to have received a fourth-generation cephalosporin (33% vs. 19%, p = 0.02) or intravenous vancomycin (46% vs. 29%, p = 0.01) than patients with community-onset HCFA CDI.
Table 3.
Univariate comparison of patients with hospital onset and community onset HCFA CDI
| Hospital Onset n = 148 n (%) |
Onset ≤ 60 days from Discharge n = 99 n (%) |
p | |
|---|---|---|---|
| Age [median (range) | 69 (21 – 97) | 71 (22 – 95) | 0.66 |
| Female | 81 (55) | 58 (59) | 0.55 |
| White | 78 (53) | 41 (41) | 0.08 |
| Charlson Comorbidity Index ≥3 | 69 (47) | 38 (38) | 0.20 |
| First generation cephalosporin | 16 (11) | 11 (11) | 0.94 |
| Third generation cephalosporin | 35 (24) | 20 (20) | 0.52 |
| Fourth-generation cephalosporin | 49 (33) | 19 (19) | 0.02 |
| Clindamycin | 9 (6) | 2 (2) | 0.21 |
| Carbapenem | 6 (4) | 3 (3) | 0.74 |
| Piperacillin/tazobactam | 14 (10) | 8 (8) | 0.71 |
| Fluoroquinolones | 67 (45) | 48 (49) | 0.62 |
| Intravenous Vancomycin | 68 (46) | 29 (29) | 0.01 |
| Any Antibiotic | 131 (89) | 86 (87) | 0.70 |
| H2 Antagonists | 16 (11) | 16 (16) | 0.22 |
| Proton Pump Inhibitors | 117 (79) | 69 (70) | 0.10 |
Discussion
The CDI rate increased significantly when an expanded definition of CDI that included patients with a positive toxin assay within the first 48 hours of hospital admission and a recent discharge from a study ward was used, compared to the more traditional definition of healthcare onset CDI. There was a 52% increase in the CDI rate with the HCFA-30 definition and a 67% increase in the CDI rate with the HCFA-60 definition. However, all of the CDI rates showed good correlation over time. Although the expanded definitions may capture more cases, it may not be necessary to track community onset HCFA CDI in order to detect a CDI outbreak.
Our findings that 40% of identified CDI cases had an onset in the community is similar to other recent studies examining the onset of CDI (2–4). Kutty et al also examined the correlation between HO/CDI and community-onset HCFA CDI (2). They found a correlation of 0.7 (P < 0.01) between HO/CDI and HCFA-30 rates. This is remarkably similar to our study where the correlation of rates over time was 0.69 (Table 2). The good correlation between definitions has important practical implications. HO/CDI surveillance can be conducted with minimal time and effort if only diarrheal stools are accepted for C. difficile testing. In this setting it is only necessary to determine stool collection date in relation to hospital admission (1). Tracking community-onset HCFA CDI requires review of all charts of patients with CDI onset ≤ 48 hours after hospital admission. Although it may be important to track community-onset CDI to completely understand the epidemiology of CDI, it may not be necessary to track community-onset HCFA CDI to identify a healthcare facility associated CDI outbreak. We also examined HO/CDI and HCFA-30 rates using an objective measure, run control chart methodology, to determine if an abnormal increase in CDI was detected by either definition. Since an abnormal increase in CDI was not detected, it is not possible to determine if one definition may be more sensitive than the other for detecting CDI outbreaks.
We also compared CDI risk factors between patients with HO/CDI and community-onset HCFA CDI. There were no differences in patient age, gender, race, or overall comorbidities. Overall antibiotic exposures were also very similar: 89% of patients with HO/CDI had documented antibiotic exposures during the hospitalization when CDI occurred, and 87% of community onset HCFA CDI had documented antibiotic exposures during the hospitalization immediately prior to the one in which CDI was diagnosed. However, despite similarities in overall antibiotic exposures, HO/CDI cases were more likely to receive a fourth-generation cephalosporin or intravenous vancomycin than community-onset HCFA CDI cases. Several possible explanations for this finding exist. Cefepime in addition to vancomycin is the preferred empiric antibiotic formulary regimen for patients with suspected healthcare associated infections at our facility. It is possible this may represent differences in how rapidly these antibiotics are able to alter the intestinal flora, resistance of C. difficile strains in our healthcare facility to these antibiotics, or differences in patient acuity of illness on admission to the hospital. Further study of these antibiotic issues is warranted since this may have implications on initial empiric antibiotic choices for patients at high risk for HO/CDI.
There are some limitations to this study. Although all CDI cases were identified and categorized prospectively for CDI surveillance purposes, all data on patient demographics and medication exposures were collected retrospectively from electronic databases. There are limitations to data collected from administrative databases as well as data gathered retrospectively. A key limitation was our lack of data on outpatient antibiotic exposures. This study was limited to medicine wards at a tertiary care facility and therefore the results may not be generalizable to other settings. However, as many as 85% of CDI cases within a healthcare facility occur on medical wards (7).
Standardized surveillance definitions are necessary to track disease incidence over time and to compare disease incidence in different settings. Unfortunately there are no universally accepted surveillance definitions for CDI. However, the recently published recommendations provide an important starting point (1). Because of the limited knowledge regarding the exact pathogenesis of CDI, several different surveillance definitions have been recommended. Healthcare exposure is a well described risk factor for CDI, presumably because of increased risk of C. difficile exposure (8;9). It is also recognized that the period of risk for developing CDI extends up to 90 days after discharge from a healthcare facility. This is in contrast to prior studies that demonstrated the incubation period of CDI to be less than seven days (10;11). It is possible that community onset HCFA CDI represents community acquisition of C. difficile in a patient still at risk for CDI after hospital discharge.
The decision of whether or not to track community-onset HCFA CDI is an important one for a healthcare facility. Tracking community-onset HCFA CDI takes more time, it may not enhance CDI outbreak detection, C. difficile may not have been acquired in the healthcare facility, it will increase the incidence of CDI associated with a healthcare facility, and it may change the relative incidence of CDI compared to other healthcare facilities (2). On the other hand, tracking community-onset HCFA CDI is necessary to have a complete understanding of CDI epidemiology. Methods to prevent community-onset HCFA CDI may be different from methods to prevent HO/CDI, and the incidence of community-onset CDI may impact the incidence of HO/CDI (12;13). Even if community-onset HCFA CDI does not represent healthcare acquisition of C. difficile, it may be possible to prevent it if specific modifiable risk factors for community onset HCFA CDI, such as type of antibiotic exposure, are identified.
The perspective of this study was that of the hospital-based infection prevention and control department. In order to maximize the practical applicability of this study, data were collected and analyzed in a manner analogous to “real world” infection prevention and control practices: positive toxin results of a diarrheal stool were considered a case of CDI without additional chart review to assess duration and frequency of diarrhea (which is often so poorly recorded in the medical record this information is not useful), efforts to control for false positive toxin assays with repeat testing were not performed, and no efforts were made to determine which recurrent cases were due to a new acquisition of C. difficile versus relapse of a prior infection. Also, no efforts were made to assess CDI surveillance from a public health, population-based perspective. This is an important distinction because it is not clear which CDI surveillance definition is ideal from this perspective either. Factors that may influence the perceived burden of CDI from a healthcare facility include length of stay (shorter length of stay may falsely lower HO/CDI rates), ability to identify community-onset HCFA CDI not readmitted to the same healthcare facility, prescribing practices that may influence the setting of CDI onset, and CDI with onset >7 days from healthcare facility discharge may not represent C. difficile acquired from that healthcare facility (10;11). These are in addition to the usual confounders present when comparing infection rates across healthcare facilities (14).
At a minimum all healthcare facilities should track HO/CDI (1). Additional study is necessary to determine whether tracking community-onset HCFA CDI improves the ability to detect a CDI outbreak and to determine the impact tracking community-onset HCFA CDI has on healthcare facilities. Of note, differences in antibiotic exposures between HO/CDI and community-onset HCFA CDI indicate important differences may exist in C. difficile acquisition and/or CDI pathogenesis based on the CDI onset setting. Efforts to control this costly pathogen will continue to be hampered until we have a more complete understanding of C. difficile epidemiology and pathogenesis.
Acknowledgments
Financial support: This work was supported by grants from the Centers for Disease Control and Prevention (UR8/CCU715087-06/1, 1U01C1000333-01) and the National Institutes of Health (KL2RR024994-01, K24AI067794-01)
Footnotes
Preliminary data were presented in part at the 44th Annual Meeting of the Infectious Diseases Society of America, Toronto, ON, Canada.
Potential conflicts of interest:
E.R.D.: consultant – Merck, Becton-Dickenson, Salix; research – Viropharma
V.J.F.: speakers’ bureaus Verimetrix, Steris
All other authors: no conflict
Reference List
- 1.McDonald LC, Coignard B, Dubberke E, Song X, Horan T, Kutty PK. Recommendations for surveillance of Clostridium difficile-associated disease. Infect Control Hosp Epidemiol. 2007;28(2):140–145. doi: 10.1086/511798. [DOI] [PubMed] [Google Scholar]
- 2.Kutty PK, Benoit SR, Woods CW, Sena AC, Naggie S, Frederick J, et al. Assessment of Clostridium difficile-associated disease surveillance definitions, North Carolina, 2005. Infect Control Hosp Epidemiol. 2008;29(3):197–202. doi: 10.1086/528813. [DOI] [PubMed] [Google Scholar]
- 3.Paltansing S, van den Berg RJ, Guseinova RA, Visser CE, van der Vorm ER, Kuijper EJ. Characteristics and incidence of Clostridium difficile-associated disease in The Netherlands, 2005. Clin Microbiol Infect. 2007;13(11):1058–1064. doi: 10.1111/j.1469-0691.2007.01793.x. [DOI] [PubMed] [Google Scholar]
- 4.Price MF, Dao-Tran T, Garey KW, Graham G, Gentry LO, Dhungana L, et al. Epidemiology and incidence of Clostridium difficile-associated diarrhoea diagnosed upon admission to a university hospital. J Hosp Infect. 2007;65(1):42–46. doi: 10.1016/j.jhin.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 6.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 7.The Association for Professionals in Infection Control and Epidemiology, Inc. [Accessed November 17, 2008];National Prevalence Study of Clostridium difficile in US Healthcare Facilities. Available at: http://www.apic.org/AM/CM/ContentDisplay.cfm?ContentFileID=11410.
- 8.Dubberke ER, Reske KA, Yan Y, Olsen MA, McDonald LC, Fraser VJ. Clostridium difficile--associated disease in a setting of endemicity: identification of novel risk factors. Clin Infect Dis. 2007;45(12):1543–1549. doi: 10.1086/523582. [DOI] [PubMed] [Google Scholar]
- 9.Dubberke ER, Reske KA, Olsen MA, McMullen KM, Mayfield JL, McDonald LC, et al. Evaluation of Clostridium difficile-associated disease pressure as a risk factor for C difficile-associated disease. Arch Intern Med. 2007;167(10):1092–1097. doi: 10.1001/archinte.167.10.1092. [DOI] [PubMed] [Google Scholar]
- 10.Clabots CR, Johnson S, Olson MM, Peterson LR, Gerding DN. Acquisition of Clostridium difficile by hospitalized patients: evidence for colonized new admissions as a source of infection. J Infect Dis. 1992;166(3):561–567. doi: 10.1093/infdis/166.3.561. [DOI] [PubMed] [Google Scholar]
- 11.McFarland LV, Mulligan ME, Kwok RY, Stamm WE. Nosocomial acquisition of Clostridium difficile infection. N Engl J Med. 1989;320(4):204–210. doi: 10.1056/NEJM198901263200402. [DOI] [PubMed] [Google Scholar]
- 12.Gerding DN, Johnson S, Peterson LR, Mulligan ME, Silva J., Jr Clostridium difficile-associated diarrhea and colitis. Infect Control Hosp Epidemiol. 1995;16(8):459–477. doi: 10.1086/648363. [DOI] [PubMed] [Google Scholar]
- 13.Simor AE, Bradley SF, Strausbaugh LJ, Crossley K, Nicolle LE. Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol. 2002;23(11):696–703. doi: 10.1086/501997. [DOI] [PubMed] [Google Scholar]
- 14.Wong ES, Rupp ME, Mermel L, Perl TM, Bradley S, Ramsey KM, et al. Public Disclosure of Healthcare-Associated Infections: The Role of the Society for Healthcare Epidemiology of America. Infect Control Hosp Epidemiol. 2005;26(2):210–212. doi: 10.1086/502528. [DOI] [PubMed] [Google Scholar]