Abstract
Epidural electrical stimulation (ES) at spinal cord segment L2 can produce coordinated step-like movements in completely spinalized adult rats [R.M. Ichiyama, Y.P. Gerasimenko, H. Zhong, R.R. Roy, V.R. Edgerton, Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation, Neurosci. Lett. 383 (2005) 339–344]. Plantar placement of the paws, however, was rarely observed. Here, we sought to determine the dose dependence of a 5-HT agonist (quipazine) on stepping kinematics when administered in combination with ES. Six adult female Sprague–Dawley rats received a complete mid-thoracic spinal cord transection and were implanted with epidural electrodes at the L2 spinal cord level. Quipazine (i.p.) was tested at doses of 0.1, 0.2, 0.3, 0.4, and 0.5 mg/kg. Rats were placed in a body weight support system, allowing them to walk bipedally on a moving treadmill belt (7 cm/s). 3D step kinematics analysis revealed that coordinated alternating bilateral stepping was induced by L2 stimulation (50 Hz) alone and by quipazine alone. Furthermore, the combination treatment produced significantly greater numbers of plantar steps and improved quality of stepping compared to either intervention alone. Both number and quality of stepping peaked at the intermediate dose of 0.3–0.4 mg/kg. The results indicate that quipazine and ES can have complementary effects on spinal circuits and that quipazine dosage is an important factor in differentially modulating these circuitries to improve the quality of the bipedal stepping on a treadmill belt.
Keywords: Epidural electrical stimulation, Serotonin, Locomotion, Spinal cord, Rats
Engaging the neural circuits within the spinal cord to produce oscillating, step-like movements in vivo has been achieved both pharmacologically and/or electrically in adult spinal cats [3,4,8,11,12,15,16,18] and rats [1,2,11]. We recently reported that in completely spinalized adult rats epidural stimulation (ES) of spinal cord segment L2 evokes alternating locomotor movements of the hindlimbs [11]. ES elicited left–right alternation and intralimb coordination conducive to bipedal stepping over a moving treadmill belt. Most of those movements, however, were not plantar-supported steps.
The purpose of the present study was to determine whether administration of quipazine combined with ES could have complementary effects on locomotion of spinal adult rats and to determine the dose response to quipazine in the presence or absence of ES. We observed that compared to ES alone or quipazine alone, the combination strategy resulted in the largest number of plantar steps. The combination strategy also resulted in the most improvement in the quality of the stepping based on kinematics and measures of stepping rhythm and consistency.
Procedures for spinal cord transection and epidural electrode implantation have been described in detail previously [11] and comply with the guidelines of the National Institute of Health Guide for the Care and Use of Laboratory Animals. Briefly, six adult female Sprague–Dawley rats (200–250 g) were anesthetized with a combination of ketamine (100 mg/kg) and xylazine (10 mg/kg) and maintained in deep anesthesia with supplemental doses of ketamine. Under aseptic conditions, a mid-dorsal skin incision was made and the paravertebral muscles and fascia were separated from the vertebral column. A partial laminectomy was performed at a mid-thoracic level (~T7–T9) and the spinal cord was completely transected using fine scissors and forceps. The completeness of the transection was verified by two surgeons. Gel foam was inserted into the gap created by the transection as a coagulant and to separate the cut ends of the spinal cord. A second partial laminectomy exposed L2 for ES electrode implantation. Two Teflon-coated stainless steel wires (AS632; Cooner Wire, Chatsworth, CA) were passed under the spinous processes and above the dura mater of the remaining vertebrae between the partial laminectomy sites. After removing a small portion (~1 mm notch) of the Teflon coating and exposing the wire on the surface facing the spinal cord, electrodes were sutured to the dura mater above and below the segment of interest. The two wires were placed as far lateral to the midline, one on each side, of the spinal cord as possible. A common ground (indifferent) wire was inserted subcutaneously in the mid-back region. The appropriate location of the stimulating electrode was verified post-mortem. Electrode wires were connected to an amphenol connector that was cemented to the animals’ skull [19], which was connected to a Grass S88 Stimulator (Grass Instruments) through a stimulus isolation unit (Grass SIU5, Grass Instruments).
All experimental manipulations were performed 4–5 weeks after spinal cord transection and ES electrode implantation. Muscle-twitch threshold and the threshold for inducing locomotor activity under ES were determined. ES was delivered at 50 Hz continuously as established in previous experiments [11]. Rats were placed in an upper-body harness [5] and an automated body weight support system [21] provided appropriate amounts of support to perform bipedal locomotion (80–90%) at 7 cm/s. Video analysis (Motus, Peak Performance Technologies, Englewood, CO) of the hip, knee, and ankle joints was obtained for the determination of segmental motion and joint angular kinematics. A four-camera system with retro-reflective markers placed on the iliac crest, greater trochanter, lateral condyle, lateral malleolus, and the distal end of the fifth metatarsal on both legs was used, which provided 3D reconstruction models in several anatomical planes. Before any ES, the two stimulating electrodes were connected (via an isolation unit) and used as a single electrode. Rectangular pulses (200 μs duration) were delivered at 50 Hz. The intensity of stimulation ranged from 1 to 13 V. The duration of a testing session for each rat was between 30 and 40 min.
Quipazine was tested at doses of 0.1, 0.2, 0.3, 0.4 and 0.5 mg/kg in all rats using a repeated measures design, administered i.p. 15 min prior to the start of a recording session. Each dose was tested on separate days in a random order between rats. At the beginning of each testing session, the most effective ES intensity to induce alternating locomotor behavior was determined before quipazine administration. Locomotor movements were recorded for three conditions: ES alone, quipazine alone, and their combination. Within each testing session at a specific dose of quipazine, the order of the tests was kept constant between days, i.e., ES alone, then quipazine alone, and finally the combination, due to the constraints imposed by the administration of quipazine. For each experimental condition, two individuals (blinded to the experimental conditions) analyzed 10 s of the best locomotor movements for each rat in each condition.
For joint angular kinematics all comparisons between groups were made at a quipazine dose of 0.3 mg/kg, based on the results presented below. Movement extent was characterized by differentiating the kinematics time series for each joint angle with respect to time to yield velocity, then calculating the average velocity over time and across joint angles for the entire 10 s trial (Vave). Movement frequency was measured by calculating the fast Fourier transform (FFT) of each joint angle time series, and identifying the average frequency at peak power (FPP) across joint angles [17]. Joint coordination (JC) was calculated using a technique based on the peak cross-correlation of joint kinematics time series, and movement shape (MS) was evaluated using a method based on the continuous wavelet transform [17]. The peak cross-correlation is an indication of joint coordination without considering how movement shapes match normal locomotion, whereas the movement shape measure compares joint angle kinematics to those of intact control rats irrespective of frequency.
Comparison of the three treatment groups were conducted using SAS 9.1 PROC MIXED which fits a linear mixed effects model with the treatment indicator as a covariate, where the stimulation alone group was treated as the baseline. Paired t-tests were used to compare the five levels (doses) within the quipazine alone and combination groups. For the kinematics measurements, which were made at the quipazine dose of 0.3 mg/kg, paired t-tests were used to compare the three conditions. The coefficients of variability of joint angles were calculated as standard deviations divided by the mean angles for all step cycles during the 10 s period with the best locomotor movements for each condition. Results are expressed as mean (±S.E.M.), unless otherwise indicated.
Quipazine alone, ES alone, and their combination were all capable of stimulating the spinal cord to generate stepping movements when rats were placed on a moving treadmill belt. Five out of the six rats were able to produce alternating bilateral locomotor movements under all three conditions tested. The stepping characteristics, however, were different for each condition. The combination of quipazine and ES generated significantly more plantar steps than either intervention alone (p < 0.001). The combination also exhibited stepping kinematics that were robust, coordinated, and of intermediate frequency between quipazine alone and ES alone.
ES alone (50 Hz) resulted in a greater number of alternating bilateral locomotor movements than quipazine alone or the combination (p < 0.001), which is reflected in the higher movement frequencies observed during ES alone (Figs. 1A (x-axis) and 2). ES alone resulted in a mean of 18 (±1.9) total locomotor movements (right and left combined) within the 10 s trial. A low percentage of these locomotor movements (2.8 ± 2.3 steps; 15.7%), however, were plantar steps (Fig. 2).
Fig. 1.
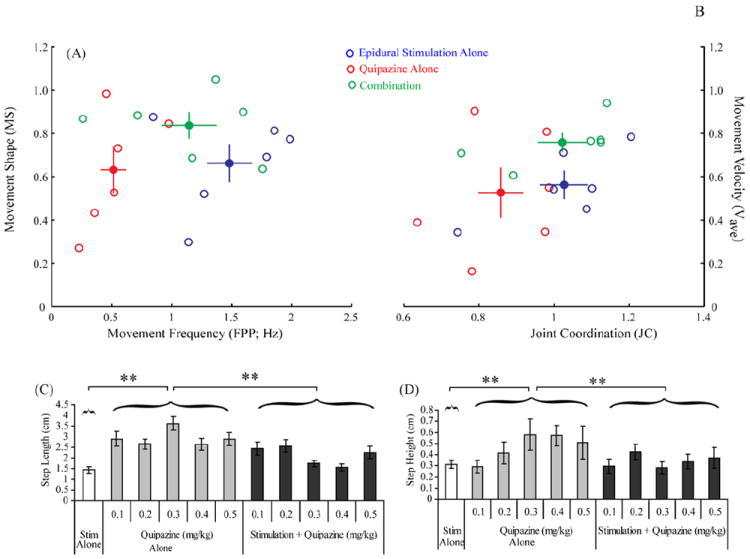
Kinematics of bipedal weight-supported treadmill stepping at 7 cm/s for animals given epidural stimulation (50 Hz at L2) alone, quipazine alone (0.3 mg/kg), and a combination of epidural stimulation and quipazine. The distribution and mean group values for the relationships between movement frequency and movement shape (A) and between joint coordination and movement velocity (B) are shown. Note that the combination treatment has intermediate movement frequency and higher movement velocity values. The mean step length (C) and step height (D) showed significant group differences (** p < 0.01). Stim, stimulation.
Fig. 2.
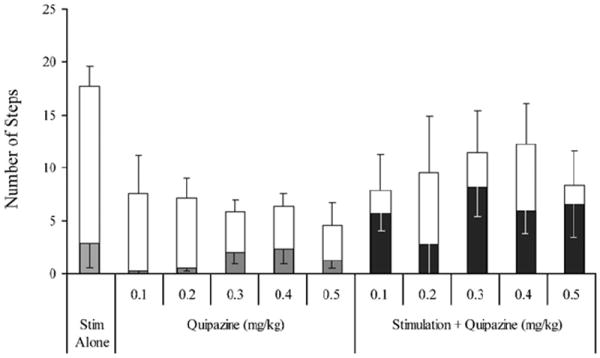
Total number of locomotor movements (open bars) and plantar steps (filled bars) for each intervention and each dosage of quipazine. As a group, stimulation alone produced a significantly greater number of locomotor movements (p < 0.001). However, as a group, the combination of stimulation and quipazine produced a significantly greater number of plantar steps than either quipazine alone or stimulation alone (p < 0.001).
Although quipazine alone produced locomotor movements, the resulting steps occurred at a lower frequency (Fig. 1A) than with ES alone or the combination (p < 0.05). Step length was longer (Fig. 1C) and step height was higher (Fig. 1D) in rats treated with quipazine alone than in rats receiving ES alone (p < 0.001) or the combination (p < 0.001). At the highest dose of quipazine (0.5 mg/kg), most of the motor activity was extreme flexion or extension of the hindlimbs and not coordinated stepping. Across all doses of quipazine alone, significantly fewer total locomotor movements were generated than in the other two conditions (p < 0.0003) (Fig. 2). A low percentage of locomotor movements were plantar steps, ranging from 3.3% (at 0.1 mg/kg) to 36.9% (at 0.4 mg/kg) (Fig. 2).
The range of angles for all joints analyzed was greatest during stepping under quipazine alone, except for the right hip (Fig. 3D). The range of right knee angles (p < 0.005) and left ankle angles (p < 0.01) were significantly larger after quipazine alone than ES alone. The coefficient of variability for all joints also was highest after quipazine alone (Fig. 3E). The variability of the left ankle joint angles after quipazine alone was significantly higher (p < 0.01) than with ES alone. These results combined suggest that under quipazine alone, even at the medium dose of 0.3 mg/kg, stepping movements were more erratic and irregular when compared to the other two conditions.
Fig. 3.
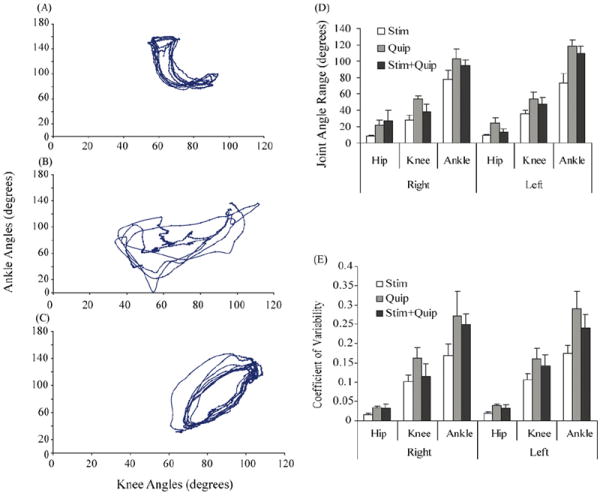
Relative motion plots of ankle and knee joint angles during stepping at 7 cm/s with epidural stimulation alone (A), quipazine alone (B), and the combination of stimulation + quipazine (C). (D) The maximum angle range for the hip, knee, and ankle joints for both the left and right hindlimbs during locomotion. (E) The coefficient of variability under the same conditions. Values in (D) and (E) are means (±S.E.M.). Quip, quipazine; Stim, stimulation.
Quipazine increased the sensitivity of the spinal cord to ES. The stimulation threshold to elicit muscle twitch as detected visually and by palpation was lower after quipazine administration (Table 1). The largest decrease in the threshold for a muscle twitch was observed at the highest quipazine dose tested (0.5 mg/kg) (7.6%, p < 0.05). The effective ES intensity required to produce locomotor movements refers to the lowest intensity of electrical stimulation that resulted in alternating locomotor-like movements of the legs, including both dorsal and plantar placement stepping. There was a significant decrease in the effective ES intensity after administration of quipazine at dosages of 0.2, 0.3, and 0.5 mg/kg (Table 1).
Table 1.
Percent change in muscle twitch and locomotor thresholds after compared to before quipazine administration at various dosages with intensity of stimulation ranging from 1 to 13 V
| Dose of quipazine (mg/kg) | Muscle twitch threshold (% change) | Difference in voltage to facilitate locomotion (% change) |
|---|---|---|
| 0.1 | −4.0 (2.9) | 9.9 (17.1) |
| 0.2 | −4.9 (2.4) | −17.6 (8.7)* |
| 0.3 | −4.4 (1.5) | −17.3 (10.1)* |
| 0.4 | −3.7 (2.6) | −1.6 (1.6) |
| 0.5 | −7.6 (2.6)* | −25.3 (0.3)* |
Values are mean (±S.E.M.).
Significant differences after quipazine administration at p < 0.05.
Combining quipazine and ES resulted in movements of intermediate frequency but robust amplitudes as indicated by high average velocities (Fig. 1A). Overall movement velocity for the combination treatment was significantly higher than for ES alone (p < 0.01; Fig. 1A). There was a trend (p = 0.057; Fig. 1A) for the combination to yield higher-velocity movements than quipazine alone. The combination treatment resulted in movements of significantly higher frequency and greater coordination than quipazine alone (FPP: p < 0.05; JC: p < 0.04), and significantly more normal step movements than ES alone (MS: p < 0.01). The stepping produced with the combination treatment had more consistent joint angle kinematics (compare Fig. 3B and C) and more consistent intralimb coordination (Fig. 1B) compared to quipazine alone. Although the joint angle kinematics with ES alone also was greater than with quipazine alone, the movement shape was the most abnormal with ES alone (Fig. 1A). Increases in frequency and coordination over both separate treatments allowed the combination group to generate a significantly larger number of plantar steps (p < 0.0005). The number of plantar steps showed an apparent peak at 0.3 mg/kg (Fig. 2), but there were no significant differences in the total number of plantar steps between the different doses of quipazine, when quipazine administration was combined with ES.
We have demonstrated that ES in combination with a specific range of quipazine dosages 4–5 weeks after a complete spinal cord transection in adult rats can markedly improve the quality and consistency of hindlimb stepping. Our main finding was that the combination strategy resulted in a larger number of bipedal alternating plantar steps, when compared to either ES alone or quipazine administration alone. The data show that either the pharmacological or ES intervention alone was able to engage spinal circuitries that can facilitate stepping when placed on a moving treadmill belt. These observations also emphasize the importance of the sensory feedback associated with weight-bearing stepping in manifesting the dose-dependent modulation of quipazine in the presence of ES. Thus, there were complementary influences of ES, quipazine, and proprioceptive processing within the spinal circuitries that led to the improved stepping.
Quipazine acts as an agonist to 5-HT2 receptors and facilitates locomotion on a moving treadmill belt after a spinal cord injury in adult animals [1,2,6,7]. In those studies, consistent stepping was observed only after days of daily quipazine administration (i.p. or i.t.). In contrast, a single i.p. injection of quipazine in combination with ES resulted in consistent, coordinated, and alternating plantar stepping in the present study. Quipazine alone was not as effective in eliciting locomotor movements on the treadmill as the other two interventions, not even at the higher doses. In fact, the higher doses of quipazine seemed to interfere with the production of coordinated movements and the highest dosage (0.5 mg/kg) resulted in the least number of plantar steps generated. In those instances, rats showed exaggerated flexion and extension of the hindlimbs that seemed to interfere with stepping. Low doses of quipazine (0.1 and 0.2 mg/kg) were ineffective in facilitating stepping, particularly plantar steps. The most consistent optimum dose of quipazine in combination with ES to facilitate coordinated alternating plantar steps was 0.3 mg/kg.
5-HT affects the membrane properties of neurons, spinal cord reflexes, and locomotor function (reviewed by [20]). In chronic spinal cats [3] and rats [14] there is a significant increase in responses to serotonergic agonists. Plantar stepping requires the coordinated activation of flexors and extensors of the hip, knee, ankle, and the digits. At the stimulation levels and quipazine dosages used in the present study neither ES alone nor quipazine alone induced the same number of plantar steps as their combination. Perhaps quipazine elevated the net excitability of the motoneuronal pools via interneurons as well as directly on motoneurons. This heightened net level of excitability could have enabled the activation of the locomotor circuitry initiated by ES at L2. 5-HT also may have enhanced peripheral feedback, providing necessary conditions for more effective locomotor activity in the presence of ES. The actual mechanisms underlying the interaction between ES and quipazine administration, however, remain unclear.
The present results with ES alone after a complete spinal cord transection confirm our previous results [11]. We have demonstrated that one of the features of ES is that it engages motoneurons directly via monosynaptic pathways and polysynaptic pathways [10]. We subsequently observed a close correlation between the time of recovery of stepping movements following a complete spinal cord transection and when ES can induce polysynaptic responses to a single stimulus during locomotion on a treadmill [13]. The implication of the present results, given these previous observations, is that ES can activate motor pools via both monosynaptic and polysynaptic pathways, both of which are modulated in a phase-dependent manner during stepping. Evidence also suggests that quipazine can facilitate both the monosynaptic and polysynaptic responses, but the responses are differentially modulated in flexor and extensor motor pools [9]. A logical interpretation of all of these results is that there are different components of the spinal circuitries that are activated by ES and/or by quipazine that can cooperatively facilitate stepping. Furthermore, based on all of these results it would appear that a “fine tuning” of the presentation of these two means of activating the spinal circuitry can be achieved by optimizing the agonist dosage.
Acknowledgments
This work was supported by CDRF International Scientific Consortium, California Roman Reed Funds, NIH NS16333, NIH HD44830, RFBR-CRDF Grant 07-04-91106 and RFBR Grant 07-04-00526.
References
- 1.Antri M, Barthe JY, Mouffle C, Orsal D. Long-lasting recovery of locomotor function in chronic spinal rat following chronic combined pharmacological stimulation of serotonergic receptors with 8-OHDPAT and quipazine. Neurosci Lett. 2005;384:162–167. doi: 10.1016/j.neulet.2005.04.062. [DOI] [PubMed] [Google Scholar]
- 2.Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT2 agonist. Eur J Neurosci. 2002;16:467–476. doi: 10.1046/j.1460-9568.2002.02088.x. [DOI] [PubMed] [Google Scholar]
- 3.Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Res. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- 4.de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol. 1998;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- 5.de Leon RD, Reinkensmeyer DJ, Timoszyk WK, London NJ, Roy RR, Edgerton VR. Use of robotics in assessing the adaptive capacity of the rat lumbar spinal cord. Prog Brain Res. 2002;137:141–149. doi: 10.1016/s0079-6123(02)37013-4. [DOI] [PubMed] [Google Scholar]
- 6.Feraboli-Lohnherr D, Orsal D, Yakovleff A, Gimenez y Ribotta M, Privat A. Recovery of locomotor activity in the adult chronic spinal rat after sublesional transplantation of embryonic nervous cells: specific role of serotonergic neurons. Exp Brain Res. 1997;113:443–454. doi: 10.1007/pl00005597. [DOI] [PubMed] [Google Scholar]
- 7.Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci. 2005;25:11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerasimenko YP, Avelev VD, Nikitin OA, Lavrov IA. Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci Behav Physiol. 2003;33:247–254. doi: 10.1023/a:1022199214515. [DOI] [PubMed] [Google Scholar]
- 9.Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol. 2007;98:2525–2536. doi: 10.1152/jn.00836.2007. [DOI] [PubMed] [Google Scholar]
- 10.Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Method. 2006;157:253–263. doi: 10.1016/j.jneumeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Spinal cord stimulation-induced locomotion in the adult cat. Brain Res Bull. 1992;28:99–105. doi: 10.1016/0361-9230(92)90235-p. [DOI] [PubMed] [Google Scholar]
- 13.Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol. 2006;96:1699–1710. doi: 10.1152/jn.00325.2006. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Murray K, Harvey PJ, Ballou EW, Bennett DJ. Serotonin facilitates a persistent calcium current in motoneurons of rats with and without chronic spinal cord injury. J Neurophysiol. 2007;97:1236–1246. doi: 10.1152/jn.00995.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mushahwar VK, Aoyagi Y, Stein RB, Prochazka A. Movements generated by intraspinal microstimulation in the intermediate gray matter of the anesthetized, decerebrate, and spinal cat. Can J Physiol Pharmacol. 2004;82:702–714. doi: 10.1139/y04-079. [DOI] [PubMed] [Google Scholar]
- 16.Mushahwar VK, Gillard DM, Gauthier MJ, Prochazka A. Intraspinal micro-stimulation generates locomotor-like and feedback-controlled movements. IEEE Trans Neural Syst Rehabil Eng. 2002;10:68–81. doi: 10.1109/TNSRE.2002.1021588. [DOI] [PubMed] [Google Scholar]
- 17.Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy RR, Edgerton VR, Mendell LM. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J Neurosci. 2007;27:4460–4471. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prochazka A, Mushahwar V, Yakovenko S. Activation and coordination of spinal motoneuron pools after spinal cord injury. Prog Brain Res. 2002;137:109–124. doi: 10.1016/s0079-6123(02)37011-0. [DOI] [PubMed] [Google Scholar]
- 19.Roy RR, Hutchison DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol. 1991;70:2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- 21.Timoszyk WK, Nessler JA, Acosta C, Roy RR, Edgerton VR, Reinkens-meyer DJ, de Leon R. Hindlimb loading determines stepping quantity and quality following spinal cord transection. Brain Res. 2005;1050:180–189. doi: 10.1016/j.brainres.2005.05.041. [DOI] [PubMed] [Google Scholar]


