Abstract
Background
Erythropoietin (Epo) increases and maintains hematocrit using once weekly dosing in adults with anemia due to end stage renal disease. Epo is used in preterm infants to treat the anemia of prematurity, but has not been studied using once weekly dosing, a schedule which might offer neuroprotection in addition to increasing red cell mass. We compared reticulocyte responses of once weekly Epo dosing with thrice weekly dosing in preterm infants.
Methods
Infants ≤1,500 grams and ≥7 days of age were randomized to once weekly Epo, 1,200 units/kg/dose, or thrice weekly Epo, 400 units/kg/dose, subcutaneously for 4 weeks, along with iron and vitamin supplementation. Complete blood counts, absolute reticulocyte counts (ARC), transfusions, phlebotomy losses, and adverse events were recorded.
Results
Twenty preterm infants (962±55 grams, 27.9±0.4 weeks, 17±3 days of age) were enrolled. Groups were similar at baseline. Infants in both groups increased ARC (p<0.01, thrice weekly Epo group). ARC were similar between treatment groups at the start and end of 4 weeks. Hematocrit remained stable, and similar numbers of transfusions were administered. No adverse effects of either dosing schedule were noted.
Conclusions
Preterm infants respond to weekly Epo by increasing ARC and maintaining hematocrit. We speculate that once weekly Epo dosing might be beneficial to preterm infants requiring increased erythropoiesis.
Keywords: erythropoietin, transfusions, dosing schedule, anemia of prematurity, neuroprotection
Introduction
Erythropoietin (Epo) has been shown to be effective in increasing and maintaining hematocrit using once weekly dosing in adults with anemia due to end stage renal disease, and in adults with cancer. Dosing schedules in adults have progressed from three times a week dosing 2 decades ago, to once a week dosing (1, 2). Epo has been used in preterm infants to prevent and treat the anemia of prematurity, and is usually given three times a week as a subcutaneous injection (3). Alternatively, Epo can also be given intravenously by adding it daily to parenteral nutrition solution (4). While the pharmacokinetics of Epo in preterm infants would suggest more frequent dosing (4–6), the erythrokinetics of Epo--the response of red cell precursors to Epo--may allow for less frequent dosing. In addition, recent studies evaluating non-hematopoietic, neuroprotective effects of Epo have used higher doses to achieve elevated serum and presumably CSF concentrations (7, 8). Once a week dosing of Epo might also achieve increased serum concentrations, thus potentially providing neuroprotection in addition to erythropoietic stimulus. We investigated whether weekly Epo would result in a similar reticulocyte response to three times a week dosing in preterm infants.
Materials and methods
Subjects
Infants were eligible for study if they met the following entry criteria: birth weight ≤1,500 grams, gestational age ≤32 weeks, hematocrit ≤48%, ≥7 days of age, and informed consent obtained from a parent or guardian. Infants were ineligible for study if they were already receiving Epo or were enrolled in an Epo study, if they were not expected to survive, if they had Coombs positive hemolytic disease, if clinical seizures were present, if they had known thromboses, if they had a major congenital malformation (such as trisomy 21, 18 or 13, or complex cyanotic congenital heart disease), or if they had systolic blood pressures >100 mm Hg while not on pressor support. The study was approved by the Human Research Review Committee at the University of New Mexico.
Randomization
Infants were randomized using a random number table generated by a statistical program (SysStat) to one of two treatment groups: Epo (Amgen, Thousand Oaks, CA) 1,200 units/kg once a week subcutaneously (SC), or Epo 400 units/kg/dose three times a week SC (our current clinical dosing schedule). All providers aside from the research nurse were masked to the treatment group. Infants in the once a week dosing group received sham subcutaneous injections for the other two dosing periods each week. An adhesive bandage covered the true and sham injection sites. The study drug was brought to the bedside in a closed container, and injections were shielded from the caregivers by screens. Twins were randomized to the same treatment group.
Epo Dosing
Epo was dispensed from the pharmacy in 29½ gauge, 0.3 mL syringes for SC dosing. Infants weighing ≤1,000 grams at any time during the study received the following dose: using a concentration of 2,000 units/mL, the thrice weekly dosing volume was 0.2 mL/kg body weight. Using a concentration of 4,000 units/mL, the once weekly dosing volume was 0.3 mL/kg body weight. Infants weighing >1,000 grams at any time during the study received the following dose: using a concentration of 4,000 units/mL, the thrice weekly dosing volume was 0.1 mL/kg body weight. Using a concentration of 10,000 units/mL, the once weekly dosing volume was 0.12 mL/kg body weight. The rationale for using different concentrations of Epo was to maintain the total dosing volume between 0.1 mL and 0.3 mL. Administering a dose less than 0.1 mL is technically difficult, and administering a dose greater than 0.3 mL in a small infant increases discomfort. The dose was initially based on study entry weight, and was adjusted weekly for changes in weight. The dosing continued until the infant reached study day 28, was discharged from the hospital, or was transferred to another hospital. Only the research pharmacist and the research nurse giving the SC injection knew what the infant was receiving.
Supplemental iron and vitamins
All infants received 6 mg/kg/day elemental iron while on at least 60 mL/kg/day of enteral feedings. For infants who were on less than 60 mL/kg/day enteral feeds and had total parenteral nutrition (TPN) ordered, parenteral iron in the form of iron dextran was administered once a week in the TPN. A baseline ferritin was measured, and parenteral iron dosing was based on the results (dosing was determined by the research nurse) (3). For infants with a baseline ferritin between 100 and 400 ng/mL, parenteral iron dosing was given at a dose of 3 mg/kg once a week in TPN, for up to 2 doses. For infants with a baseline ferritin less than 100, a third parenteral iron dose was given if the infant was still on TPN. For infants with a baseline ferritin greater than 400, no parenteral iron was given. All infants on any enteral feeds received 15 IU of oral Vitamin E each day, and 50 micrograms of oral folate each day.
Laboratory evaluation
Each infant had a complete blood count with differential (CBC) and reticulocyte count performed at baseline (drawn prior to study drug), day 14 and day 28 of study (labs were not redrawn if sufficient blood could not be obtained, or if the sample clotted). Absolute reticulocyte counts (ARC) were determined based on the percent reticulocyte count x red blood cell count (106 cells/mL). Circulating erythrocyte volume (mL) was calculated based on an estimated blood volume of 85 mL/kg, the weight of the infant on the day the CBC was drawn, and the hematocrit, in order to evaluate the effect of Epo on erythropoiesis. In addition, each subject had a serum ferritin measured at the beginning and end of study. During the study period the standard NICU transfusion protocol implemented in 2004 at the University of New Mexico was used to administer PRBC transfusions (Table 1). Transfusions were considered (but not mandated) when infants reached the hematocrit levels listed.
Table 1.
Transfusion Guidelines
| 1) Infants requiring moderate or significant mechanical ventilation (conventional: mean airway pressure [MAP] >8 cm H2O AND FiO2 >0.40; high frequency: MAP >14 AND FiO2 >0.40) | hematocrit <30% hemoglobin ≤10 gm/dL |
| 2) Infants requiring minimal mechanical ventilation (conventional: MAP ≤8 cm H2O and/or FiO2 ≤0.40; high frequency: MAP ≤14 and/or FiO2 ≤0.40) | hematocrit <25% hemoglobin≤ 8 gm/dL |
3) Infants on supplemental oxygen who are not requiring mechanical ventilation, and one or more of the following is present:
|
hematocrit <21% hemoglobin ≤7 gm/dL |
| 4) Infants without any symptoms, and the absolute reticulocyte count is <100,000 cells/μL (<2%) | hematocrit ≤18% hemoglobin ≤6 gm/dL |
Data analysis and power analysis
Statistical analyses were performed using R (4a). Differences in hematocrit, reticulocyte count, and transfusion number and volumes were compared using paired and unpaired t-tests or ANOVA. For this study, the primary outcome variable was change in reticulocyte count from baseline. Previous studies in preterm infants receiving Epo (compared to placebo) have shown a difference in reticulocyte counts of 150±70 x103 cells/μl during a two to four week period (3). We previously evaluated two dosing schedules and reported a difference in reticulocyte response of 75 x103 cells/μl (4). Based on a difference in the means of 75 x103 cells/μl and a standard deviation of 50 x103 cells/μl, a total of 8 infants in each group were required to obtain an α of 0.05 with 80% power. As this was a pilot study, we did not determine a sample size based on equivalence between the two dosing schedules. An equivalence study would require 223 infants in each arm to determine if the two dosing schedules were similar, using an equivalence interval for mean differences of ±25 x103 cells/μl and observed standard deviation of approximately 90 x103 cells/μl for differences between baseline and endpoint values. For this pilot study we planned to enroll 10 infants in each group to allow for patient drop out.
Results
Twenty preterm infants (10 in each treatment group; one set of twins in the once weekly treatment group) were enrolled at the University of New Mexico between April 2006 and March 2009. One infant in each of the treatment groups died from necrotizing enterocolitis (NEC). Infant characteristics (birth weight, gestational age, age upon entry into study when first dose of study drug was administered, hematologic parameters) were similar between the two groups at baseline (Table 2). Four of 10 infants in each group were still receiving TPN. All surviving infants were fed breast milk plus fortifier (6/9 in each group) or premature formula, enriched to 24–26 calories per ounce.
Table 2.
Subject Characteristics at Study Entry
| Once/Week (n=10) | Three Times/Week (n=10) | |
|---|---|---|
| Birth Weight (grams) | 922±75 | 1045±83 |
| Gestation (weeks) | 27.9±0.6 | 27.8±0.6 |
| Age on day 1 of study drug (days) | 18±3 | 15±4 |
| Hematocrit (%) | 29.9±1.5 | 31.7±1.9 |
| ARC (x103/μL) | 122±12 | 140±24 |
| ANC (x103/μL) | 10.8±3.6 | 10.1±3.8 |
| Platelets (x103/μL) | 403±49 | 291±45 |
| Ferritin (ng/mL) | 119±18 | 276±99 |
| Transfusions prior to study (#/pt) | 0.8±0.4 | 0.8±0.4 |
| Transfusion volume prior to study (mL/kg) | 10±6 | 15±8 |
| Phlebotomy losses prior to study (mL/kg) | 18±3 | 26±9 |
ARC: absolute reticulocyte count; ANC: absolute neutrophil count. Values represent mean±SE
Absolute reticulocyte counts (ARC, Figure 1) increased during the study period (NS for baseline versus week 2 in both groups; NS for baseline vs. week 4 in once weekly group; p<0.01, baseline versus week 4 in thrice weekly group). ARC were similar between groups at baseline, two weeks and four weeks of the study. Because of a lack of data points due to insufficient blood samples provided, the difference between baseline ARC and ARC at 4 weeks was not significant in the weekly Epo group.
Figure 1.
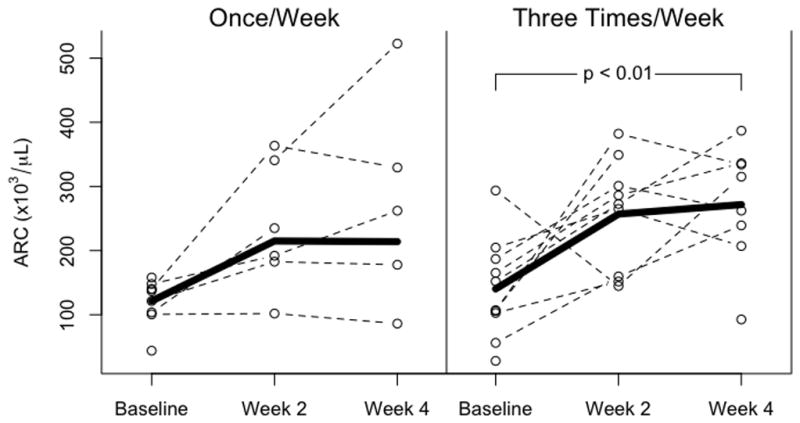
Absolute reticulocyte counts in infants receiving once weekly Epo, 1,200 units/kg/dose (left panel) or thrice weekly Epo, 400 units/kg/dose. There were no significant differences between groups in ARC at the beginning or end of the study. ARC increased with both dosing schedules, achieving significance in the three times weekly dosing (p<0.01).
There were no significant differences between groups in hematocrit during the 4 week study period, and no significant differences within groups over the four weeks of study (Figure 2). Circulating erythrocyte volumes (CEV) were calculated by multiplying study hematocrit with an estimated blood volume of 85 mL/kg and an estimated weight increase of 2% per day. CEV in both treatment groups increased significantly from baseline over the 4 week study period (Figure 3).
Figure 2.
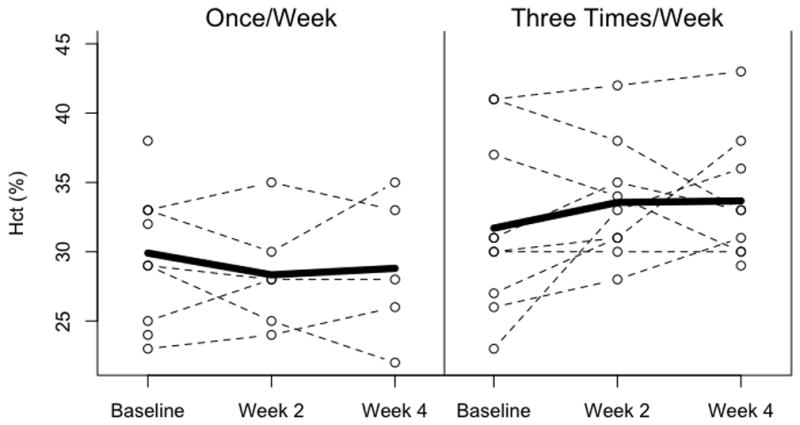
Hematocrit in infants receiving once weekly dosing (left panel) and thrice weekly dosing (right panel). Hematocrit remained stable in both treatment groups, and no significant differences were noted in hematocrit between groups during the four week study.
Figure 3.
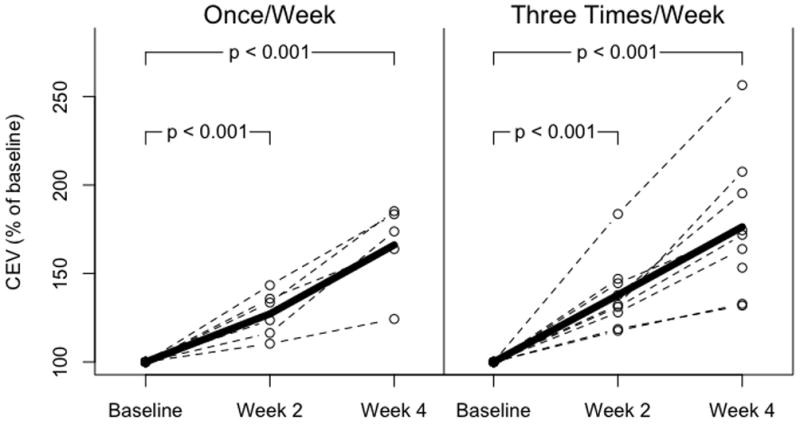
Circulating erythrocyte volume (CEV in infants receiving once weekly dosing (left panel) and thrice weekly dosing (right panel). CEV is expressed as percent of baseline. CEV increased significantly in both treatment groups to a similar extent (p<0.001, baseline versus week 4 for both groups).
There were no statistical differences in absolute neutrophil counts between groups throughout the study. For all subjects, there was an overall decrease in ANC from baseline to week 4 of study (p<0.05, data not shown). There were no differences in platelet counts between groups or within groups throughout the study.
Infants had similar number and volume of transfusions (Table 3). Phlebotomy losses were similar between groups (29±6 mL/kg once weekly, 33±10 mL/kg thrice weekly). A total of 9 transfusions were administered during the 4 week study period, for an average of 0.6 transfusions per patient in the once weekly group and 0.4 transfusions in the thrice weekly group (p=NS). Ferritin concentrations were similar at the beginning of study, and decreased significantly in both treatment groups despite iron supplementation (p<0.05, day 1 versus day 28).
Table 3.
Transfusions and phlebotomy losses
| Once/Week | Three Times/Week | |
|---|---|---|
| Un-transfused infants day 1 | 6/10 | 6/10 |
| Un-transfused infants day 28 | 5/10 | 6/10 |
| Number of infants transfused during study | 3/10 | 2/10 |
| Transfusions during study (per pt) | 0.6±0.3 | 0.4±0.3 |
| Transfusions during hospitalization (per pt) | 1.5±0.6 | 1.4±0.7 |
| Transfusion volume during study (mL/kg) | 9±6 | 8±6 |
| Total transfusion volume (mL/kg) | 13±9 | 20±12 |
| Phlebotomy during study (mL/kg) | 10±3 | 13±4 |
| Total phlebotomy (mL/kg) | 29±6 | 33±10 |
Values represent mean±SE
Morbidities associated with prematurity were similar in both groups (Table 4), and were similar to previous studies of the same weight and gestation (10). No infant was diagnosed with thromboses or hypertension (defined as systolic blood pressure consistently greater than 100 mm Hg) in either treatment group during the study. One infant in the once weekly dosing group and three infants in the thrice weekly dosing group had grade 3–4 intraventricular hemorrhage (IVH), all diagnosed prior to study entry. The incidence of retinopathy of prematurity (ROP) was low: 5 infants with stage 1 ROP and 1 infant with stage 2 ROP in the thrice weekly group. In the once weekly group, 5 infants had stage 1 ROP. None of the infants enrolled required surgical intervention, and all ROP had resolved by 36 weeks corrected gestation. A similar number of infants in each treatment group had bronchopulmonary dysplasia (oxygen requirement at 36 weeks corrected age).
Table 4.
Morbidities and Mortality
| Once/Week | Three Times/Week | |
|---|---|---|
| ROP ≥ stage 3 | 0/9 | 0/9 |
| IVH ≥ grade 3 | 1/10 | 3/10 |
| Hypertension | 0 | 0 |
| BPD | 5/9 | 6/9 |
| Death | 1/10 | 1/10 |
ROP: retinopathy of prematurity; IVH: intraventricular hemorrhage; BPD: bronchopulmonary dysplasia
Discussion
Numerous studies evaluating the use of Epo to prevent and treat the anemia of prematurity have shown that Epo is successful in preterm infants in stimulating erythropoiesis, and transfusion requirements are decreased (3). Success rates in decreasing and preventing transfusions in preterm infants are dependent in part on starting hematocrit, transfusion criteria and the frequency of phlebotomy (11). A variety of doses and dosing schedules have been evaluated. Similar to adults, a weekly dosing schedule, if shown to be effective, would be beneficial to preterm infants, in that the number of total doses with be significantly decreased. We designed a randomized, masked study to evaluate the response of preterm infants receiving once a week Epo dosing, compared to three times a week dosing.
Erythroid progenitor responsiveness appeared similar between once weekly and thrice weekly dosing. Hematocrits in both treatment groups were maintained during the four week study due to active erythropoiesis, resulting in an approximate 60% increase in CEV. No adverse effects were noted with a higher dose given once a week, and morbidities were similar between the two dosing groups. Although the study was not powered to identify differences in infrequent side effects or morbidities, including ROP (12), the incidence of ROP was similar to other infants in our NICU not receiving Epo.
For most intensive care patients, neonatal through adult, the number of transfusions and donors has decreased over the past twenty years. The decrease is due to a combination of factors, including newer blood banking practices, instituting more restrictive transfusion guidelines, an improved understanding of the pathophysiology of anemia, and a greater perception of the risks versus benefits of red cell transfusions, especially risks associated with prematurity, such as NEC and IVH (13, 14).
Despite these changes, the number of transfusions given to preterm infants remains significant (15, 16). The largest randomized study performed in Canadian centers (Preterm Infants in Need of Transfusion, or PINT Study) evaluating restrictive transfusion guidelines in over 400 ELBW infants showed no difference between restrictive and liberal transfusion guidelines in the primary outcome of death or morbidity (17). Concerns were initially raised about possible negative effects of restrictive transfusion practices in the Iowa transfusion study (18), however long term follow up of those infants revealed lower performance scores on measures of associative verbal fluency, visual memory, and reading in the liberally-transfused group compared to the restrictively-transfused group (19, 20), bringing into question the benefit of liberal transfusion guidelines. In a follow up study of infants enrolled in the original PINT study, there were no differences in neurodevelopmental outcomes between the restrictive and liberal transfusion groups (21), however post hoc analyses revealed an increased number of infants with a mental developmental index score <85 in the restrictive transfusion group. The transfusion guidelines reported here have been used clinically at the University of New Mexico since 2004, and are being evaluated in a randomized, masked study of weekly darbepoetin in preterm infants enrolled at high-altitude (>5000 ft) NICUs (NCT00334737). Further study is required to determine the most appropriate transfusion guidelines for preterm infants, and the role erythropoiesis stimulating agents will play in increasing the red cell mass.
Interest has grown regarding the non-erythropoietic effects of Epo in term and preterm infants. In addition to stimulating erythropoiesis, Epo has been shown to be protective in the developing brain in animal models (22–26), making it possibly beneficial for very premature infants who are at risk for IVH, hypoxic-ischemic injury, and developmental delay. The neuroprotective mechanisms of Epo include increased neurogenesis (27), decreased neuronal susceptibility to glutamate toxicity (28, 29), decreased neuronal apoptosis (30–34), decreased inflammation (35, 36), decreased nitric oxide-mediated injury (37–39), and increased protective effects on glia (40–42).
A small number of studies evaluating Epo in neonates have shown improved recovery from HIE in term infants (43), decreased nitric oxide-mediated injury (8), and long term neurodevelopmental improvement in preterm infants with IVH (44). Although we did not measure peak Epo concentrations in these infants, based on our previous study evaluating Epo concentrations in preterm infants receiving 400 units/kg three times a week SC (45), peak Epo concentrations in preterm infants receiving once weekly dosing would be in the range of 4,000 to 6,000 mU/mL. This range might be high enough to provide neuroprotection in preterm infants (46). Evaluation of long term development (4 and 6 years) of preterm infants 500–1,250 grams randomized to Epo, darbepoetin (a long acting biologically modified version of Epo) or placebo is currently underway (NCT01207778).
This pilot study was designed to determine if further investigation of weekly Epo administration is warranted, with respect to reducing transfusion requirements, and with the possibility of generating higher Epo concentrations that might provide neuroprotection. The study is limited in the number of infants enrolled, and in the somewhat limited time course (4 weeks) of Epo treatment. We speculate that the significant increase in reticulocyte counts and CEV seen in our study might also occur when Epo is administered earlier during the hospital course, and for a longer duration. Further study is required to identify what, if any neuroprotective affects this Epo dosing schedule might have in preterm infants, and to perform equivalency studies (accompanied by measurement of peak Epo concentrations) in a larger prospective study.
Acknowledgments
The authors are indebted to the parents for their willingness to allow their infants to participate in this study, and to the staff and nurses at the University of New Mexico Newborn Intensive Care Unit and the CTSC Research Coordinators for their support and contributions to this study. We also wish to thank Rebecca Moran, MD, for her thoughtful review and comments, and Ann Chavez for her assistance in editing the manuscript.
Supported by a grant from The University of New Mexico Clinical Translational Science Center, 1UL1RR031977
Abbreviations
- Epo
erythropoietin
- TPN
total parenteral nutrition
- Hct
hematocrit
- ARC
absolute reticulocyte count
- CEV
circulating erythrocyte volume
References
- 1.Locatelli F, Baldamus CA, Villa G, Ganea A, Martin de Francisco AL. Once weekly compared with three times weekly subcutaneous epoetin : results from a randomized, multicenter, therapeutic-equivalence study. Am J Kidney Dis. 2002;40:119–125. doi: 10.1053/ajkd.2002.33920. [DOI] [PubMed] [Google Scholar]
- 2.Weiss LG, Clyne N, Fihlho JD, Frisenette-Fich C, Kurkus J, Svensson B. The efficacy of once weekly compared with two or three times weekly subcutaneous epoetin : results from a randomized controlled multicenter trial. Nephrol Dial Transplant. 2000;15:2014–2019. doi: 10.1093/ndt/15.12.2014. [DOI] [PubMed] [Google Scholar]
- 3.Ohls RK, Ehrenkranz RA, Wright LL, Lemons JA, Korones SB, Stoll BJ, Stark AR, Shankaran S, Donovan EF, Close NC, Das A. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birthweight: a multi-center, randomized controlled trial. Pediatrics. 2001;108:934–942. doi: 10.1542/peds.108.4.934. [DOI] [PubMed] [Google Scholar]
- 4.Ohls RK, Veerman MW, Christensen RD. Pharmacokinetics and effectiveness of recombinant erythropoietin administered to preterm infants by continuous infusion in parenteral nutrition solution. J Pediatr. 1996;128:518–23. doi: 10.1016/s0022-3476(96)70363-3. [DOI] [PubMed] [Google Scholar]
- 4a.R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. URL http://www.R-project.org/ [Google Scholar]
- 5.Brown MS, Jones MA, Ohls RK, Christensen RD. Single-dose pharmacokinetics of recombinant human erythropoietin in preterm infants after intravenous and subcutaneous administration. J Pediatr. 1993;122:655–7. doi: 10.1016/s0022-3476(05)83559-0. [DOI] [PubMed] [Google Scholar]
- 6.Freise KJ, Widness JA, Veng-Pedersen P. Erythropoietic response to endogenous erythropoietin in premature very low birth weight infants. J Pharmacol ExpTher. 2010 Jan;332(1):229–37. doi: 10.1124/jpet.109.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics. 2008 Aug;122(2):383–91. doi: 10.1542/peds.2007-2711. [DOI] [PubMed] [Google Scholar]
- 8.Elmahdy H, El-Mashad AR, El-Bahrawy H, El-Gohary T, El-Barbary A, Aly H. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics. 2010;125:e1135–42. doi: 10.1542/peds.2009-2268. [DOI] [PubMed] [Google Scholar]
- 9.Maier RF, Obladen M, Kattner E, Natzschka J, Messer J, Regazzoni BM, Speer CP, Fellman V, Grauel EL, Groneck P, Wagner M, Moriette G, Salle BL, Verellen G, Scigalla P. High-versus low-dose erythropoietin in extremely low birth weight infants. The European Multicenter rhEPO Study Group. J Pediatr. 1998 May;132(5):866–70. doi: 10.1016/s0022-3476(98)70320-8. [DOI] [PubMed] [Google Scholar]
- 10.Ohls RK, Ehrenkranz RA, Das A, Dusick AM, Yolton K, Sherwonit E, Delaney-Black V, Papile LA, Simon NP, Steichen JJ, Lee KG. Neurodevelopmental outcome and growth at 18–22 months corrected age in extremely low birth weight infants treated with early erythropoietin and iron. Pediatrics. 2004;114:1287–91. doi: 10.1542/peds.2003-1129-L. [DOI] [PubMed] [Google Scholar]
- 11.Ohls RK. Erythropoietin treatment in extremely low birth weight infants: blood in versus blood out. J Pediatr. 2002;140:3–6. doi: 10.1067/mpd.2002.125853. [DOI] [PubMed] [Google Scholar]
- 12.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;3:CD004863. doi: 10.1002/14651858.CD004863.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Blau J, Calo JM, Dozor D, Sutton M, Alpan G, La Gamma EF. Transfusion-related acute gut injury: necrotizing enterocolitis in very low birth weight neonates after packed red blood cell transfusion. J Pediatr. 2011;158:403–9. doi: 10.1016/j.jpeds.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Baer VL, Lambert DK, Henry E, Snow GL, Butler A, Christensen RD. Among very-low-birth-weight neonates is red blood cell transfusion an independent risk factor for subsequently developing a severe intraventricular hemorrhage? Transfusion. 2011 Mar 7; doi: 10.1111/j.1537-2995.2011.03081.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK. Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics. 2005 May;115(5):1299–306. doi: 10.1542/peds.2004-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier RF, Sonntag J, Walka MM, Liu G, Metze BC, Obladen M. Changing practices of red blood cell transfusions in infants with birth weights less than 1000 g. J Pediatr. 2000 Feb;136(2):220–4. doi: 10.1016/s0022-3476(00)70105-3. [DOI] [PubMed] [Google Scholar]
- 17.Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, Peliowski A, Rios A, LaCorte M, Connelly R, Barrington K, Roberts RS. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006 Sep;149(3):301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Bell EF, Strauss RG, Widness JA, Mahoney LT, Mock DM, Seward VJ, Cress GA, Johnson KJ, Kromer IJ, Zimmerman MB. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005 Jun;115(6):1685–91. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nopoulos PC, Conrad AL, Bell EF, Strauss RG, Widness JA, Magnotta VA, Zimmerman MB, Georgieff MK, Lindgren SD, Richman LC. Long-term outcome of brain structure in premature infants: effects of liberal vs. restricted red blood cell transfusions. Arch Pediatr Adolesc Med. 2011 Jan 3; doi: 10.1001/archpediatrics.2010.269. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF. Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol. 2011 Feb 24;:1–21. doi: 10.1080/09297049.2010.544647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whyte RK, Kirpalani H, Asztalos EV, et al. Neurodevelopmental outcome of extremely low birth weight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusion. Pediatrics. 2009;123:207. doi: 10.1542/peds.2008-0338. [DOI] [PubMed] [Google Scholar]
- 22.Sola A, Wen TC, Hamrick SEG, Ferriero DM. Potential for protection and repair following injury to the developing brain: a role for erythropoietin? Pediatr Res. 2005;57:110R–117R. doi: 10.1203/01.PDR.0000159571.50758.39. [DOI] [PubMed] [Google Scholar]
- 23.Yu X, Shacka JJ, Eells JB, Suarez-Quian C, Przygodzki RM, Beleslin-Cokic B, Lin CS, Nikodem VM, Hempstead B, Flanders KC, Costantini F, Noguchi CT. Erythropoietin receptor signaling is required for normal brain development. Development. 2002;129:505–516. doi: 10.1242/dev.129.2.505. [DOI] [PubMed] [Google Scholar]
- 24.Sirén AL, Knerlich F, Poser W, Gleiter CH, Brück W, Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. 2001;101:271–6. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- 25.Juul SE. Erythropoietin in the central nervous system and its use to prevent hypoxic-ischemic brain damage. Acta Paediatr Suppl. 2002;91:36–42. doi: 10.1111/j.1651-2227.2002.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 26.Wen TC, Sadamoto Y, Tanaka J, Zhu PX, Nakata K, Ma YJ, Hata R, Sakanaka M. Erythropoietin protects neurons against chemical hypoxia and cerebral ischemic injury by up-regulating Bcl-xL expression. J Neurosci Res. 2002;67:795–803. doi: 10.1002/jnr.10166. [DOI] [PubMed] [Google Scholar]
- 27.Campana WM, Misasi R, O’Brien JS. Identification of a neurotrophic sequence in erythropoietin. Int J Mol Med. 1998;1:235–241. doi: 10.3892/ijmm.1.1.235. [DOI] [PubMed] [Google Scholar]
- 28.Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami M, Sekiguchi M, Sato K, Kozaki S, Takahashi M. Erythropoietin receptor-mediated inhibition of exocytotic glutamate release confers neuroprotection during chemical ischemia. J Biol Chem. 2001;276:39469–39475. doi: 10.1074/jbc.M105832200. [DOI] [PubMed] [Google Scholar]
- 30.Villa P, Bigini P, Mennini T, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198:971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva M, Benito A, Sanz C, et al. Erythropoietin can induce the expression of bcl-x(L) through Stat5 in erythropoietin-dependent progenitor cell lines. J Biol Chem. 1999;274:22165–22169. doi: 10.1074/jbc.274.32.22165. [DOI] [PubMed] [Google Scholar]
- 33.Celik M, Gokmen N, Erbayraktar S, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci U S A. 2002;99:2258–2263. doi: 10.1073/pnas.042693799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renzi MJ, Farrell FX, Bittner A, et al. Erythropoietin induces changes in gene expression in PC-12 cells. Brain Res Mol Brain Res. 2002;104:86–95. doi: 10.1016/s0169-328x(02)00323-6. [DOI] [PubMed] [Google Scholar]
- 35.Gorio A, Gokmen N, Erbayraktar S, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci U S A. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agnello D, Bigini P, Villa P, et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 2002;952:128–134. doi: 10.1016/s0006-8993(02)03239-0. [DOI] [PubMed] [Google Scholar]
- 37.Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves crosstalk between Jak2 and NF-kappaB signaling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- 38.Calapai G, Marciano MC, Corica F, et al. Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. Eur J Pharmacol. 2000;401:349–356. doi: 10.1016/s0014-2999(00)00466-0. [DOI] [PubMed] [Google Scholar]
- 39.Kumral A, Baskin H, Gokmen N, et al. Selective Inhibition of Nitric Oxide in Hypoxic-Ischemic Brain Model in Newborn Rats: Is It an Explanation for the Protective Role of Erythropoietin? Biol Neonate. 2004;85:51–54. doi: 10.1159/000074958. [DOI] [PubMed] [Google Scholar]
- 40.Nagai A, Nakagawa E, Choi HB, Hatori K, Kobayashi S, Kim SU. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J Neuropathol Exp Neurol. 2001;60:386–392. doi: 10.1093/jnen/60.4.386. [DOI] [PubMed] [Google Scholar]
- 41.Vairano M, Dello Russo C, Pozzoli G, et al. Erythropoietin exerts anti-apoptotic effects on rat microglial cells in vitro. Eur J Neurosci. 2002;16:584–592. doi: 10.1046/j.1460-9568.2002.02125.x. [DOI] [PubMed] [Google Scholar]
- 42.Sugawa M, Sakurai Y, Ishikawa-Ieda Y, Suzuki H, Asou H. Effects of erythropoietin on glial cell development; oligodendrocyte maturation and astrocyte proliferation. Neurosci Res. 2002;44:391–403. doi: 10.1016/s0168-0102(02)00161-x. [DOI] [PubMed] [Google Scholar]
- 43.Zhu C, Kang W, Xu F, Cheng X, Zhang Z, Jia L, Ji L, Guo X, Xiong H, Simbruner G, Blomgren K, Wang X. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics. 2009 Aug;124(2):e218–26. doi: 10.1542/peds.2008-3553. Epub 2009 Jul 27. [DOI] [PubMed] [Google Scholar]
- 44.Neubauer AP, Voss W, Wachtendorf M, Jungmann T. Erythropoietin improves neurodevelopmental outcome of extremely preterm infants. Ann Neurol. 2010 May;67(5):657–66. doi: 10.1002/ana.21977. [DOI] [PubMed] [Google Scholar]
- 45.Bierer R, Roohi M, Peceny C, Ohls RK. Erythropoietin increases reticulocyte counts and maintains hematocrit in neonates requiring surgery. J Pediatr Surg. 2009;44:1540–5. doi: 10.1016/j.jpedsurg.2008.10.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McPherson RJ, Juul SE. Recent trends in erythropoietin mediated neuroprotection. Int J Dev Neurosci. 2008 Feb;26(1):103–11. doi: 10.1016/j.ijdevneu.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


