Abstract
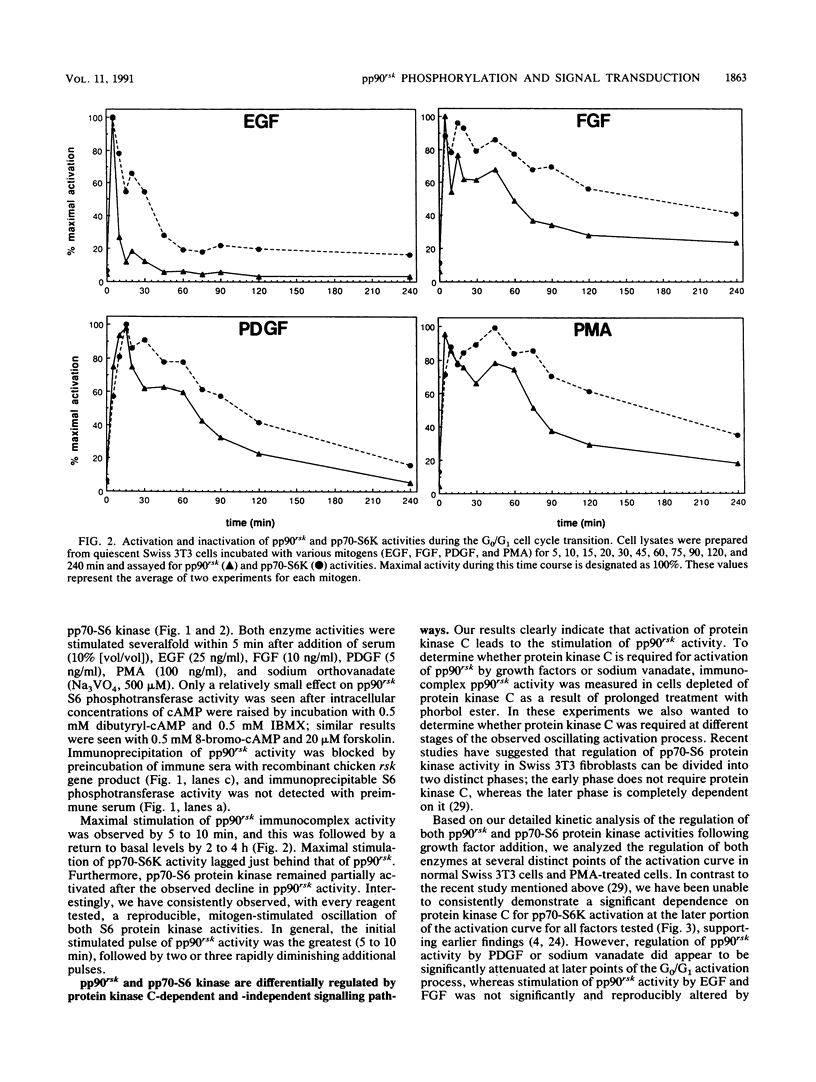
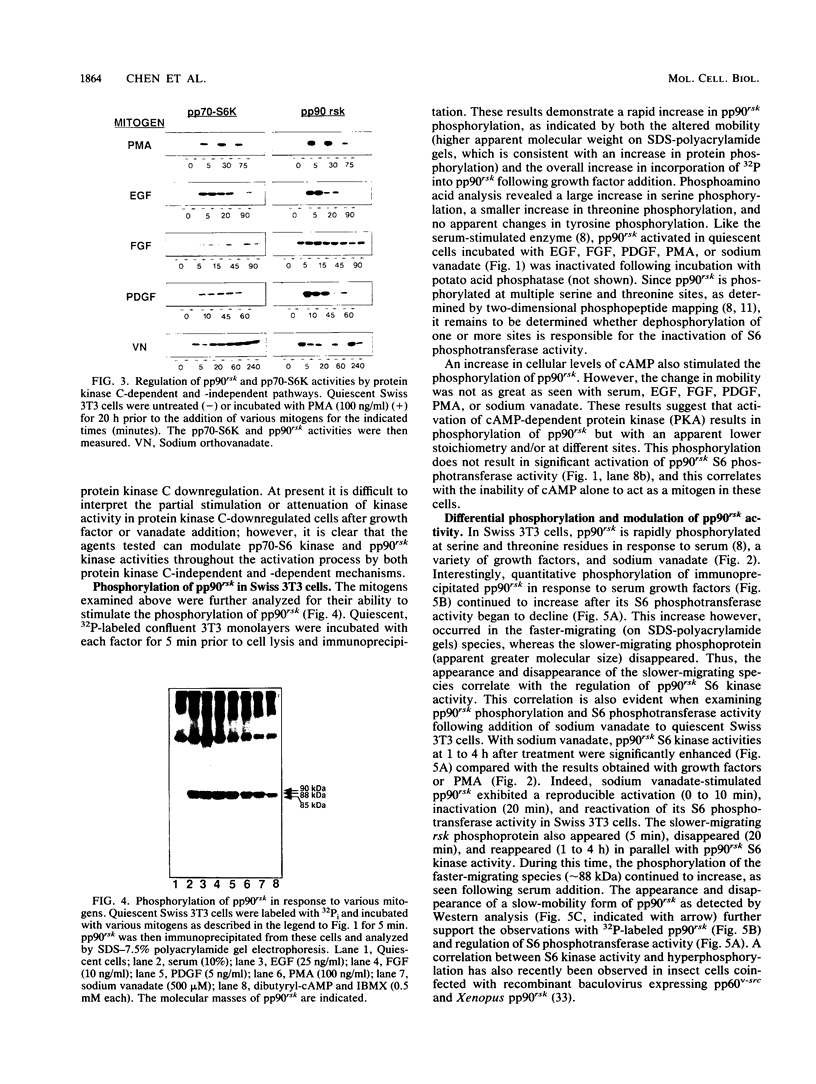
Somatic cell homologs to the Xenopus laevis S6 protein kinases (referred to collectively as pp90rsk) have recently been identified and partially characterized. Here we examine alterations in pp90rsk phosphorylation and S6 phosphotransferase activity in response to regulators of multiple signal transduction systems: purified growth factors, phorbol ester, changes in cyclic AMP (cAMP) levels, and sodium vanadate. All reagents tested increased pp90rsk serine and threonine phosphorylation, but only those agents that regulate cell proliferation and sodium vanadate activated its S6 kinase activity. In addition to the cAMP-stimulated phosphorylation of pp90rsk, a simple correlation between the extent of growth-regulated pp90rsk phosphorylation and S6 phosphotransferase activity was not observed. Quantitative phosphorylation of pp90rsk continued to increase after its S6 kinase activity began its return towards basal levels. However, a close correlation between the appearance and disappearance of a slow-mobility form of phosphorylated pp90rsk (by electrophoresis) and pp90rsk activity was observed. In addition, pp90rsk was regulated by both protein kinase C-independent and -dependent signaling mechanisms. The extent of protein kinase C participation, however, varied depending on which growth factor receptor was activated. Furthermore, growth factor-specific differences in the temporal regulation of pp90rsk S6 phosphotransferase activity were also observed. These results support the notion that the complex regulation of the rsk gene product constitutes one of the primary responses of animal cells to mitogenic signals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcorta D. A., Crews C. M., Sweet L. J., Bankston L., Jones S. W., Erikson R. L. Sequence and expression of chicken and mouse rsk: homologs of Xenopus laevis ribosomal S6 kinase. Mol Cell Biol. 1989 Sep;9(9):3850–3859. doi: 10.1128/mcb.9.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Regulation of a ribosomal protein S6 kinase activity by the Rous sarcoma virus transforming protein, serum, or phorbol ester. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7621–7625. doi: 10.1073/pnas.82.22.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J., Erikson R. L. Stimulation of ribosomal protein S6 kinase activity by pp60v-src or by serum: dissociation from phorbol ester-stimulated activity. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1733–1737. doi: 10.1073/pnas.83.6.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenis J., Kuo C. J., Alcorta D. A., Erikson R. L. Role of second messengers in regulating the growth-associated S6 protein kinase activity. Prog Clin Biol Res. 1987;249:181–192. [PubMed] [Google Scholar]
- Blenis J., Kuo C. J., Erikson R. L. Identification of a ribosomal protein S6 kinase regulated by transformation and growth-promoting stimuli. J Biol Chem. 1987 Oct 25;262(30):14373–14376. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Blenis J. Identification of Xenopus S6 protein kinase homologs (pp90rsk) in somatic cells: phosphorylation and activation during initiation of cell proliferation. Mol Cell Biol. 1990 Jun;10(6):3204–3215. doi: 10.1128/mcb.10.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J., Chen R. H., Blenis J. Coordinate regulation of pp90rsk and a distinct protein-serine/threonine kinase activity that phosphorylates recombinant pp90rsk in vitro. Mol Cell Biol. 1991 Apr;11(4):1868–1874. doi: 10.1128/mcb.11.4.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Erikson E., Maller J. L. A protein kinase from Xenopus eggs specific for ribosomal protein S6. Proc Natl Acad Sci U S A. 1985 Feb;82(3):742–746. doi: 10.1073/pnas.82.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Maller J. L. In vivo phosphorylation and activation of ribosomal protein S6 kinases during Xenopus oocyte maturation. J Biol Chem. 1989 Aug 15;264(23):13711–13717. [PubMed] [Google Scholar]
- Erikson E., Stefanovic D., Blenis J., Erikson R. L., Maller J. L. Antibodies to Xenopus egg S6 kinase II recognize S6 kinase from progesterone- and insulin-stimulated Xenopus oocytes and from proliferating chicken embryo fibroblasts. Mol Cell Biol. 1987 Sep;7(9):3147–3155. doi: 10.1128/mcb.7.9.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. S., Boulton T. G., Sang B. C., Cobb M. H. An insulin-stimulated ribosomal protein S6 kinase from rabbit liver. J Biol Chem. 1989 Nov 5;264(31):18397–18401. [PubMed] [Google Scholar]
- Halsey D. L., Girard P. R., Kuo J. F., Blackshear P. J. Protein kinase C in fibroblasts. Characteristics of its intracellular location during growth and after exposure to phorbol esters and other mitogens. J Biol Chem. 1987 Feb 15;262(5):2234–2243. [PubMed] [Google Scholar]
- Hanocq-Quertier J., Baltus E. Phosphorylation of ribosomal proteins during maturation of Xenopus laevis oocytes. Eur J Biochem. 1981 Nov;120(2):351–355. doi: 10.1111/j.1432-1033.1981.tb05711.x. [DOI] [PubMed] [Google Scholar]
- Jenö P., Ballou L. M., Novak-Hofer I., Thomas G. Identification and characterization of a mitogen-activated S6 kinase. Proc Natl Acad Sci U S A. 1988 Jan;85(2):406–410. doi: 10.1073/pnas.85.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. W., Erikson E., Blenis J., Maller J. L., Erikson R. L. A Xenopus ribosomal protein S6 kinase has two apparent kinase domains that are each similar to distinct protein kinases. Proc Natl Acad Sci U S A. 1988 May;85(10):3377–3381. doi: 10.1073/pnas.85.10.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma S. C., Ferrari S., Thomas G. Unmasking a growth factor/oncogene-activated S6 phosphorylation cascade. Cell Signal. 1989;1(3):219–225. doi: 10.1016/0898-6568(89)90039-9. [DOI] [PubMed] [Google Scholar]
- Kozma S. C., Lane H. A., Ferrari S., Luther H., Siegmann M., Thomas G. A stimulated S6 kinase from rat liver: identity with the mitogen activated S6 kinase of 3T3 cells. EMBO J. 1989 Dec 20;8(13):4125–4132. doi: 10.1002/j.1460-2075.1989.tb08597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luttrell D. K., Luttrell L. M., Parsons S. J. Augmented mitogenic responsiveness to epidermal growth factor in murine fibroblasts that overexpress pp60c-src. Mol Cell Biol. 1988 Jan;8(1):497–501. doi: 10.1128/mcb.8.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemenoff R. A., Price D. J., Mendelsohn M. J., Carter E. A., Avruch J. An S6 kinase activated during liver regeneration is related to the insulin-stimulated S6 kinase in H4 hepatoma cells. J Biol Chem. 1988 Dec 25;263(36):19455–19460. [PubMed] [Google Scholar]
- Nielsen P. J., Thomas G., Maller J. L. Increased phosphorylation of ribosomal protein S6 during meiotic maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1982 May;79(9):2937–2941. doi: 10.1073/pnas.79.9.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak-Hofer I., Küng W., Eppenberger U. Role of extracellular electrolytes in the activation of ribosomal protein S6 kinase by epidermal growth factor, insulin-like growth factor 1, and insulin in ZR-75-1 cells. J Cell Biol. 1988 Feb;106(2):395–401. doi: 10.1083/jcb.106.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelech S. L., Krebs E. G. Mitogen-activated S6 kinase is stimulated via protein kinase C-dependent and independent pathways in Swiss 3T3 cells. J Biol Chem. 1987 Aug 25;262(24):11598–11606. [PubMed] [Google Scholar]
- Pelech S. L., Meier K. E., Krebs E. G. Rapid microassay for protein kinase C translocation in Swiss 3T3 cells. Biochemistry. 1986 Dec 30;25(26):8348–8353. doi: 10.1021/bi00374a002. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Hallam T. J. Calcium signalling in non-excitable cells: notes on oscillations and store refilling. Cell Calcium. 1989 Jul;10(5):385–395. doi: 10.1016/0143-4160(89)90064-x. [DOI] [PubMed] [Google Scholar]
- Stith B. J., Maller J. L. The effect of insulin on intracellular ph and ribosomal protein S6 phosphorylation in oocytes of Xenopus laevis. Dev Biol. 1984 Mar;102(1):79–89. doi: 10.1016/0012-1606(84)90176-3. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Sweet L. J., Alcorta D. A., Erikson R. L. Two distinct enzymes contribute to biphasic S6 phosphorylation in serum-stimulated chicken embryo fibroblasts. Mol Cell Biol. 1990 Jun;10(6):2787–2792. doi: 10.1128/mcb.10.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet L. J., Alcorta D. A., Jones S. W., Erikson E., Erikson R. L. Identification of mitogen-responsive ribosomal protein S6 kinase pp90rsk, a homolog of Xenopus S6 kinase II, in chicken embryo fibroblasts. Mol Cell Biol. 1990 May;10(5):2413–2417. doi: 10.1128/mcb.10.5.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarini D., Garcia de Herreros A., Heinrich J., Rosen O. M. Purification of a bovine liver S6 kinase. Biochem Biophys Res Commun. 1987 Apr 29;144(2):891–899. doi: 10.1016/s0006-291x(87)80048-7. [DOI] [PubMed] [Google Scholar]
- Vik T. A., Sweet L. J., Erikson R. L. Coinfection of insect cells with recombinant baculovirus expressing pp60v-src results in the activation of a serine-specific protein kinase pp90rsk. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2685–2689. doi: 10.1073/pnas.87.7.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]