Abstract
Previous preclinical and clinical studies have demonstrated the efficacy of group II metabotropic glutamate receptor (mGluR) agonists as potential antipsychotics. Recent studies utilizing mGluR2-, mGluR3-, and double knockout mice support that the antipsychotic effects of those compounds are mediated by mGluR2. Indeed, biphenyl indanone-A (BINA), an allosteric potentiator of mGluR2, is effective in experimental models of psychosis, blocking phencyclidine (PCP)-induced hyperlocomotion and prepulse inhibition deficits in mice. In this study, we administered the NMDA receptor antagonist PCP (5.6 mg/kg i.p.) to rats, an established animal model predictive of schizophrenia. Here, we show that BINA (32 mg/kg i.p.) attenuated PCP-induced locomotor activity in rats. Using behaviorally relevant doses of BINA and PCP, we performed pharmacological magnetic resonance imaging (phMRI) to assess the specific brain regions that underlie the psychotomimetic effects of PCP, and examined how BINA modulated the PCP-induced functional changes in vivo. In anesthetized rats, acute administration of PCP produced robust, sustained blood oxygenation level-dependent (BOLD) activation in specific cortical, limbic, thalamic, and striatal regions. Pretreatment with BINA suppressed the amplitude of the BOLD response to PCP in the prefrontal cortex, caudaute–putamen, nucleus accumbens, and mediodorsal thalamus. Our results show key brain structures underlying PCP-induced behaviors in a preclinical model of schizophrenia, and, importantly, its reversal by potentiation of mGluR2 by BINA, revealing specific brain regions functionally involved in its pharmacological action. Finally, our findings bolster the growing body of evidence that mGluR2 is a viable target for the treatment of schizophrenia.
Keywords: mGluR2, BINA, phMRI, BOLD, antipsychotic, schizophrenia
Glutamate, the primary excitatory neurotransmitter of the mammalian central nervous system (CNS), not only elicits fast excitatory synaptic responses via ionotropic glutamate receptors (Hollmann et al., 1994; Nakanishi, 1998), but also activates G protein-coupled metabotropic glutamate receptors (mGluRs), which induce neuromodulatory effects (Sugiyama et al., 1989; Cha et al., 1990). The eight subtypes of mGluRs are divided into three specific groups (Group I: mGluR1, 5; Group II: mGluR2, 3; Group III: mGluR4, 6, 7, 8) based on their molecular structure, signal transduction, and pharmacology (Conn and Pin, 1997; Schoepp et al., 1999). Of the group II mGluRs, mGluR2 localizes presynaptically in the cortex, thalamus, striatum, amygdala, and hippocampus (Moghaddam and Adams, 1998; Gu et al., 2008), reducing transmission at glutamatergic synapses (Anwyl, 1999; Cartmell and Schoepp, 2000), while mGluR3 is primarily expressed by glia (Ohishi et al., 1993; Gu et al., 2008). Based on their function as neuromodulatory autoreceptors and their forebrain and limbic distribution, group II mGluRs represent viable pharmacological targets for the treatment of schizophrenia, having shown promising results in preclinical and clinical studies (Moghaddam and Adams, 1998; Galici et al., 2005, 2006; Krystal et al., 2005; Patil et al., 2007). Pharmacological analyses in mGluR2-, mGluR3-, and double knockout mice support the hypothesis that the mGluR2 subtype mediates the antipsychotic-like effects of group II (mGluR2/3) agonists (Spooren et al., 2000; Woolley et al., 2008; Fell et al., 2008), suggesting that selective mGluR2 agonists should be similarly effective. An alternative approach to group II mGluR agonists is subtype-selective positive allosteric modulation of mGluR2. Interestingly, mGluR2 potentiators do not activate the receptor directly but act at an allosteric site, inducing a leftward shift of the glutamate concentration-repsonse curve (Johnson et al., 2003, 2005; Galici et al., 2005, 2006) and can mimic many of the mGluR2/3 agonists in animal models predictive of antipsychotic activity, including reversal of phencyclidine (PCP)-induced hyperlocomotor activity in mice (Galici et al., 2005, 2006; Johnson et al., 2005).
Due to its psychotomimetic effects, acute administration of the noncompetitive N-methyl-d-aspartate receptor (NMDAR) antagonists, such as PCP, is a widely used method for establishing animal models predictive of schizophrenia for the purpose of evaluating antipsychotics (Bakshi et al., 1994; Moghaddam and Adams, 1998; Cartmell et al., 1999; Spooren et al., 2000; Adams and Moghaddam, 2001; Risterucci et al., 2005; Gozzi et al., 2008a; Mouri et al., 2007). In rodents, PCP dramatically alters motor behavior, increasing horizontal locomotion, and inducing repetitive head and limb movements (stereotypy) (Sturgeon et al., 1979; Sams-Dodd, 1996). These behaviors are associated with alterations in limbic striatal function (Matthysse, 1986), and such “dysfunctional repetitive motor behavior” occurs in schizophrenia (Morrens et al., 2006). PCP also produces “schizophrenia-like symptoms” in rodents, including disruption of prepulse inhibition and working memory (Bakshi et al., 1994), analogous to its effects in healthy humans (Krystal et al., 1994). In fact, PCP administration to healthy volunteers triggers a behavioral state that mimics specific aspects of schizophrenia, such as thought disorder and hallucinations (Javitt and Zukin, 1991; Tamminga, 1998; Krystal et al., 1999).
In rodents, PCP administration leads to excess glutamate release in forebrain and limbic regions (Schoepp and Marek, 2002). Since agonist activation of group II mGluRs decreases PCP-evoked glutamate release (Schoepp and Marek, 2002), we hypothesized that positive allosteric modulation of mGluR2 with biphenyl indanone-A (BINA), which potentiates the effects of glutamate (Galici et al., 2006) should suppress PCP-mediated brain activation and resulting behavior. Reversal of PCP-induced locomotor activity is a standard experiment for testing antipsychotic-like activity of compounds, while pharmacological magnetic resonance imaging (phMRI) can reveal the specific sites of action of NMDAR antagonists and antipsychotics (Risterucci et al., 2005; Littlewood et al., 2006; Gozzi et al., 2008a). The central hemodynamic response serves as a biomarker of brain activity (Ogawa et al., 1990; Detre and Wang, 2002; Gore, 2003), so phMRI is an extensively used neuroimaging technique for mapping the spatio–temporal effects of psychoactive drugs in vivo (Chen et al., 1997; Schwarz et al., 2004; Febo et al., 2005; Gozzi et al., 2006; Williams et al., 2007). Here we utilized behaviorally relevant doses of BINA and PCP in our phMRI studies to assess the specific brain regions activated by PCP and to determine whether pretreatment with BINA blocks regional PCP-induced brain activation. This is the first report to characterize the specific brain regions integral to the ability of selective potentiation of mGluR2 to suppress PCP-induced hyperlocomotion.
EXPERIMENTAL PROCEDURES
Animals
Adult male Sprague–Dawley rats (250–375 g) (Harlan, Indianapolis, IN, USA) were tested in PCP-hyperlocomotion and phMRI studies. Rats were housed three per cage and maintained on a 12-h light/dark cycle (lights on at 6:00 am) with water and food available ad libitum. All procedures were approved by the VUMC Institutional Animal Care and Use Committees and were conducted according to the NIH Guide for the Care and Use of Laboratory Animals.
Drugs
PCP hydrochloride (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.9% saline and administered at a dose of 5.6 mg/kg interperitoneally (i.p.). BINA was synthesized by the Vanderbilt Institute for Chemical Biology Core as previously described (Galici et al., 2006). For both behavioral and phMRI experiments, BINA was dissolved in 10% 1 N NaOH and 10% Tween-80 with the pH adjusted to 7.4 using 8.5% lactic acid. BINA was administered as a single dose of 32 mg/kg (i.p.).
PCP-induced hyperlocomotion
Apparatus
Locomotor experiments were performed in open field chambers (27×27×20 cm3) equipped with 16 horizontal (x- and y-axes) infrared photobeams located 2 cm above the chamber floor (Med Associates, Inc., St. Albans, VT, USA). Photobeam breaks, indicating changes in locomotor activity, were recorded with a Pentium I computer equipped with rodent activity monitoring system software (Med Associates).
Blockade of PCP-induced hyperlocomotion
PCP-induced hyperlocomotion experiments were performed using a between-subject counterbalanced design (animals were tested only once). All test sessions took place between 12:00 pm and 6:00 pm. First, PCP dose-response studies were performed using 5.6 and 10 mg/kg (i.p.). 5.6 mg/kg was determined to be the optimal dose (data not shown), and was used for the subsequent behavioral experiments. To assess the effects of 30 min pretreatment with BINA on PCP-induced hyperlocomotion, four groups of drug naive, adult male Sprague–Dawley rats (8–12 per dose group) were tested: Vehicle/Vehicle (Veh/Veh), Vehicle/PCP (Veh/PCP), BINA/PCP, and BINA/Vehicle (BINA/Veh). Animals were individually placed in separate open field chambers, and after 30 min were pretreated with either vehicle (1 ml/kg i.p.) or BINA (32 mg/kg i.p.) and returned to their chambers for another 30 min, for a total habituation period of 60 min to measure baseline motor activity. After the habituation period, each rat received a second injection of either vehicle (1 ml/kg saline, i.p.) or PCP (5.6 mg/kg i.p.), and their stimulant-driven locomotor activity was measured for 60 min. Data were collected as average counts (i.e., average total number of beam breaks) in 5-min time bins.
Pharmacological MRI
Animal surgery and preparation
For the phMRI studies, animal surgery and preparation for phMRI were performed in adult male, Sprague–Dawley rats as described previously (Hackler et al., 2007). Briefly, rats were anesthetized with 2% isoflurane and a P50 catheter (Braintree Scientific, Braintree, MA, USA) was inserted into the intraperitoneal cavity for drug administration. The rat was tracheotomized (14-gauge, 3 cm long; Johnson & Johnson, New Brunswick, NJ, USA) and connected to a mechanical ventilator (Kent Scientific, Litchfield, CT, USA), which delivered a gas mixture of 33:67% O2/N2O. Heart rate, respiration rate, and temperature were measured using a magnetic resonance-compatible monitoring system, which controlled a thermocoupled heating unit for maintenance of body temperature (SAM-PC; SA Instruments, Encinitas, CA, USA). Ten minutes prior to the scan, rats were paralyzed with a bolus of pancuronium bromide (2 mg/kg i.p.; Sigma-Aldrich, St. Louis, MO, USA), and isoflurane was reduced to 1% for the duration of the experiment. Oxygen (Sp02) and end-tidal carbon dioxide (etC02) levels were continuously monitored using the magnet-compatible Magnitude monitoring system™ (Invivo Research, Orlando, FL, USA).
Acquisition
All phMRI measurements were acquired using a 7T Varian magnet (16 cm bore) controlled by a Varian Inova console running VnmrJ 1.1D software (Varian Instruments, Palo Alto, CA, USA). The animals were placed on a custom-built stereotaxic plexiglas platform and fixed into position with an incisor bar and Teflon ear bars. A 20-mm dual transmit-receive radio frequency surface coil (Varian) was secured to the dorsal surface of the rat’s head. Prior to drug administration, a full set of high resolution fast spin-echo structural MR images were collected [repetition time (TR), 4000 ms; echo spacing (TE), 10 ms; number of excitations (NEX), 2; 256×128×8 matrix; 30×30×2 mm3 field of view (FOV)]. Following a 20 min baseline period, pretreatment drug, either BINA or vehicle, was administered via i.p. catheter. Challenge drug, PCP or corresponding vehicle, saline, was administered 30 min after the first injection. All drugs were delivered through tubing attached to the i.p. catheter. Functional images were acquired using a gradient-echo sequence (TR, 200 ms; TE, 12 ms; flip angle, 20°; NEX, 2; 64×64×8 matrix; 30×30×2 mm3 FOV). Functional images following drug administration were superimposed on structural images taken prior to drug injection to identify regions of interest (ROI), that is, specific areas that show significant changes in the blood oxygenation level dependent (BOLD) signal.
Data analysis
Motor activity data were calculated as the average number of beam breaks in each 5-min period (counts/5 min) during the 60 min following PCP. The mean total locomotor activity during the 60 min after PCP injection was compared by one way ANOVA followed by post hoc Dunnett’s multiple comparison post hoc test to determine group differences. All results are expressed as mean±SEM with a P<0.05 considered significant. Data were compiled, analyzed, and plotted using GraphPad Prism 2.01 (GraphPad Software, Inc., San Diego, CA, USA).
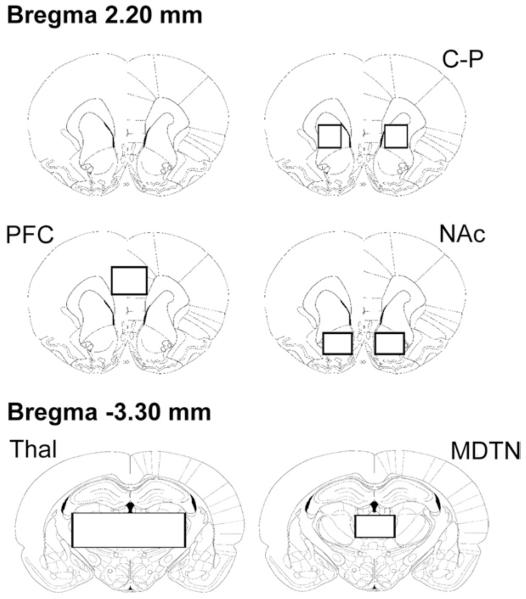
Time course analyses of phMRI data were performed using MATLAB software (Version 7.0.4; MathWorks, Inc., Natick, MA, USA). To measure changes in specific brain regions, ROI analyses were performed using structural markers and a rat brain atlas as a guide (Paxinos and Watson, 1998). ROIs were drawn to include the caudate–putamen (C–P), nucleus accumbens (NAc) (core and shell), prefrontal cortex (PFC), thalamus (Thal) (including mediodorsal, lateral, ventral, ventromedial nuclei), and the mediodorsal thalamic nucleus (MDTN) (including the medial, central, lateral, and paralaminar parts) (Fig. 1). Raw image intensities from each subject were converted to a percentage BOLD signal change relative to the pre-PCP baseline period (ΔS/S0), with extraneous signal stemming from physiological sources (i.e., respiration, heart beat) extracted using principal component analysis (Andersen et al., 1999; Thomas et al., 2002). Data were further processed by linear detrending to correct for baseline signal drift. For group analyses, ROI BOLD signal intensities (ΔS/So) from individuals were averaged across animals for each treatment group. Area under the curve (AUC) was calculated for the first 15 min post-challenge injection period for each animal. The values from both the left and right hemispheres were averaged and data were analyzed using one-way ANOVA and Dunnett’s multiple comparison post hoc test. Differences were considered significant at P<0.05. Group mean AUC maps were generated using Analysis of Functional NeuroImages (AFNI) (afni.nimh.nih.gov/afni). Raw image intensities from each animal were converted to a percent BOLD signal change relative to baseline (pre-PCP injection period) (% ΔS/S0) on a voxel-wise basis and linearly detrended to correct for baseline signal drift. These processed images for each treatment condition were group-averaged and coregistered to a set of high-resolution anatomic reference images. To create group mean AUC maps, individual AUC values were calculated for each voxel for the 15 min post-PCP period then averaged for each group. Maps were colorized according to the voxel AUC value and AFNI’s linear interpolation was applied.
Fig. 1.
Regions of interests (ROIs) for BOLD time course and AUC analyses. Boxes indicate ROIs covering the caudate–putamen (C–P), prefrontal cortex (PFC), and nucleus accumbens (NAc) at the level of Bregma 2.20 mm, and thalamus (Thal), and the mediodorsal thalamic nucleus (MDTN) at Bregma −3.30 mm (figures are based on Paxinos and Watson, 1998).
RESULTS
BINA suppresses PCP-induced hyperlocomotion
Previous studies in mice have shown that BINA, a highly selective allosteric potentiator of mGluR2, blocked PCP-induced hyperlocomotion (Galici et al., 2006). We performed PCP-induced locomotor activity studies in Sprague–Dawley rats to determine whether BINA could elicit similar effects with a shorter pretreatment interval. First, the locomotor profiles induced by two different doses of PCP (5.6 and 10 mg/kg i.p.) were assessed to determine the optimal subanesthetic dose that would produce a behavioral phenotype (i.e., significant increases in locomotor activity) since high levels of PCP elicits a sedative effect (Sturgeon et al., 1979). We found that 5.6 mg/kg was an optimal dose, generating more motor activity than at 10 mg/kg (data not shown).
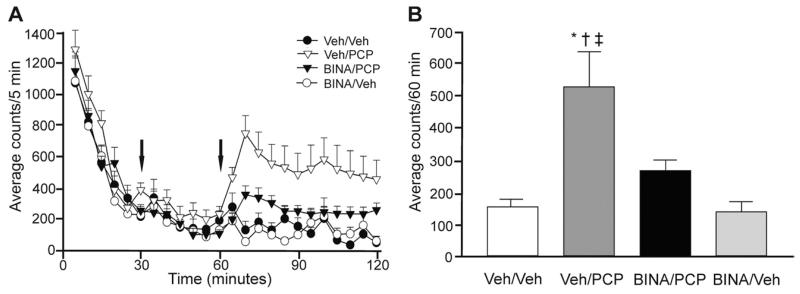
The locomotor activities of animals pretreated with BINA (BINA/PCP group) were measured to evaluate the effectiveness of mGluR2 potentiation when compound was administered 30 min before PCP injection. Locomotor activity of all animals placed in the novel open field chambers decreased rapidly over time reaching very low levels, from over 1000 beam breaks/5 min, due to exploratory activity, to under 300 by the end of the 1 h habituation period (Fig. 2A). Pretreatment drug BINA (32 mg/kg i.p.) or corresponding vehicle was administered 30 min after placement into the chamber, followed by PCP (5.6 mg/kg i.p.) or saline injection after the last 30 min of habituation. As expected, in vehicle pretreated animals, PCP triggered a rapid surge of horizontal locomotion and other distinct PCP-mediated behaviors, such as stereotopy (i.e., head-weaving and back-treading), circling, and falling, which were not separately assessed, but included in the total number of beam breaks. PCP-induced hyperactivity reached a maximum approximately 10 min after injection in the Veh/PCP group (Fig. 2A). One-way ANOVA demonstrated a drug-dependent effect in average horizontal counts during the 60 min post-PCP period [F(3,39)=6.841, P=0.0009], and post hoc analysis indicated a significant effect of BINA (32 mg/kg i.p.) pretreatment with motor activity significantly greater in the Veh/PCP group (530.3±107.6 counts) over the BINA/PCP (267.5±34.16 counts; P<0.05), Veh/Veh (159.9±21.72 counts; P<0.01), and BINA/Veh (142.3±31.34 counts; P<0.01) groups (Fig. 2B). The mean number of beam breaks post-PCP was not different per se between the BINA/PCP group and their Veh/Veh or BINA/Veh cohorts (P>0.05) (Fig. 2B). BINA itself did not alter motor activity such that the locomotor profiles of saline-injected animals pretreated with vehicle (Veh/Veh) or BINA (BINA/Veh) overlapped, with animals remaining habituated to the chamber (Fig. 2A).
Fig. 2.
BINA suppresses PCP-induced hyperlocomotion in rats. (A) Pretreatment with BINA (32 mg/kg i.p.) 30 min after animals were placed in the open field chamber and 30 min before PCP (5.6 mg/kg i.p.) administration attenuated PCP-induced hyperlocomotor activity. Times of injections (t=30 min for BINA and t=60 min for PCP) are indicated by arrows. (B) The average locomotor activity during the 60 min after PCP administration is significantly higher in the Veh/PCP group than in the Veh/Veh (* P<0.01), BINA/PCP († P<0.01), and BINA/Veh (‡ P<0.01) groups. Data (average counts/60 min) is presented as mean±SEM (n=8–12 per dose group).
PCP-induced BOLD signal changes in cortical, striatal, thalamic, and limbic regions
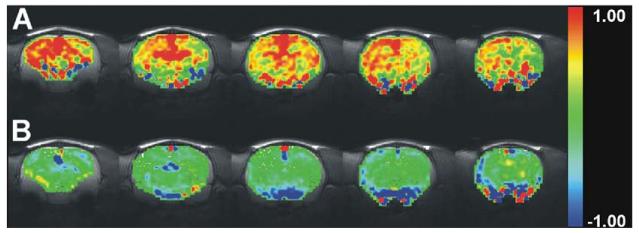
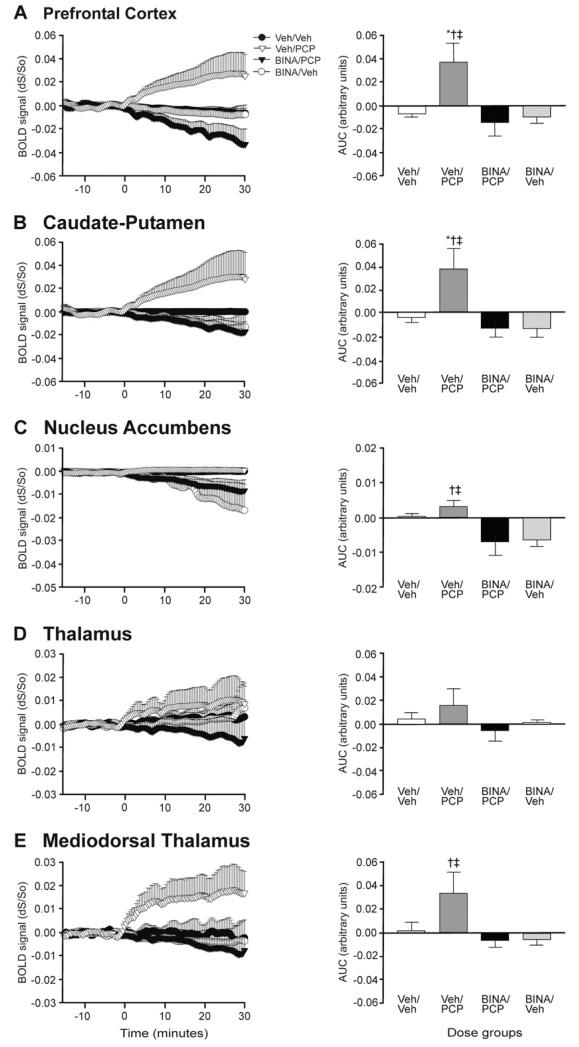
We pharmacologically challenged subjects with PCP (5.6 mg/kg i.p.) to assess the brain regions activated by the psychotomimetic agent. Acute PCP administration in drug naive rats led to robust, sustained activation of cortical, striatal, and mediodorsal thalamic brain regions as shown by mean AUC activation maps depicting the 15 min post-stimulant window of the PCP-induced BOLD response in five coronal brain slices (Fig. 3A). These brain sections include the PFC, C–P, Thal, and NAc for which we analyzed time courses and AUCs (Fig. 4). The time courses of PCP-mediated BOLD signal changes were similar in all regions showing activation (Fig. 4). We observed statistically significant PCP-induced BOLD increases in ROIs encompassing the PFC, C–P, and MDTN, but not in the NAc (Fig. 4C) by one way ANOVA and post hoc analyses.
Fig. 3.
Group AUC maps. Group maps depict mean AUC on a voxel-wise basis for the 15 min post-PCP interval for the (A) Veh/PCP group, which show widespread positive mean AUC values and (B) BINA/PCP, which show lower mean AUC values. Colored pixels correspond to AUC values. The five brain slices include the following regions: hippocampus, thalamus, retrosplenial cortex, motor cortex, cingulate cortex, medial prefrontal cortex, caudate-putamen, and nucleus accumbens.
Fig. 4.
Time course and AUC analyses in specific ROIs. PCP-induced BOLD signal (fractional change relative to baseline dS/So) increases in the (A) PFC (vs. Veh/Veh * P<0.05; vs. BINA/PCP † P<0.01; vs. BINA/Veh ‡ P<0.05) and (B) C–P (vs. Veh/Veh * P<0.05; vs. BINA/PCP † P<0.01; vs. BINA/Veh ‡ P<0.01) are suppressed by BINA pretreatment. BINA pretreatment decreases BOLD activity in the (C) NAc and (E) MDTN (vs. BINA/PCP † P<0.05; vs. BINA/Veh ‡ P<0.05). (D) For the entire thalamus, drug effects were not significant. (n=5–7 per treatment group.) AUC was calculated for the first 15 min after PCP injection.
BINA suppresses PCP-induced BOLD signal changes
For the phMRI studies, BINA was administered at the same dose (32 mg/kg) and administration route (i.p.) as in the locomotor behavior experiments to evaluate the ability of mGluR2 potentiation to modulate PCP-induced brain activation. The group mean AUC maps (Fig. 3B) show that potentiation of mGluR2 with BINA 30 min before PCP administration suppressed the PCP response, as demonstrated by the lower mean AUC values for BINA/PCP compared to the Veh/PCP group in the PFC, C–P, NAc, MDTN, as well as the retrosplenial and motor cortices. We characterized the extent of BOLD activation induced by PCP and the suppressive effects of mGluR2 potentiation by time course and AUC analyses. Time series and AUC data from specific ROIs revealed marked BINA-mediated inhibition in the PFC and C–P (Fig. 4A, B). BINA pretreatment completely blunted the amplitude of PCP-induced activation in these regions for the entire scan duration (Fig. 4A, B). One-way ANOVA demonstrated a drug-dependent effect in post-challenge AUC for the PFC [F(3,23)=7.313, P=0.0017] and C–P [F(3,23)=5.630, P=0.0058] with post hoc analysis indicating that the AUC values for the BINA/PCP group were significantly different from that of Veh/PCP (PFC and C–P, P<0.01). Similarly, there was a significant difference between the Veh/PCP group compared to Veh/Veh (PFC and C–P, P<0.05) and BINA/Veh (PFC, P<0.05; C–P, P<0.01) control groups. Although there was a significant difference in BOLD AUC in the NAc [F(3,23)=0.4.086, P=0.025], post hoc analysis demonstrated that the nominal increase in PCP-mediated activation was not significant compared to vehicle challenge (P>0.05) in that region. BINA pretreatment, however, significantly decreased brain activation in the NAc in both BINA/PCP and BINA/Veh groups compared to the Veh/Veh control (P<0.05) (Fig. 4C). In the mediodorsal thalamic nucleus, a region with projections to the frontal cortex, we found significant decreases in brain activity in subjects pretreated with BINA (BINA/PCP) compared to animals treated only with PCP (Veh/PCP) [F(3,23)=3.440, P=0.0364] (Fig. 4E).
DISCUSSION
The present findings represent the first report of the characterization of a novel mGluR2 positive allosteric modulator, BINA, on changes in brain activation by phMRI. Our results demonstrate that selective positive allosteric modulation of mGluR2 can inhibit abnormal hyperglutamatergic brain activity induced by the NMDAR antagonist PCP, a widely used pharmacological challenge that induces symptoms similar to schizophrenia in healthy humans. The ability of BINA to suppress PCP-mediated brain activity correlated with results from the behavioral studies, reversal of PCP-induced hyperlocomotor activity, similar to the actions of clinical antipsychotics and mGluR2/3 agonists. Our findings provide further evidence that the antipsychotic-like effects of mGluR2/3 agonists in animal models can be mimicked by selective potentiation of mGluR2 by BINA, raising the exciting possibility that selective allosteric potentiators of mGluR2 may provide antipsychotic efficacy in patients. In fact, recent studies in mGluR2-, mGluR3-, and double knockout mice have demonstrated that the antipsychotic effects of mGluR2/3 agonists are mediated by mGluR2 (Spooren et al., 2000; Woolley et al., 2008; Fell et al., 2008), and interestingly, our results support previous work by Gozzi et al. (2008a) on the reversal of PCP-induced brain activation by the mGluR2/3 agonist LY354740, also suggesting that selective mGluR2 potentiation is sufficient to produce the antipsychotic effects observed in phMRI studies with group II agonists. The lack of specific orthosteric agonists that discriminate between the two subtypes due to the high conservation of the glutamate binding site underscores the importance of selective allosteric modulators; thus the synthesis and characterization of BINA by our group (Galici et al., 2006) was a major advance in further demonstrating the feasibility of targeting individual mGluRs systemically, and revealed the antipsychotic-like efficacy of selective mGluR2 potentiation in murine models, which could be abolished by the mGluR2/3 antagonist LY341495 (Galici et al., 2006). Here, we were able to replicate the suppressive effects of BINA on PCP-induced hyperlocomotor activity in rat with a shorter 30-min pretreatment, which confirmed its behavioral efficacy and also served as a benchmark for compound dosing for our phMRI studies. Interestingly, BINA has also been shown to suppress hallucinogen-induced behaviors (Benneyworth et al., 2007). Its success in two distinct preclinical paradigms of schizophrenia warranted further investigation on its sites of action.
Unlike the various mGluR2/3 agonists previously evaluated, BINA’s discrimination between the two subtypes (Galici et al., 2006) renders it a useful tool to study the role of mGluR2 in the CNS with particular relevance to schizophrenia due to its presynaptic expression pattern and function. In order to better understand the underlying circuitry mediated by pharmacologic agents, we utilized phMRI using BOLD contrast, which is sensitive to fluctuations in the oxygenation state of hemoglobin in blood and blood flow that follows changes in neuronal activity and is directly translatable to human imaging (Ogawa et al., 1990; Logothetis et al., 2001; Gore, 2003). Clinical phMRI studies have reported NMDAR antagonist-mediated increases in regional BOLD signal (Deakin et al., 2008) as well as BOLD signal changes associated with task performance relative to control subjects (Abel et al., 2003; Northoff et al., 2005; Daumann et al., 2008; Honey et al., 2008). For example, Deakin et al. (2008) recently demonstrated that ketamine induced BOLD signal increases in frontal gyri, middle temporal gyrus, and thalamus in healthy volunteers similar to results from an earlier PET study showing significant increases in regional cerebral blood flow in the frontal cortex, anterior cingulate, caudate, putamen, and thalamus (Langsjo et al., 2003). Acute NMDAR antagonist administration has also been recently adapted for rodent phMRI studies in which a similar sensitivity to NMDAR antagonism has been demonstrated, highlighting the relevance and utility of the rodent PCP model. Our findings are consistent with these animal and clinical phMRI data with acute PCP drug challenge producing a robust, sustained BOLD response in distinct brain structures of anesthetized rats, specifically the PFC, C–P, and thalamus, converging with cortico–striato–thalamic circuitry implicated in the pathophysiology of schizophrenia. The phMRI results correlate with the behavioral hyperactivity we observed upon PCP administration in the locomotor studies and are also consistent with PCP-induced regional cerebral blood volume (CBV) increases observed by contrast-enhanced phMRI in anesthetized rat (Gozzi et al., 2008a), microdialysis results in free-moving animals (Nishijima et al., 1996; Adams and Moghaddam, 2001), and [14 C]-2-deoxyglucose autoradiographic studies after administration of NMDAR antagonists ketamine and MK-801 in unanesthetized rats (Duncan et al., 1999). The nominal rise in PCP-mediated BOLD signal we observed in the NAc, however, was not significant compared to the vehicle treated (Veh/Veh) group, a similar finding to that of Gozzi et al. (2008a).
Utilizing the PCP-induced BOLD signal readout, we examined whether selective potentiation of mGluR2 with the positive allosteric modulator BINA could modulate the response. BINA blocked PCP-evoked brain activation in the PFC, C–P, and MDTN, revealing underlying circuitry consistent with the behavioral effects and expression pattern of mGluR2 (Ohishi et al., 1993; Gu et al., 2008). The ability of mGluR2 potentiation to block PCP-evoked BOLD signal in these specific regions are compelling as this circuitry is found to be abnormal both structurally and functionally in schizophrenic patients (Andersen et al., 1999; Shenton et al., 2001; Meyer-Lindenberg and Weinberger, 2006). A balance of excitatory input from the cortex and thalamus to the dorsal striatum, dopaminergic innervation from the striatum to the PFC, and reciprocal thalamo–cortical connections all control normal processing, which is dysfunctional in schizophrenia (Meyer-Lindenberg and Weinberger, 2006). The reversal of the PCP-induced increases in BOLD signal in the PFC appears to correspond to BINA’s suppressive effects in PCP-induced hyperactivity experiments as the PFC has been shown to mediate this behavior (Takahata and Moghaddam, 2003), and, thus, may serve a functional readout for treatment of symptoms of schizophrenia. Interestingly, BINA significantly decreased BOLD signal in the NAc whether it was followed by vehicle (BINA/Veh) or PCP (BINA/PCP). These results are consistent with the ability of the mixed mGluR2/3 agonist LY354740 to inhibit PCP-induced CBV increases (Gozzi et al., 2008a). Unlike Gozzi et al. (2008a) we did not find significant activation in the dorsal hippocampus by PCP. LY354740 could reverse this regional response (Gozzi et al., 2008a), while BINA pretreatment led to a trend toward decrease (data not shown). Although this difference could be attributed to mGluR3 in the dorsal hippocampus, it is likely due to the higher sensitivity of the CBV phMRI method utilizing exogenous contrast agent compared to the less invasive BOLD contrast method we employed. Since BOLD functional magnetic resonance imaging (fMRI) is based on hemodynamic response, drug-induced changes in cerebrovascular tone independent of neural activity and on global cardiovascular function can confound results. No significant changes in heart rate or etCO2 were observed following BINA administration in the present study, and Gozzi et al. (2008a) reported that the mGluR2/3 agonist LY354740 did not significantly alter mean arterial blood pressure, suggesting that, as for the direct mGluR2/3 agonist LY354740, there were no confounding cardiovascular effects of BINA.
While the mechanism underlying the effects of BINA are unclear, it is possible that BINA potentiates presynaptic mGluR2 in the presence of glutamate leading to decreased glutamatergic transmission, resulting in the inhibition of PCP-induced neural activity and behavioral output by dampening excitatory neurotransmission in specific brain regions. Its known function as an autoreceptor that decreases glutamatergic output supports this model. Moreover, the decreased levels of mGluR2 transcript in postmortem brains of untreated schizophrenic patients (Gonzalez-Maeso et al., 2008) support the hypothesis of abnormally hyperexcitatory tone in the schizophrenic brain, which could be effectively normalized through potentiation or activation of mGluR2. The adverse effects associated with current schizophrenia treatments, including severe extrapyramidal symptoms (typical antipsychotics) as well as weight gain and agranulocytosis (atypical drugs) and failure to address cognitive impairment (Kurz et al., 1995; Meltzer, 2004; Henderson, 2007) underscore the need for alternative targets outside of the dopaminergic and serotonergic systems. The advantages of BINA over an earlier mGluR2 receptor potentiator, LY487379 include its higher potency and longer duration of action (Galici et al., 2005, 2006), so that the question of whether chronic mGluR2 potentiation leads to tolerance can now be tested. Since positive allosteric modulators of mGluR2 decrease excitatory neurotransmission only when there is sufficient glutamatergic tone, they may overcome the problem of potential receptor desensitization from prolonged receptor activation. Indeed, tolerance developed to the behavioral effects of the agonist LY379268 (Galici et al., 2005). In future studies, we need to determine whether BINA continues to be efficacious in chronic dosing paradigms. That mGluR2/3 agonists alleviated the cognitive deficits induced by the NMDAR antagonist ketamine in healthy humans undergoing working memory tasks (Krystal et al., 2005) is very compelling, and the potential role of mGluR2 on cognition needs to be addressed to determine the subtype that can mediate procognitive effects.
Across numerous neuroimaging studies in awake volunteers and anesthetized rodents, a similar pattern of NMDAR antagonist generated brain activation has emerged, so although anesthesia attenuates neural activity, the level of isoflurane we utilized for phMRI does not appear to have significantly altered or reduced the activation pattern produced by NMDAR antagonists. It is important to recognize, however, that anesthesia could potentially dampen functional response as inhalation and injectable anesthetics have been shown to alter cerebral glucose metabolism (Dudley et al., 1982; Ori et al., 1986) and increase inhibitory neurotransmission (Richards, 1995). However, since movement-associated artifacts would have precluded analysis and interpretation of BOLD signal data (Hajnal et al., 1994), it was not possible to perform these experiments in awake, free-breathing rats. Although Risterucci et al. (2005) reported that PCP decreased perfusion in both the PFC and striatum, this observation is likely due to the higher level of isoflurane (1.2%) used in their study (Risterucci et al., 2005). A recent study on anesthesia-PCP interactions highlighted the need to balance concentrations of both (i.e., 0.8% halothane or 1% isoflurane with subanesthetic doses of PCP) to prevent negative responses in areas that are normally activated (Gozzi et al., 2008b). In our studies, 1% isoflurane, the minimum concentration we could allow for proper maintenance anesthesia in all animals, did not result in negative BOLD patterns of response to PCP at 5.6 mg/kg, a concentration that led to robust increases in locomotor output.
CONCLUSION
In conclusion, the current studies bolster the growing body of evidence that mGluR2 is a promising target for the treatment of schizophrenia. By utilizing the psychotomimetic PCP, we were able to show the antipsychotic-like activity of the selective mGluR2 allosteric potentiator BINA. Its efficacy in alleviating a hyperglutamatergic state was demonstrated by its ability to suppress PCP-mediated hyperlocomotion and reduce PCP-induced brain activity in the rat. By utilizing phMRI, we were able to map the PCP-induced BOLD response and evaluate its blockade by BINA pretreatment in vivo. By resolving PCP activation and BINA extinction maps, we showed that potentiation of the mGlu2 receptor can block PCP-mediated brain activity in structures linked to schizophrenia, defining the mechanism of this compound at the brain circuitry level, which has important implications for the development of novel antipsychotic drugs. This study also underscores the utility of phMRI to determine neuropharmacological mechanisms underlying the effects of therapeutics for treating psychiatric and neurological disorders.
Acknowledgments
This work was supported by grants from the National Institutes of Health, NIBIB and NIMH. Vanderbilt is a site in the National Institutes of Health-Supported Molecular Libraries Probe Center Network. We would like to thank Jarrod True of the Vanderbilt University Institute of Imaging Science for his technical support and assistance.
Abbreviations
- AFNI
Analysis of Functional NeuroImages
- AUC
area under the curve
- BINA
biphenyl indadone-A
- BOLD
blood oxygenation level-dependent
- CBV
cerebral blood volume
- CNS
central nervous system
- C–P
caudate–putamen
- fMRI
functional magnetic resonance imaging
- FOV
field of view
- MDTN
mediodorsal thalamic nuclei
- mGluR
metabotropic glutamate receptor
- NAc
nucleus accumbens
- NEX
number of excitations
- NMDAR
N-methyl-d-aspartate receptor
- PCP
phencyclidine
- PFC
prefrontal cortex
- phMRI
pharmacological magnetic resonance imaging
- ROI
region of interest
- TE
echo spacing
- Thal
thalamus
- TR
repetition time
- Veh
vehicle
REFERENCES
- Abel KM, Allin MP, Kucharska-Pietura K, Andrew C, Williams S, David AS, Phillips ML. Ketamine and fMRI BOLD signal: distinguishing between effects mediated by change in blood flow versus change in cognitive state. Hum Brain Mapp. 2003;18:135–145. doi: 10.1002/hbm.10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams BW, Moghaddam B. Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol Psychiatry. 2001;50:750–757. doi: 10.1016/s0006-3223(01)01195-7. [DOI] [PubMed] [Google Scholar]
- Andersen AH, Gash DM, Avison MJ. Principal component analysis of the dynamic response measured by fMRI: a generalized linear systems framework. Magn Reson Imaging. 1999;17:795–815. doi: 10.1016/s0730-725x(99)00028-4. [DOI] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Swerdlow NR, Geyer MA. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J Pharmacol Exp Ther. 1994;271:787–794. [PubMed] [Google Scholar]
- Benneyworth MA, Xiang Z, Smith RL, Garcia EE, Conn PJ, Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol Pharmacol. 2007;72:477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–170. [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Cha JH, Makowiec RL, Penney JB, Young AB. L-[3H]glutamate labels the metabotropic excitatory amino acid receptor in rodent brain. Neurosci Lett. 1990;113:78–83. doi: 10.1016/0304-3940(90)90498-x. [DOI] [PubMed] [Google Scholar]
- Chen YC, Galpern WR, Brownell AL, Matthews RT, Bogdanov M, Isacson O, Keltner JR, Beal MF, Rosen BR, Jenkins BG. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med. 1997;38:389–398. doi: 10.1002/mrm.1910380306. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Daumann J, Heekeren K, Neukirch A, Thiel CM, Moller-Hartmann W, Gouzoulis-Mayfrank E. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology (Berl) 2008;200:573–583. doi: 10.1007/s00213-008-1237-1. [DOI] [PubMed] [Google Scholar]
- Deakin JF, Lees J, McKie S, Hallak JE, Williams SR, Dursun SM. Glutamate and the neural basis of the subjective effects of ketamine: a pharmaco-magnetic resonance imaging study. Arch Gen Psychiatry. 2008;65:154–164. doi: 10.1001/archgenpsychiatry.2007.37. [DOI] [PubMed] [Google Scholar]
- Detre JA, Wang J. Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol. 2002;113:621–634. doi: 10.1016/s1388-2457(02)00038-x. [DOI] [PubMed] [Google Scholar]
- Dudley RE, Nelson SR, Samson F. Influence of chloralose on brain regional glucose utilization. Brain Res. 1982;233:173–180. doi: 10.1016/0006-8993(82)90938-6. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA. Comparison of brain metabolic activity patterns induced by ketamine, MK-801 and amphetamine in rats: support for NMDA receptor involvement in responses to subanesthetic dose of ketamine. Brain Res. 1999;843:171–183. doi: 10.1016/s0006-8993(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Febo M, Ferris CF, Segarra AC. Estrogen influences cocaineinduced blood oxygen level-dependent signal changes in female rats. J Neurosci. 2005;25:1132–1136. doi: 10.1523/JNEUROSCI.3801-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell MJ, Svensson KA, Johnson BG, Schoepp DD. Evidence for the role of metabotropic glutamate (mGlu)2 not mGlu3 receptors in the preclinical antipsychotic pharmacology of the mGlu2/3 receptor agonist (−)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]hexane-4,6-dicarboxylic acid (LY404039) J Pharmacol Exp Ther. 2008;326:209–217. doi: 10.1124/jpet.108.136861. [DOI] [PubMed] [Google Scholar]
- Galici R, Echemendia NG, Rodriguez AL, Conn PJ. A selective allosteric potentiator of metabotropic glutamate (mGlu) 2 receptors has effects similar to an orthosteric mGlu2/3 receptor agonist in mouse models predictive of antipsychotic activity. J Pharmacol Exp Ther. 2005;315:1181–1187. doi: 10.1124/jpet.105.091074. [DOI] [PubMed] [Google Scholar]
- Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, de Paulis T, Conn PJ. Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J Pharmacol Exp Ther. 2006;318:173–185. doi: 10.1124/jpet.106.102046. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452:93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore JC. Principles and practice of functional MRI of the human brain. J Clin Invest. 2003;112:4–9. doi: 10.1172/JCI19010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Large CH, Schwarz A, Bertani S, Crestan V, Bifone A. Differential effects of antipsychotic and glutamatergic agents on the phMRI response to phencyclidine. Neuropsychopharmacology. 2008a;33:1690–1703. doi: 10.1038/sj.npp.1301547. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Schwarz A, Crestan V, Bifone A. Drug-anaesthetic interaction in phMRI: the case of the psychotomimetic agent phencyclidine. Magn Reson Imaging. 2008b;26:999–1006. doi: 10.1016/j.mri.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Gozzi A, Schwarz A, Reese T, Bertani S, Crestan V, Bifone A. Region-specific effects of nicotine on brain activity: a pharmacological MRI study in the drug-naive rat. Neuropsychopharmacology. 2006;31:1690–1703. doi: 10.1038/sj.npp.1300955. [DOI] [PubMed] [Google Scholar]
- Gu G, Lorrain DS, Wei H, Cole RL, Zhang X, Daggett LP, Schaffhauser HJ, Bristow LJ, Lechner SM. Distribution of metabotropic glutamate 2 and 3 receptors in the rat forebrain: implication in emotional responses and central disinhibition. Brain Res. 2008;1197:47–62. doi: 10.1016/j.brainres.2007.12.057. [DOI] [PubMed] [Google Scholar]
- Hackler EA, Turner GH, Gresch PJ, Sengupta S, Deutch AY, Avison MJ, Gore JC, Sanders-Bush E. 5-Hydroxytryptamine 2C receptor contribution to m-chlorophenylpiperazine and N-methyl-beta-carboline-3-carboxamide-induced anxiety-like behavior and limbic brain activation. J Pharmacol Exp Ther. 2007;320:1023–1029. doi: 10.1124/jpet.106.113357. [DOI] [PubMed] [Google Scholar]
- Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magn Reson Med. 1994;31:283–291. doi: 10.1002/mrm.1910310307. [DOI] [PubMed] [Google Scholar]
- Henderson DC. Weight gain with atypical antipsychotics: evidence and insights. J Clin Psychiatry. 2007;68(Suppl 12):18–26. [PubMed] [Google Scholar]
- Hollmann M, Boulter J, Maron C, Heinemann S. Molecular biology of glutamate receptors. Potentiation of N-methyl-d-aspartate receptor splice variants by zinc. Ren Physiol Biochem. 1994;17:182–183. [PubMed] [Google Scholar]
- Honey GD, Corlett PR, Absalom AR, Lee M, Pomarol-Clotet E, Murray GK, McKenna PJ, Bullmore ET, Menon DK, Fletcher PC. Individual differences in psychotic effects of ketamine are predicted by brain function measured under placebo. J Neurosci. 2008;28:6295–6303. doi: 10.1523/JNEUROSCI.0910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Baez M, Jagdmann GE, Jr, Britton TC, Large TH, Callagaro DO, Tizzano JP, Monn JA, Schoepp DD. Discovery of allosteric potentiators for the metabotropic glutamate 2 receptor: synthesis and subtype selectivity of N-(4-(2-methoxyphenoxy)phenyl)-N-(2,2,2-trifluoroethylsulfonyl)pyrid-3-ylmethylamine. J Med Chem. 2003;46:3189–3192. doi: 10.1021/jm034015u. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, McKinzie DL, Nisenbaum ES, Tizzano JP, Schoepp DD. Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s) Psychopharmacology (Berl) 2005;179:271–283. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology (Berl) 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Petrakis IL, Belger A, Berman RM, Charney DS, Abi-Saab W, Madonick S. NMDA agonists and antagonists as probes of glutamatergic dysfunction and pharmacotherapies in neuropsychiatric disorders. Harv Rev Psychiatry. 1999;7:125–143. [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Kurz M, Hummer M, Oberbauer H, Fleischhacker WW. Extrapyramidal side effects of clozapine and haloperidol. Psychopharmacology (Berl) 1995;118:52–56. doi: 10.1007/BF02245249. [DOI] [PubMed] [Google Scholar]
- Langsjo JW, Kaisti KK, Aalto S, Hinkka S, Aantaa R, Oikonen V, Sipila H, Kurki T, Silvanto M, Scheinin H. Effects of subanesthetic doses of ketamine on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:614–623. doi: 10.1097/00000542-200309000-00016. [DOI] [PubMed] [Google Scholar]
- Littlewood CL, Jones N, O’Neill MJ, Mitchell SN, Tricklebank M, Williams SC. Mapping the central effects of ketamine in the rat using pharmacological MRI. Psychopharmacology (Berl) 2006;186:64–81. doi: 10.1007/s00213-006-0344-0. [DOI] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Matthysse S. Animal models in psychiatric research. Prog Brain Res. 1986;65:259–270. doi: 10.1016/S0079-6123(08)60655-X. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. What’s atypical about atypical antipsychotic drugs? Curr Opin Pharmacol. 2004;4:53–57. doi: 10.1016/j.coph.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatrvic disorders. Nat Rev Neurosci. 2006;7:818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Morrens M, Hulstijn W, Lewi PJ, De Hert M, Sabbe BG. Stereotypy in schizophrenia. Schizophr Res. 2006;84:397–404. doi: 10.1016/j.schres.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Glutamate receptors and brain function [in Japanese] Seikagaku. 1998;70:1145–1158. [PubMed] [Google Scholar]
- Nishijima K, Kashiwa A, Hashimoto A, Iwama H, Umino A, Nishikawa T. Differential effects of phencyclidine and methamphetamine on dopamine metabolism in rat frontal cortex and striatum as revealed by in vivo dialysis. Synapse. 1996;22:304–312. doi: 10.1002/(SICI)1098-2396(199604)22:4<304::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Northoff G, Richter A, Bermpohl F, Grimm S, Martin E, Marcar VL, Wahl C, Hell D, Boeker H. NMDA hypofunction in the posterior cingulate as a model for schizophrenia: an exploratory ketamine administration study in fMRI. Schizophr Res. 2005;72:235–248. doi: 10.1016/j.schres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Nayak AS, Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14:68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Ori C, Dam M, Pizzolato G, Battistin L, Giron G. Effects of isoflurane anesthesia on local cerebral glucose utilization in the rat. Anesthesiology. 1986;65:152–156. doi: 10.1097/00000542-198608000-00004. [DOI] [PubMed] [Google Scholar]
- Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotactic coordinates. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Richards CD. The synaptic basis of general anaesthesia. Eur J Anaesthesiol. 1995;12:5–19. [PubMed] [Google Scholar]
- Risterucci C, Jeanneau K, Schoppenthau S, Bielser T, Kunnecke B, von Kienlin M, Moreau JL. Functional magnetic resonance imaging reveals similar brain activity changes in two different animal models of schizophrenia. Psychopharmacology (Berl) 2005;180:724–734. doi: 10.1007/s00213-005-2204-8. [DOI] [PubMed] [Google Scholar]
- Sams-Dodd F. Phencyclidine-induced stereotyped behaviour and social isolation in rats: a possible animal model of schizophrenia. Behav Pharmacol. 1996;7:3–23. [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Marek GJ. Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia? Curr Drug Targets CNS Neurol Disord. 2002;1:215–225. doi: 10.2174/1568007024606177. [DOI] [PubMed] [Google Scholar]
- Schwarz AJ, Zocchi A, Reese T, Gozzi A, Garzotti M, Varnier G, Curcuruto O, Sartori I, Girlanda E, Biscaro B, Crestan V, Bertani S, Heidbreder C, Bifone A. Concurrent pharmacological MRI and in situ microdialysis of cocaine reveal a complex relationship between the central hemodynamic response and local dopamine concentration. Neuroimage. 2004;23:296–304. doi: 10.1016/j.neuroimage.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooren WP, Gasparini F, van der Putten H, Koller M, Nakanishi S, Kuhn R. Lack of effect of LY314582 (a group 2 metabotropic glutamate receptor agonist) on phencyclidine-induced locomotor activity in metabotropic glutamate receptor 2 knockout mice. Eur J Pharmacol. 2000;397:R1–R2. doi: 10.1016/s0014-2999(00)00269-7. [DOI] [PubMed] [Google Scholar]
- Sturgeon RD, Fessler RG, Meltzer HY. Behavioral rating scales for assessing phencyclidine-induced locomotor activity, stereotyped behavior and ataxia in rats. Eur J Pharmacol. 1979;59:169–179. doi: 10.1016/0014-2999(79)90279-6. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Ito I, Watanabe M. Glutamate receptor subtypes may be classified into two major categories: a study on Xenopus oocytes injected with rat brain mRNA. Neuron. 1989;3:129–132. doi: 10.1016/0896-6273(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Takahata R, Moghaddam B. Activation of glutamate neurotransmission in the prefrontal cortex sustains the motoric and dopaminergic effects of phencyclidine. Neuropsychopharmacology. 2003;28:1117–1124. doi: 10.1038/sj.npp.1300127. [DOI] [PubMed] [Google Scholar]
- Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- Thomas CG, Harshman RA, Menon RS. Noise reduction in BOLD-based fMRI using component analysis. Neuroimage. 2002;17:1521–1537. doi: 10.1006/nimg.2002.1200. [DOI] [PubMed] [Google Scholar]
- Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A. Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol. 2007;5:2369–2378. doi: 10.1371/journal.pbio.0050274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley ML, Pemberton DJ, Bate S, Corti C, Jones DN. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology (Berl) 2008;196:431–440. doi: 10.1007/s00213-007-0974-x. [DOI] [PubMed] [Google Scholar]