Summary
Anaplasma phagocytophilum is an obligatory intracellular bacterium that infects neutrophils, the primary host defense cells. Consequent effects of infection on host cells result in a potentially fatal systemic disease called human granulocytic anaplasmosis. Despite ongoing reductive genome evolution and deletion of most genes for intermediary metabolism and amino acid biosynthesis, Anaplasma has also experienced expansion of genes encoding several components of the Type IV secretion (T4S) apparatus. Two A. phagocytophilum T4S effector molecules are currently known; Anaplasma translocated substrate 1 (Ats-1) and ankyrin repeat domain–containing protein A (AnkA) have C-terminal positively charged amino acid residues that are recognized by the T4S coupling protein, VirD4. AnkA and Ats-1 contain eukaryotic protein motifs and are uniquely evolved in the family Anaplasmataceae; Ats-1 contains a mitochondria-targeting signal. They are abundantly produced and secreted into the host cytoplasm, are not toxic to host cells, and manipulate host cell processes to aid in the infection process. At the cellular level, the two effectors have distinct subcellular localization and signaling in host cells. Thus in this obligatory intracellular pathogen, the T4S system has evolved as a host-subversive survival factor.
Introduction
Anaplasma phagocytophilum is a tick-borne gram-negative intragranulocytic bacterium in the order Rickettsiales and the class Alphaproteobacteria (Dumler, 2005). Infection of humans with A. phagocytophilum results in a potentially fatal acute flu-like illness called human granulocytic anaplasmosis (HGA, formerly human granulocytic ehrlichiosis). HGA is frequently accompanied by leukopenia, thrombocytopenia, anemia, and elevated levels of serum hepatic aminotransferases (Bakken et al., 2008). Wild rodents are major reservoirs for A. phagocytophilum in the United States (Telford et al., 1996). Although identified less than two decades ago, HGA is currently among the most prevalent life-threatening tick-borne zoonoses, and has been recently recognized in North America as an important and frequent cause of human fever after Ixodes tick bite.
A. phagocytophilum replicates in membrane-bound compartments (called inclusions or morulae) of neutrophil granulocytes, the most abundant type of white blood cells. Neutrophils are the primary immune defense cells responsible for powerful innate antimicrobial responses. Lipopolysaccharide and peptidoglycan, which activate the innate immune responses, have been eliminated from A. phagocytophilum at the genomic level (Lin et al., 2003). To survive and replicate inside hostile neutrophils, bidirectional signals are transduced inside A. phagocytophilum and inside host cells upon interaction (Rikihisa, 2010b). Some of these signaling events lead to the subversion of several innate neutrophil immune responses, including inhibition of NADPH oxidase activation, lysosomal fusion with bacterial inclusions, autophagy, and IFN-γ signaling (Carlyon et al., 2002, Mott et al., 2000, IJdo et al., 2004, Mott et al., 1999, Pedra et al., 2008, Webster et al., 1998, Niu et al., 2008, Rikihisa, 2010b, Rikihisa et al., 2010, Rikihisa, 2010a). Furthermore, A. phagocytophilum inhibits spontaneous and induced human neutrophil apoptosis to maximize intracellular bacterial reproduction (Yoshiie et al., 2000, Borjesson et al., 2005) and hijacks host cholesterol to stabilize the bacterial membrane (Xiong et al., 2009). Consequently, several non-antimicrobial compounds that block A. phagocytophilum or host cell signaling were shown to eliminate A. phagocytophilum infection in vitro without harming the host cells (Cheng et al., 2006, Lin et al., 2007, Lin et al., 2002, Xiong et al., 2009). A. phagocytophilum utilizes the bacterial Type IV secretion (T4S) system to subvert the host innate immune system and exploit the host cell. The T4S system is a multi-component membrane-spanning transporter machinery that translocates DNA to or from bacteria, and proteins or nucleoprotein complexes to the eukaryotic target cells (Alvarez-Martinez et al., 2009).
The T4S apparatus of A. phagocytophilum
There are at least two ancestral lineages of the T4S effector protein or nucleoprotein delivery system: the virB/virD system of Agrobacterium tumefaciens and the dot/icm system of Legionella pneumophila, sometimes referred to as the T4aS and T4bS systems, respectively. A. phagocytophilum utilizes the T4aS system. In A. tumefaciens, the single virB operon, along with virD4, encodes 12 membrane-associated proteins that form a transmembrane channel (Alvarez-Martinez et al., 2009). Despite the small genome size of A. phagocytophilum, genes encoding some virB orthologs, have expanded during evolution (Dunning Hotopp et al., 2006). In A. phagocytophilum, virB/D are distributed into three major genomic islands: sodB-virB3-virB4-virB6-1-virB6-2-virB6-3-virB6-4, virB8-1-virB9-1-virB10-virB11-virD4, and virB2-1- virB2-2-virB2-3-virB2-4-virB2-5-virB2-6-virB2-7-virB2-8-virB4-2. Between these islands lie virB8-2, virB9-2, and a putative virB7 (no open reading frame number assigned, coordinates 1033978 to 1034181). virB7 is homologous to the Anaplasma marginale putative virB7 and contains a cysteine residue and a “[I/L][K/R]SPC” motif, which are conserved among virB7 of Rickettsia spp. (Sutten et al., 2010, Gillespie et al., 2009). A genomic map of A. phagocytophilum virB/D is shown in Figure 1 inset. The split T4S genomic islands and the duplicated virB (A. marginale has 22 candidates of virB2) are characteristics conserved among the Rickettsiaceae and Anaplasmataceae families, suggesting a common ancestral origin and a requirement for preservation of these features (Dunning Hotopp et al., 2006, Gillespie et al., 2009, Gillespie et al., 2010). A. phagocytophilum virB9 has been shown to be transcribed in peripheral blood leukocytes from HGA patients and from experimentally infected animals, indicating the in vivo relevance of the T4S system (Ohashi et al., 2002). A. phagocytophilum T4S system is expressed in ISE6 tick cell culture, and in the closely related monocyte-tropic Ehrlichia canis, virB9 is expressed in infected ticks (Felek et al., 2003, Nelson et al., 2008). Thus the T4S system is believed to function in the tick stages of the bacteria.
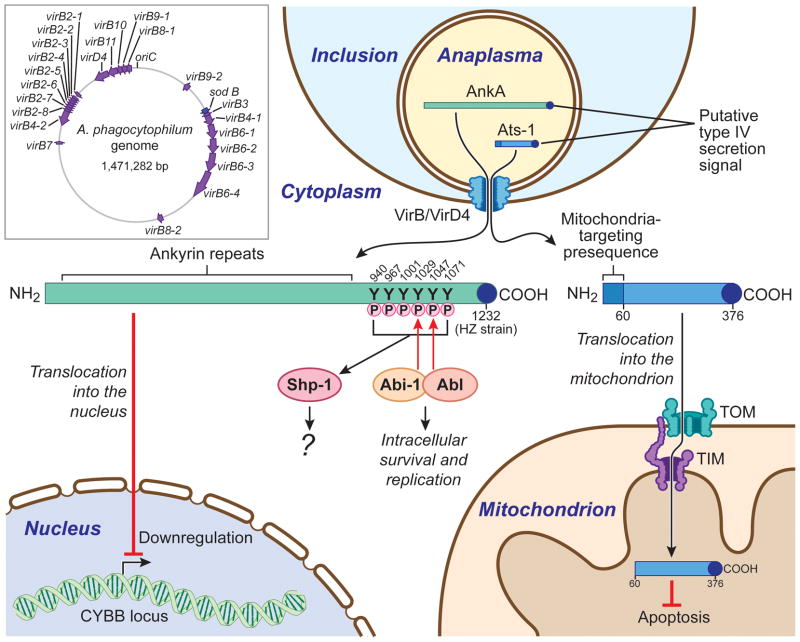
Fig. 1. virB/D loci on the A. phagocytophilum genome and subcellular location and targets of A. phagocytophilum AnkA and Ats-1 and host cell signaling pathways.
Inset. The virB/D loci are shown as colored arrows on the circular genome map of A. phagocytophilum. Genes are not drawn to scale and arrows reflect a 10-fold expansion of the gene size relative to the genome. The putative origin of replication is indicated as oriC.
AnkA and Ats-1 are depicted as green and blue bars, respectively, with the putative type IV secretion signal depicted as a dark blue dot. AnkA is secreted into the host cell cytoplasm and binds the adaptor protein Abi-1, which recruits and activates Abl-1 tyrosine kinase. AnkA from the A. phagocytophilum HZ strain contains six tyrosine phosphorylation sites, two of which (Y1029 and Y1047) are phosphorylated by Abl-1. The remaining four tyrosine phosphorylation sites are phosphorylated by Src kinases, allowing subsequent binding to the SH2 domain of Shp-1. AnkA protein translocates to the nucleus and executes gene regulation. Ats-1 lacks ankyrin repeats or tyrosine phosphorylation sites, but has a mitochondria-targeting presequence. Ats-1 binds to mitochondria via a mitochondria-targeting presequence and translocates across the outer and inner membranes. The presequence is then cleaved, and the mature Ats-l localizes in the mitochondrial matrix. Mitochondria-localized Ats-1 blocks apoptosis of eukaryotic host cells by preventing loss of mitochondrial membrane potential.
TOM: Transporter Outer Membrane complex; TIM: Transporter Inner Membrane complex
Expression of the T4S apparatus is regulated during intracellular bacterial infection. Both virB8-virD4 and sodB-virB6 operons are transcribed polycistronically during A. phagocytophilum replication in HL-60 promyelocytic leukemia cells (Ohashi et al., 2002). Because sodB encodes an iron superoxide dismutase, a protective oxidative stress response might therefore be coupled with T4S apparatus assembly in the cytoplasm of mammalian neutrophils or blood-feeding ticks, where both activities are needed for bacterial survival and/or proliferation. In Ehrlichia chaffeensis, a bacterium closely related to A. phagocytophilum, the small DNA binding protein EcxR regulates all five virB/D loci (Cheng et al., 2008); regulation of virB7 has not been studied. virB9 and virB6, which are present in different operons of A. phagocytophilum, are upregulated at the mRNA level during infection of human neutrophils in vitro; virB9 is upregulated at the protein level (Niu et al., 2006). In contrast, in the majority of A. phagocytophilum spontaneously released from infected host cells, VirB9 protein expression is minimal (Niu et al., 2006).
Recently, cryo-electron microscopy demonstrated that purified VirB7, VirB9, and VirB10 homologs encoded by Escherichia coli pKM101 assemble into a 1-MDa channel of sufficient size to span the entire gram-negative bacterial cell envelope (Fronzes et al., 2009). Assembly of the T4S apparatus in Rickettsia is poorly understood. The virB6 homologs in Rickettsia are 3- to 10-fold larger than A. tumefaciens virB6, and four to five tandem copies are present per genome (Dunning Hotopp et al., 2006, Gillespie et al., 2009). These virB6 encodes polytopic membrane proteins with large hydrophilic domains in the N-terminal, central, or C-terminal region. VirB6-1, 6-2, 6-3, and 6-4 are coexpressed and interact with each other and with VirB9 from E. chaffeensis (Bao et al., 2009), which is a bacterial surface–exposed protein in A. phagocytophilum and E. chaffeensis (Niu et al., 2006, Ge et al., 2007a, Ge et al., 2007b). E. chaffeensis VirB6-2 undergoes proteolysis, resulting in the release of an 80-kDa fragment that accumulates in E. chaffeensis–containing vacuoles (Bao et al., 2009). Differential transcription of several A. phagocytophilum T4S VirB2 pilus protein paralogs in mammalian and ISE6 tick cell cultures was recently reported (Nelson et al., 2008), suggesting that this bacterium uses different sets of VirB2 proteins in different host cells. Whether tandemly expressed VirB2 molecules function in the effector transport channel and/or host cell adhesion apparatus, as reported in Bartonella (Dehio, 2008), remains to be determined. Some T4S apparatus proteins are immunogenic in infected or immunized animals (Felek et al., 2003, Sutten et al., 2010), and therefore multiple non-identical VirB proteins may provide some advantage for immune evasion.
T4S substrates
Although the total number of T4S substrates encoded by the A. phagocytophilum genome is unknown, two substrate proteins have been partially characterized. The coupling protein VirD4 contains docking sites for these T4S substrates. The A. phagocytophilum VirD4 protein has two N-terminal transmembrane domains, a P-loop and a walker-B site for nucleotide binding, and a C-terminal region that has a ~150-residue extension compared with the Agrobacterium or Rickettsia VirD4. Whether this C-terminal extension of VirD4, also present in the E. chaffeensis VirD4 molecule, modulates substrate transfer similar to the coupling protein TcpA, which regulates conjugative transfer of pCW3 (Steen et al., 2009), remains to be determined. Using a Cre recombinase reporter assay for translocation, the A. phagocytophilum ankyrin repeat domain–containing protein AnkA was shown to be translocated into plant cells in an A. tumefaciens VirD4–dependent manner (Lin et al., 2007). A hypothetical protein, later named Anaplasma Translocation Substrate 1 (Ats-1), was discovered by screening an A. phagocytophilum genomic prey library using A. phagocytophilum VirD4 as bait in a bacterial two-hybrid system (Niu et al., 2010). Both AnkA and Ats-1 have basic C-terminal domains similar to the A. tumefaciens T4S substrates (Vergunst et al., 2005) (Table 1). Both proteins are abundantly expressed and secreted into the host cell cytoplasm (Lin et al., 2007, Niu et al., 2010). AnkA and Ats-1 have distinct subcellular localization and downstream signals, and appear to be multifunctional, as summarized in Figure 1, although detailed signaling mechanisms and pathways remain to be elucidated.
Table 1.
Characteristics of Type IV secretion effectors from the A. phagocytophilum HZ strain
| Effector | Molecular size (aa) | C-terminal residues | Protein motifs | Subcellular localization |
|---|---|---|---|---|
| AnkA | 1232 | SEGPKSVKGGRGR | Ankyrin repeats, Src/Abl tyrosine phosphorylation sites, SH2/SH3 binding motifs | Cytoplasm, nucleus |
| Ats-1 | 376 | QNRGPETHGKGTR | N-terminal mitochondria localization signal | Mitochondria, bacterial inclusions |
AnkA
AnkA was originally discovered from a genomic DNA expression library of the A. phagocytophilum USG3 strain by screening with tick-challenged A. phagocytophilum-infected dog sera (Storey et al., 1998). A. phagocytophilum infection of human leukocytes requires protein tyrosine kinase activity, and proteomics revealed that AnkA was the primary phosphotyrosine protein (Lin et al., 2007). This phosphotyrosine protein was also suspected as AnkA by size similarity with AnkA; tyrosine phosphorylation was later confirmed by immunoprecipitation (IJdo et al., 2007). AnkA migrates between 160 and 190 kDa when subjected to SDS-PAGE, but the mass predicted from the amino acid sequence is ~ 30 kDa lower. Although some researchers have proposed that this discrepancy is due to glycosylation of AnkA, no evidence of glycosylation has yet been found. The N-terminal two-thirds of AnkA contains ~11 ankyrin repeats (Rikihisa et al., 2010). The ankyrin repeat is found in a number of biologically important eukaryotic proteins. For example, IκB, the inhibitor of the inflammatory response–regulating transcription factor NF-κB, contains seven ankyrin repeats. Ankyrin is a 33-residue repeating motif, which folds into two antiparallel α-helices followed by a β-hairpin or a long loop. Similar to previously described ankyrin proteins (Mosavi et al., 2004), it is predicted that the consecutive ankyrin repeats in AnkA stack together to form an L-shaped domain that mediates specific protein-protein interactions. In contrast to other protein-protein interaction domains such as SH2 (Src Homology 2) or SH3, ankyrin repeats typically do not recognize any specific amino acid sequence or structure. AnkA orthologs have been identified among members of the Anaplasma and Ehrlichia genera (Rikihisa et al., 2010), but not in the trematode-borne Neorickettsia species, suggesting that AnkA evolved in Anaplasma and Ehrlichia during adaptation to tick and mammalian hosts, or that Neorickettsia spp. lost the AnkA homolog after diversification of these two genera and the genus Neorickettsia from the common ancestor.
Both AnkA and tyrosine phosphorylation are required for A. phagocytophilum infection (Lin et al., 2007). AnkA contains six to seven tandem tyrosine phosphorylation sites, four to five of which can bind SH2, with a single SH3 binding site in the C-terminal one-third of the protein (Rikihisa et al., 2010). In fact, in A. phagocytophilum–infected human promyelocytic leukemia HL-60 cells and peripheral blood neutrophils, AnkA is the predominant phosphotyrosine protein (IJdo et al., 2007, Lin et al., 2007). Tyrosine phosphorylation of AnkA occurs as early as 2 min after A. phagocytophilum binds to eukaryotic cells, and bacterial internalization is not required for this early phosphorylation event (IJdo et al., 2007, Lin et al., 2007). In A. phagocytophilum–infected HL-60 cells, tyrosine phosphorylation of AnkA occurs after secretion into the host cytoplasm by two non-receptor tyrosine kinases, Src and Abelson leukemia (Abl); specific inhibitors of these kinases reduces tyrosine phosphorylation of AnkA (IJdo et al., 2007, Lin et al., 2007). The Abl and Src kinase phosphorylation sites in AnkA are important for signal transduction and/or infection in host cells (IJdo et al., 2007, Lin et al., 2007). Following phosphorylation by Src, AnkA binds the SH2 domains of the non-receptor tyrosine phosphatase Src Homology Protein (SHP)-1 (IJdo et al., 2007). SHP-1 can interact with and dephosphorylate a wide spectrum of phosphoproteins and primarily downregulates cellular activation (Poole et al., 2005). The roles of AnkA recruitment and activation of SHP-1 in A. phagocytophilum infection remain to be determined.
The two Abl kinase phosphorylation sites in AnkA are not predicted to bind SH2 domains, indicating a distinct signaling role for AnkA following phosphorylation by the Abl and Src family kinases. Yeast two-hybrid screening has demonstrated that AnkA binds to Abl-interactor 1 (Abi-1), an adaptor protein and substrate of Abl-1 tyrosine kinase (Lin et al., 2007). Abi-1 is comprised of approximately 450–500 residues (several splice variants are known) and is a proline-rich protein comprised of N-terminal WAB (Wave binding) and SNARE (syntaxin binding) domains, a homeobox homology region (HHR domain) and a C-terminal SH3 domain (Dai et al., 1995). The Abi-1 SH3 domain and one of the proline-rich motifs interact with c-Abl (Shi et al., 1995). Abi-1 is a trans-acting adaptor protein known to regulate Abl-1-mediated tyrosine phosphorylation of target proteins, such as Mammalian Enabled (Mena) and B-cell adaptor for phosphoinositide 3-kinase (BCAP), by direct interaction with the target proteins (Tani et al., 2003, Maruoka et al., 2005). Similar to Mena and BCAP, the natural ligands of Abi-1, AnkA forms a complex with Abl-1 via Abi-1, stimulates Abl-1 kinase, and is phosphorylated by Abl-1 (Fig. 1). AnkA may pry the autoinhibitory Abl open into an active signaling-competent kinase conformation (Pluk et al., 2002, Nagar et al., 2003). Interestingly, AnkA, Mena, and BCAP have multiple tyrosine phosphorylation sites but do not share sequence similarity. Abl kinase was shown to be essential for A. phagocytophilum infection using the Abl kinase–specific inhibitor STI571 (also known as imatinib mesylate or Gleevec), which does not inhibit Src family kinases, and by knockdown using a small interfering RNA targeting Abl-1 mRNA (Lin et al., 2007). STI571 has been used to treat Bcr-Abl-positive chronic myelogenous leukemia patients (Druker et al., 2001, Capdeville et al., 2002), suggesting a potential novel strategy for HGA treatment with STI571.
For Listeria and Chlamydia internalization, Abi-1 recruits the Wave complex in a Rac-dependent manner to reorganize the actin cytoskeleton (Bierne et al., 2005, Carabeo et al., 2007). However, A. phagocytophilum infection downregulates Rac2 in HL-60 cells (Carlyon et al., 2002), indicating that this pathway may not be activated by A. phagocytophilum. Abl contains actin-binding repeats and phosphorylates proteins that regulate the actin cytoskeleton. Changes in subcellular localization of Abi-1 and Abl-1, and activation of Abl by AnkA, likely regulate multiple cellular processes. Further studies are needed to determine whether AnkA recruits other potential Abi-1-interacting proteins and Abl kinase substrates to facilitate A. phagocytophilum infection.
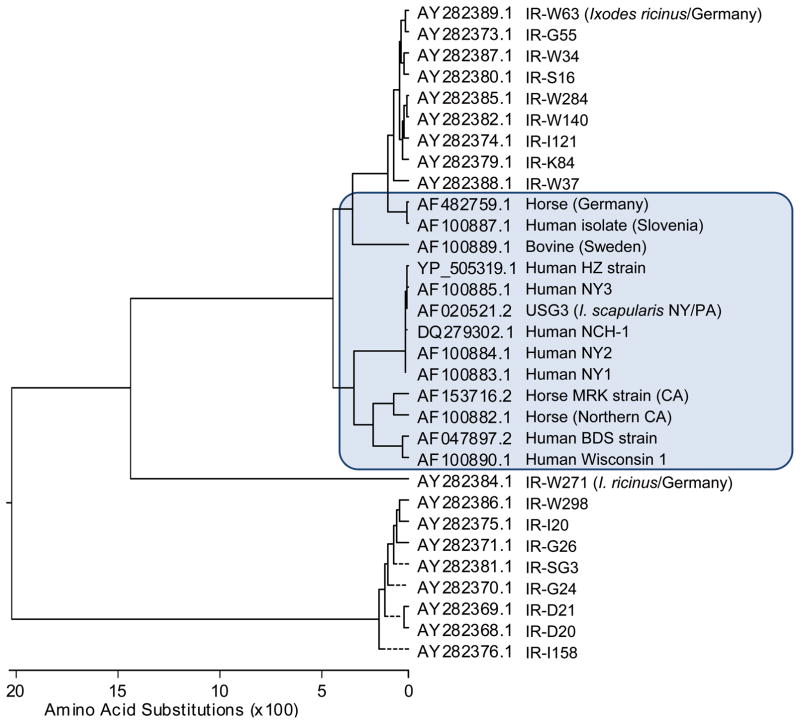
In A. phagocytophilum HZ strain, AnkA phosphorylated on tyrosine accumulates in the host cell cortical cytoplasm during infection, and very little is retained within or near the bacteria (Lin et al., 2007). In the A. phagocytophilum Webster strain, a large proportion of AnkA is contained within the nucleus (Caturegli et al., 2000, Park et al., 2004, Garcia-Garcia et al., 2009). In the nucleus, AnkA binds to a broad range of targets, including several nuclear proteins, the internucleosomal region of chromosomes in HL-60 cells, ATC-rich sequences, and transcriptional regulatory regions of the CYBB locus, to suppress the host cell innate immune response (Caturegli et al., 2000, Park et al., 2004, Garcia-Garcia et al., 2009). Although the mechanisms of AnkA nuclear translocation and binding to various nuclear targets are unclear, these reports present the fascinating possibility of global transcriptional regulation of host cells by a T4S substrate. The AnkA molecule is involved in several aspects of host-pathogen interactions. AnkA is one of several A. phagocytophilum proteins with strain-dependent amino acid variation; phylogenic analysis of AnkA protein sequences indicates that AnkA from humans and domesticated animals (cattle and horses) are clustered together, whereas the AnkA variants of A. phagocytophilum isolated from field-collected Ixodes ricinus ticks in Germany are divided into two clusters, one close to the mammalian cluster and the other quite divergent from this cluster (Massung et al., 2000, von Loewenich et al., 2003) (Fig. 2). These variations may influence the function of AnkA as a T4S effector molecule. Of note, AnkA from all human isolates and from the tick isolate USG3 lacks one glycine residue at its C-terminus which may affect AnkA T4S secretion efficiency—these isolates contain the terminal sequence PKSVKGGRGR, whereas the sequence PKSVKGGGRGR is present in the German tick and in various animal isolates from the United States and Europe. Although proteins that specifically interact with the AnkA ankyrin repeats have not been identified, the presence of the ankyrin repeats and the C-terminal tyrosine phosphorylation domain in AnkA may represent an optimal adaptation to its eukaryotic host. Further studies are needed to elucidate the mechanisms of AnkA-induced signaling and cellular regulation during A. phagocytophilum infection.
Fig. 2.
Phylogenetic analysis of A. phagocytophilum AnkA amino acid sequence variation. The GenBank number is indicated at left. Hosts and geographic regions from which strains were isolated are indicated at right. IR: Ixodes ricinus tick from Germany; modified from (von Loewenich et al., 2003). Mammalian sequences (USG3 was isolated from the dog by attaching field-collected ticks) are highlighted in a blue box.
Ats-1
Similar to AnkA, Ats-1 is abundantly expressed by A. phagocytophilum in mammalian cells, and secretion is readily visible by immunofluorescence microscopy. In contrast to AnkA, a large proportion of expressed Ats-1 colocalizes with the A. phagocytophilum inclusion; consequently, Ats-1 translocation to the host cell cytoplasm only becomes readily discernible by light microscopy at 32 h post-infection (early exponential growth stage) (Niu et al., 2010). Ats-1 is predicted to be 40.5 kDa, however SDS-PAGE analysis of lysates from A. phagocytophilum–infected HL-60 cells reveals 48-kDa and a 35-kDa Ats-1 polypeptides (Niu et al., 2010). Unlike AnkA, Ats-1 lacks any known protein motifs. However, Ats-1 contains a cleavable N-terminal mitochondria-targeting presequence, which is a hallmark of most mitochondrial matrix proteins. This sequence has been shown to direct Ats-1 localization into the mitochondrial matrix of infected human neutrophils, HL-60 cells, RF/6A monkey endothelial cells, HeLa cells, and even yeast cells via the mitochondrial protein transport system. This is an intrinsic property of Ats-1, as recombinant Ats-1 has been shown to translocate into isolated mitochondria in vitro in the absence of any other cellular proteins (Niu et al., 2010). Ats-1 is the first example of a bacterial protein that traverses five membranes (bacterial inner and outer membrane, inclusion membrane, and outer and inner membranes of mitochondria). The presequence of Ats-1 is cleaved in the mitochondrion by a mitochondrial matrix processing peptidase, releasing the mature 35-kDa form of Ats-1, whereas the uncleaved 48-kDa form of Ats-1 stays with bacterial inclusions. The functions of inclusion-colocalized Ats-1 remain to be investigated.
A. phagocytophilum infection inhibits spontaneous and induced apoptosis of isolated peripheral blood human neutrophils for up to 48 h and of neutrophils in peripheral blood leukocyte cultures for up to 96 h (Yoshiie et al., 2000). The cellular mechanisms by which A. phagocytophilum inhibits apoptosis of human neutrophils include inhibition of the loss of mitochondrial membrane potential, inhibition of Bax translocation to the mitochondria, and inhibition of activation of downstream caspase 3 (Ge et al., 2005, Ge et al., 2006). Mitochondria-translocated Ats-1 inhibits etoposide-induced apoptosis in mammalian cells (Niu et al., 2010). In the absence of the Bcl-2 family of proteins in yeast, Ats-1 was shown to inhibit Bax docking to the mitochondria and subsequent apoptosis, indicating that other Bcl-2 family members are not important in this process. However, the link between Ats-1 and inhibition of apoptosis remains unclear. The Neisseria gonorrhoeae porin protein, PorB, induces condensation of the mitochondrial matrix and the loss of cristae structures and sensitizes cells to apoptosis (Kozjak-Pavlovic et al., 2009). Therefore, a possible scenario is that Ats-1 stabilizes the mitochondrial membrane potential or even the inner-membrane cristae structure, the rearrangement of which is required for apoptosis (Scorrano et al., 2002). Although cristae stabilization by Ats-1 has not been shown, overexpression of Ats-1 does not have obvious adverse effects on mitochondria in various mammalian or yeast cells (Niu et al., 2010). Consistent with this, an insoluble, miscleaved Ats-1 mutant (deletion of residues 55–57) was shown to be defective for inhibition of apoptosis, suggesting that Ats-1 sub-mitochondrial routing and/or processing are critical for proper function (Niu et al., 2010). The absence of similarities between the amino acid sequence or the mode of action of Ats-1 and any other known cell death suppressors suggests that Ats-1 is a member of a previously undescribed class of anti-apoptotic proteins.
Concluding remarks
AnkA and Ats-1, two originally hypothetical A. phagocytophilum effector molecules, are uniquely evolved in the family Anaplasmataceae; their orthologs are encoded in the genomic sequences of other members of the genera Anaplasma and Ehrlichia. Considering the great expense of energy required to produce and secrete these effectors, they likely are of fundamental importance for survival of these bacteria. Members of the Anaplasmataceae family have a large number of hypothetical proteins, among which more effector molecules and T4S components are likely to be discovered. The identification of these molecules, and subsequent studies revealing their functions in cellular infection will be highly informative. Furthermore, future studies focusing on duplicated and modified T4S apparatus genes may uncover unusual molecular interaction and functions. Because an essential part of the life cycle of Anaplasma is the tick stage, it is also important to learn the function of the T4S system at this stage. Ongoing tick genome sequencing projects will facilitate this line of investigation.
Acknowledgments
The author thanks T. Vojt for helping preparation of Figure 1. Some of the studies from the authors’ laboratory reported in this review were supported by grants R01AI054476 and R01AI30010 to YR from the National Institutes of Health.
References
- Alvarez-Martinez CE, Christie PJ. Biological diversity of prokaryotic type IV secretion systems. Microbiol Mol Biol Rev. 2009;73:775–808. doi: 10.1128/MMBR.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken JS, Dumler S. Human granulocytic anaplasmosis. Infectious disease clinics of North America. 2008;22:433–448. viii. doi: 10.1016/j.idc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Bao W, Kumagai Y, Niu H, Yamaguchi M, Miura K, Rikihisa Y. Four VirB6 paralogs and VirB9 are expressed and interact in Ehrlichia chaffeensis-containing vacuoles. Journal of bacteriology. 2009;191:278–286. doi: 10.1128/JB.01031-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierne H, Miki H, Innocenti M, Scita G, Gertler FB, Takenawa T, Cossart P. WASP-related proteins, Abi1 and Ena/VASP are required for Listeria invasion induced by the Met receptor. Journal of cell science. 2005;118:1537–1547. doi: 10.1242/jcs.02285. [DOI] [PubMed] [Google Scholar]
- Borjesson DL, Kobayashi SD, Whitney AR, Voyich JM, Argue CM, Deleo FR. Insights into pathogen immune evasion mechanisms: Anaplasma phagocytophilum fails to induce an apoptosis differentiation program in human neutrophils. J Immunol. 2005;174:6364–6372. doi: 10.4049/jimmunol.174.10.6364. [DOI] [PubMed] [Google Scholar]
- Capdeville R, Buchdunger E, Zimmermann J, Matter A. Glivec (STI571, imatinib), a rationally developed, targeted anticancer drug. Nat Rev Drug Discov. 2002;1:493–502. doi: 10.1038/nrd839. [DOI] [PubMed] [Google Scholar]
- Carabeo RA, Dooley CA, Grieshaber SS, Hackstadt T. Rac interacts with Abi-1 and WAVE2 to promote an Arp2/3-dependent actin recruitment during chlamydial invasion. Cellular microbiology. 2007;9:2278–2288. doi: 10.1111/j.1462-5822.2007.00958.x. [DOI] [PubMed] [Google Scholar]
- Carlyon JA, Chan WT, Galan J, Roos D, Fikrig E. Repression of rac2 mRNA expression by Anaplasma phagocytophila is essential to the inhibition of superoxide production and bacterial proliferation. J Immunol. 2002;169:7009–7018. doi: 10.4049/jimmunol.169.12.7009. [DOI] [PubMed] [Google Scholar]
- Caturegli P, Asanovich KM, Walls JJ, Bakken JS, Madigan JE, Popov VL, Dumler JS. ankA: an Ehrlichia phagocytophila group gene encoding a cytoplasmic protein antigen with ankyrin repeats. Infect Immun. 2000;68:5277–5283. doi: 10.1128/iai.68.9.5277-5283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Kumagai Y, Lin M, Zhang C, Rikihisa Y. Intra-leukocyte expression of two-component systems in Ehrlichia chaffeensis and Anaplasma phagocytophilum and effects of the histidine kinase inhibitor closantel. Cellular microbiology. 2006;8:1241–1252. doi: 10.1111/j.1462-5822.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Wang X, Rikihisa Y. Regulation of type IV secretion apparatus genes during Ehrlichia chaffeensis intracellular development by a previously unidentified protein. Journal of bacteriology. 2008;190:2096–2105. doi: 10.1128/JB.01813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z, Pendergast AM. Abi-2, a novel SH3-containing protein interacts with the cAbl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 1995;9:2569–2582. doi: 10.1101/gad.9.21.2569. [DOI] [PubMed] [Google Scholar]
- Dehio C. Infection-associated type IV secretion systems of Bartonella and their diverse roles in host cell interaction. Cellular microbiology. 2008;10:1591–1598. doi: 10.1111/j.1462-5822.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. The New England Journal of Medicine. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- Dumler JS, Rikihisa Y, Dasch GA. Anaplasmataceae. In: Brenner, Krieg, Staley, Garrity, editors. The Proteobacteria, Part C, Bergey’s Manual of Systematic Bacteriology. 2. Vol. 2. Vol. 2. Springer; New York, NY: 2005. p. 117.p. 117. [Google Scholar]
- Dunning Hotopp JC, Lin M, Madupu R, Crabtree J, Angiuoli SV, Eisen J, et al. Comparative Genomics of Emerging Human Ehrlichiosis Agents. PLoS genetics. 2006;2:e21. doi: 10.1371/journal.pgen.0020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felek S, Huang H, Rikihisa Y. Sequence and expression analysis of virB9 of the type IV secretion system of Ehrlichia canis strains in ticks, dogs, and cultured cells. Infect Immun. 2003;71:6063–6067. doi: 10.1128/IAI.71.10.6063-6067.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fronzes R, Christie PJ, Waksman G. The structural biology of type IV secretion systems. Nat Rev Microbiol. 2009;7:703–714. doi: 10.1038/nrmicro2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia JC, Rennoll-Bankert KE, Pelly S, Milstone AM, Dumler JS. Silencing of Host Cell CYBB Gene Expression by the Nuclear Effector AnkA of the Intracellular Pathogen Anaplasma phagocytophilum. Infect Immun. 2009 doi: 10.1128/IAI.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Rikihisa Y. Anaplasma phagocytophilum delays spontaneous human neutrophil apoptosis by modulation of multiple apoptotic pathways. Cellular microbiology. 2006;8:1406–1416. doi: 10.1111/j.1462-5822.2006.00720.x. [DOI] [PubMed] [Google Scholar]
- Ge Y, Rikihisa Y. Identification of novel surface proteins of Anaplasma phagocytophilum by affinity purification and proteomics. Journal of bacteriology. 2007a;189:7819–7828. doi: 10.1128/JB.00866-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Rikihisa Y. Surface-exposed proteins of Ehrlichia chaffeensis. Infect Immun. 2007b;75:3833–3841. doi: 10.1128/IAI.00188-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Yoshiie K, Kuribayashi F, Lin M, Rikihisa Y. Anaplasma phagocytophilum inhibits human neutrophil apoptosis via upregulation of bfl-1, maintenance of mitochondrial membrane potential and prevention of caspase 3 activation. Cellular microbiology. 2005;7:29–38. doi: 10.1111/j.1462-5822.2004.00427.x. [DOI] [PubMed] [Google Scholar]
- Gillespie JJ, Ammerman NC, Dreher-Lesnick SM, Rahman MS, Worley MJ, Setubal JC, et al. An anomalous type IV secretion system in Rickettsia is evolutionarily conserved. PLoS ONE. 2009;4:e4833. doi: 10.1371/journal.pone.0004833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie JJ, Brayton KA, Williams KP, Diaz MA, Brown WC, Azad AF, Sobral BW. Phylogenomics reveals a diverse Rickettsiales type IV secretion system. Infect Immun. 2010;78:1809–1823. doi: 10.1128/IAI.01384-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJdo J, Carlson AC, Kennedy EL. Anaplasma phagocytophilum AnkA is tyrosine-phosphorylated at EPIYA motifs and recruits SHP-1 during early infection. Cellular microbiology. 2007;9:1284–1296. doi: 10.1111/j.1462-5822.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- IJdo J, Mueller AC. Neutrophil NADPH oxidase is reduced at the Anaplasma phagocytophilum phagosome. Infect Immun. 2004;72:5392–5401. doi: 10.1128/IAI.72.9.5392-5401.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozjak-Pavlovic V, Dian-Lothrop EA, Meinecke M, Kepp O, Ross K, Rajalingam K, et al. Bacterial porin disrupts mitochondrial membrane potential and sensitizes host cells to apoptosis. PLoS pathogens. 2009;5:e1000629. doi: 10.1371/journal.ppat.1000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, den Dulk-Ras A, Hooykaas PJ, Rikihisa Y. Anaplasma phagocytophilum AnkA secreted by type IV secretion system is tyrosine phosphorylated by Abl-1 to facilitate infection. Cellular microbiology. 2007;9:2644–2657. doi: 10.1111/j.1462-5822.2007.00985.x. [DOI] [PubMed] [Google Scholar]
- Lin M, Rikihisa Y. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect Immun. 2003;71:5324–5331. doi: 10.1128/IAI.71.9.5324-5331.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Zhu MX, Rikihisa Y. Rapid activation of protein tyrosine kinase and phospholipase C-g2 and increase in cytosolic free calcium are required by Ehrlichia chaffeensis for internalization and growth in THP-1 cells. Infect Immun. 2002;70:889–898. doi: 10.1128/IAI.70.2.889-898.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruoka M, Suzuki J, Kawata S, Yoshida K, Hirao N, Sato S, et al. Identification of B cell adaptor for PI3-kinase (BCAP) as an Abl interactor 1-regulated substrate of Abl kinases. FEBS letters. 2005;579:2986–2990. doi: 10.1016/j.febslet.2005.04.052. [DOI] [PubMed] [Google Scholar]
- Massung RF, Owens JH, Ross D, Reed KD, Petrovec M, Bjoersdorff A, et al. Sequence analysis of the ank gene of granulocytic ehrlichiae. Journal of clinical microbiology. 2000;38:2917–2922. doi: 10.1128/jcm.38.8.2917-2922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosavi LK, Cammett TJ, Desrosiers DC, Peng ZY. The ankyrin repeat as molecular architecture for protein recognition. Protein Sci. 2004;13:1435–1448. doi: 10.1110/ps.03554604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J, Barnewall RE, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–1378. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott J, Rikihisa Y. Human granulocytic ehrlichiosis agent inhibits superoxide anion generation by human neutrophils. Infect Immun. 2000;68:6697–6703. doi: 10.1128/iai.68.12.6697-6703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar B, Hantschel O, Young MA, Scheffzek K, Veach D, Bornmann W, et al. Structural basis for the autoinhibition of c-Abl tyrosine kinase. Cell. 2003;112:859–871. doi: 10.1016/s0092-8674(03)00194-6. [DOI] [PubMed] [Google Scholar]
- Nelson CM, Herron MJ, Felsheim RF, Schloeder BR, Grindle SM, Chavez AO, et al. Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis. BMC genomics. 2008;9:364. doi: 10.1186/1471-2164-9-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Kozjak-Pavlovic V, Rudel T, Rikihisa Y. Anaplasma phagocytophilum Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction. PLoS pathogens. 2010;6:e1000774. doi: 10.1371/journal.ppat.1000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Rikihisa Y, Yamaguchi M, Ohashi N. Differential expression of VirB9 and VirB6 during the life cycle of Anaplasma phagocytophilum in human leucocytes is associated with differential binding and avoidance of lysosome pathway. Cellular microbiology. 2006;8:523–534. doi: 10.1111/j.1462-5822.2005.00643.x. [DOI] [PubMed] [Google Scholar]
- Niu H, Yamaguchi M, Rikihisa Y. Subversion of cellular autophagy by Anaplasma phagocytophilum. Cellular microbiology. 2008;10:593–605. doi: 10.1111/j.1462-5822.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Zhi N, Lin Q, Rikihisa Y. Characterization and transcriptional analysis of gene clusters for a type IV secretion machinery in human granulocytic and monocytic ehrlichiosis agents. Infect Immun. 2002;70:2128–2138. doi: 10.1128/IAI.70.4.2128-2138.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kim KJ, Choi KS, Grab DJ, Dumler JS. Anaplasma phagocytophilum AnkA binds to granulocyte DNA and nuclear proteins. Cellular microbiology. 2004;6:743–751. doi: 10.1111/j.1462-5822.2004.00400.x. [DOI] [PubMed] [Google Scholar]
- Pedra JH, Mattner J, Tao J, Kerfoot SM, Davis RJ, Flavell RA, et al. c-Jun NH2-terminal kinase 2 inhibits gamma interferon production during Anaplasma phagocytophilum infection. Infect Immun. 2008;76:308–316. doi: 10.1128/IAI.00599-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluk H, Dorey K, Superti-Furga G. Autoinhibition of c-Abl. Cell. 2002;108:247–259. doi: 10.1016/s0092-8674(02)00623-2. [DOI] [PubMed] [Google Scholar]
- Poole AW, Jones ML. A SHPing tale: perspectives on the regulation of SHP-1 and SHP-2 tyrosine phosphatases by the C-terminal tail. Cell Signal. 2005;17:1323–1332. doi: 10.1016/j.cellsig.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y. Anaplasma phagocytophilum and Ehrlichia chaffeensis: subversive manipulators of host cells. Nat Rev Microbiol. 2010a;8:328–339. doi: 10.1038/nrmicro2318. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y. Molecular events involved in cellular invasion by Ehrlichia chaffeensis and Anaplasma phagocytophilum. Vet Parasitol. 2010b;167:155–166. doi: 10.1016/j.vetpar.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y, Lin M. Anaplasma phagocytophilum and Ehrlichia chaffeensis type IV secretion and Ank proteins. Current opinion in microbiology. 2010;13:59–66. doi: 10.1016/j.mib.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Shi Y, Alin K, Goff SP. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 1995;9:2583–2597. doi: 10.1101/gad.9.21.2583. [DOI] [PubMed] [Google Scholar]
- Steen JA, Bannam TL, Teng WL, Devenish RJ, Rood JI. The putative coupling protein TcpA interacts with other pCW3-encoded proteins to form an essential part of the conjugation complex. Journal of bacteriology. 2009;191:2926–2933. doi: 10.1128/JB.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JR, Doros-Richert LA, Gingrich-Baker C, Munroe K, Mather TN, Coughlin RT, et al. Molecular cloning and sequencing of three granulocytic Ehrlichia genes encoding high-molecular-weight immunoreactive proteins. Infect Immun. 1998;66:1356–1363. doi: 10.1128/iai.66.4.1356-1363.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutten EL, Norimine J, Beare PA, Heinzen RA, Lopez JE, Morse K, et al. Anaplasma marginale type IV secretion system proteins VirB2, VirB7, VirB11, and VirD4 are immunogenic components of a protective bacterial membrane vaccine. Infect Immun. 2010;78:1314–1325. doi: 10.1128/IAI.01207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani K, Sato S, Sukezane T, Kojima H, Hirose H, Hanafusa H, Shishido T. Abl interactor 1 promotes tyrosine 296 phosphorylation of mammalian enabled (Mena) by c-Abl kinase. J Biol Chem. 2003;278:21685–21692. doi: 10.1074/jbc.M301447200. [DOI] [PubMed] [Google Scholar]
- Telford SR, 3rd, Dawson JE, Katavolos P, Warner CK, Kolbert CP, Persing DH. Perpetuation of the agent of human granulocytic ehrlichiosis in a deer tick-rodent cycle. Proc Natl Acad Sci USA. 1996;93:6209–6214. doi: 10.1073/pnas.93.12.6209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergunst AC, van Lier MCM, den Dulk-Ras A, Grosse Stuve TA, Ouwehand A, Hooykaas PJJ. Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc Natl Acad Sci USA. 2005;102:832–837. doi: 10.1073/pnas.0406241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Loewenich FD, Baumgarten BU, Schroppel K, Geissdorfer W, Rollinghoff M, Bogdan C. High diversity of ankA sequences of Anaplasma phagocytophilum among Ixodes ricinus ticks in Germany. Journal of clinical microbiology. 2003;41:5033–5040. doi: 10.1128/JCM.41.11.5033-5040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster P, IJdo JW, Chicoine LM, Fikrig E. The agent of Human Granulocytic Ehrlichiosis resides in an endosomal compartment. J Clin Invest. 1998;101:1932–1941. doi: 10.1172/JCI1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Q, Lin M, Rikihisa Y. Cholesterol-dependent Anaplasma phagocytophilum exploits the low-density lipoprotein uptake pathway. PLoS pathogens. 2009;5:e1000329. doi: 10.1371/journal.ppat.1000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiie K, Kim HY, Mott J, Rikihisa Y. Intracellular infection by the human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect Immun. 2000;68:1125–1133. doi: 10.1128/iai.68.3.1125-1133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]