INTRODUCTION
The pulmonary circulation is a unique system that differs from the systemic circulation in structure, function, and regulation. For example, hypoxia causes pulmonary vaso-constriction but dilates the systemic circulation. In neonates with hypoxemic respiratory failure (HRF), circulatory changes in the lung can be primary, as in idiopathic persistent pulmonary hypertension of the newborn (PPHN), or secondary to lung disease. This article provides a brief overview of normal pulmonary circulation, changes in neonatal pulmonary circulation in common causes of neonatal HRF, and its response to therapeutic interventions in the neonatal intensive care unit (NICU).
FETAL CIRCULATION
Gas exchange is the primary function of the postnatal lung. The low-resistance, high-volume pulmonary circulation, which receives half of the combined ventricular output, is a crucial factor in achieving efficient gas exchange by the aerated lung during post-natal life. During fetal life, the placenta serves as the organ of gas exchange; placental vascular resistance is low and receives nearly half of fetal combined ventricular output. During this period, fetal pulmonary vascular resistance (PVR) is high (physiologic pulmonary hypertension), and blood flow is diverted from the pulmonary artery to the aorta and umbilical arteries toward the placenta.1 Fetal pulmonary circulation must prepare the lungs for adequate structural growth and functional maturation in anticipation for the switch to air breathing in the postnatal period. During the normal transition at birth, PVR decreases and is associated with an increase in pulmonary blood flow. Abnormal pulmonary transition leads to sustained increase of PVR, similar to the fetal state, resulting in PPHN. Parenchymal lung diseases such as meconium aspiration syndrome can result in ventilation-perfusion (V/Q) mismatch, hypoxemia, and structural and functional changes in pulmonary circulation resulting in HRF.
Most of the knowledge of fetal pulmonary hemodynamics is derived from studies in fetal lambs. Data from fetal lambs suggest that PVR is high, with only 8% to 10% of combined ventricular output entering the lungs during fetal life.2,3 More recently, Doppler flow studies in human fetuses have shown significantly higher flow into the left and right pulmonary arteries with 13% of combined ventricular output at 20 weeks’ gestation (canalicular stage), increasing to 25% at 30 weeks (saccular stage) and 21% at 38 weeks (alveolar stage).4 The fetal PVR is high during the canalicular stage secondary to paucity of pulmonary vascular network and reduced cross-sectional area of an immature pulmonary vascular bed (Fig. 1). Rasanen and colleagues5 showed that, between 20 and 26 weeks of gestation, maternal hyperoxygenation using 60% humidified oxygen by face mask does not result in pulmonary vasodilation in human fetuses, suggesting a lack of sensitivity to oxygen in early gestation. During the early saccular stage, rapid proliferation of pulmonary vessels decreases fetal PVR. During late preterm and early term gestation (34–36 and 37–38 weeks gestational age [GA], respectively), there is a marked increase in cross-sectional area of the pulmonary vascular bed. However, pulmonary vessels become more sensitive to vaso-constrictive mediators, such as endothelin (ET) and relative hypoxemia, resulting in active pulmonary vasoconstriction and an increase in PVR.6,7 During this period, maternal hyperoxygenation increases pulmonary blood flow in human fetuses5 and fetal lambs.8
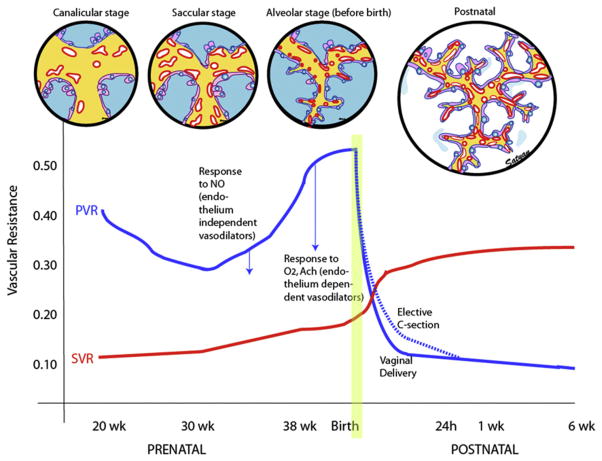
Fig. 1.
Changes in PVR and systemic vascular resistance (SVR) during the last half of gestation and the postnatal period. During the canalicular phase of lung development, high PVR is caused by low density of the vasculature. In the saccular stage, broad intersaccular septae contain the double capillary network and, with increasing vascular density, PVR decreases. In the alveolar phase, despite the rapid increase in the number of small pulmonary arteries, high PVR is maintained by active vasoconstriction. Fetal pulmonary vasodilator response to endothelium-independent (direct smooth muscle relaxant) vasodilators such as NO precedes the maturation of the vasodilator response to oxygen and acetylcholine (Ach), endothelium-dependent vasodilators. After birth, lung liquid is absorbed and an air-liquid interphase is established with juxtaposition of capillaries and alveolar epithelium to promote effective gas exchange. The dashed line represents the delay in decrease of PVR observed following elective cesarean section. SVR markedly increases after occlusion of the umbilical cord and removal of the low-resistance placental circuit from the systemic circulation. (Copyright © Satyan Lakshminrusimha.)
In fetal lambs, pulmonary vasodilation in response to endothelium-independent mediators, such as nitric oxide (NO), precedes responses to endothelium-dependent mediators, such as acetylcholine and oxygen. Response to NO depends on activity of its target enzyme, soluble guanylate cyclase (sGC), in the smooth muscle cell. In the ovine fetus, sGC messenger RNA levels are low during early preterm (126 days) gestation and increase during late preterm and early term gestation (137 days).9 In rats, abundant sGC activity is present in the lung at late gestation and early newborn periods and gradually decreases in adulthood.10 Low levels of pulmonary arterial sGC activity during late canalicular and early saccular stages of lung development are probably responsible for the poor response to iNO observed in preterm infants delivered at less than 29 weeks GA.11
Modulation of Fetal PVR
Conditions such as congenital diaphragmatic hernia (CDH), antenatal closure of the ductus arteriosus, and idiopathic PPHN are often associated with vascular remodeling and increased PVR during fetal life. Studies in animal models suggest that maternal therapy can alter fetal PVR. Loong and colleagues reported that antenatal administration of sildenafil improved lung structure (decreased mean linear intercept) and reduced pulmonary hypertension (decreased right ventricle/left ventricle + septum ratio) in nitrofen-induced CDH rat pups.12 Maternal betamethasone similarly reduces oxidative stress and improves relaxation response to adenosine triphosphate (ATP) and NO donors in fetal lambs with PPHN induced by ductal ligation.13 Antenatal tracheal occlusion in animal models of CDH reduces pulmonary circulatory impedance and pulmonary arterial remodeling.14–17 Further translational and clinical research into reducing fetal PVR and improving lung structure and function by antenatal medical and surgical intervention is critical to reduce mortality and morbidity in CDH. Maternal medications can also increase fetal PVR and increase the risk of PPHN. Two classes of medications, antidepressants and antiinflammatory agents, have been well studied.
Selective serotonin uptake inhibitors
Maternal intake of selective serotonin uptake inhibitors (SSRIs) during the last half of pregnancy has been associated with an increased risk of PPHN.18 Exposure of pregnant rats to fluoxetine resulted in pulmonary hypertension in rat pups (more profound in female pups) and was associated with hypoxia and increased mortality.19,20 The mechanism by which fluoxetine induces pulmonary hypertension in newborns is unknown. It is speculated that higher drug-induced serotonin levels result in pulmonary vasoconstriction. A more recent retrospective analysis has questioned this association.21 Obstetricians must weigh the maternal psychological benefits of antidepressant therapy during pregnancy against the risk of adverse neonatal effects.
Nonsteroidal antiinflammatory medications
Ingestion of nonsteroidal antiinflammatory drugs (NSAIDs), such as aspirin, during late gestation may be associated with in utero closure of the fetal ductus arteriosus.22 Experimental ligation of the ductus arteriosus in lambs during fetal life is associated with pulmonary vascular remodeling and PPHN.23 Prostaglandins maintain ductal patency in utero and are important mediators of pulmonary vasodilation in response to ventilation at birth. Pharmacologic blockade of prostaglandin production by NSAIDs can result in PPHN. Analysis of meconium from newborn infants with PPHN revealed the presence of NSAID in approximately half of the samples,24 linking antenatal NSAID exposure to PPHN.
TRANSITION AT BIRTH
The entry of air into the alveoli with crying and breathing improves oxygenation of the pulmonary vascular bed, decreasing PVR and increasing pulmonary blood flow.25 The increase in pulmonary blood flow raises left atrial pressures more than right atrial pressures, closing the foramen ovale. Removal of the low-resistance placental bed from the systemic circulation at birth increases systemic vascular resistance (SVR; see Fig. 1). As PVR decreases to less than SVR, flow reverses across the ductus. Oxygen-induced vasodilation and lung expansion decrease PVR to approximately half of SVR within a few minutes after birth. Over the first few hours after birth, the ductus arteriosus closes, largely in response to the increase in oxygen tension, and with this the normal postnatal circulatory pattern is established. The recognition of the role of NO in mediating pulmonary vascular transition at birth26 has led to the development of inhaled NO (iNO) as a therapeutic strategy in the life-threatening clinical disorder of PPHN. A detailed review of NO and other mediators of pulmonary vascular transition at birth is presented in a previous issue.1
Factors Altering Pulmonary Vascular Transition at Birth
Mode of delivery
Vaginal delivery is associated with reduction in fetal PVR at birth. Delivery by elective cesarean section27,28 delays the decrease in pulmonary arterial pressure (see Fig. 1), as shown by prolonged right-sided systolic time intervals, and increases the risk for PPHN.21 Compared with matched controls, infants with PPHN are more likely to have been delivered by cesarean section.29
Timing of delivery
Timing of delivery influences the risk and outcome of HRF in neonates. Delivery during late preterm or early term gestation is associated with a higher risk of admission to the NICU with respiratory distress.30 Among patients with severe HRF requiring extracorporeal membrane oxygenation (ECMO), mortality is higher among late preterm and early term infants compared with term infants.31 However, infants with CDH without other anomalies have been observed to have reduced need for ECMO and marginally better survival when delivered early term compared with late term.32 More recent population-based studies have not confirmed these findings.33
Antenatal glucocorticoids
Administration of glucocorticoids, such as betamethasone, before elective cesarean section has been shown to reduce the incidence of respiratory distress and admission to the NICU.34,35 This regimen is being adapted in some centers in Europe.36 Preliminary data from our laboratory suggest that antenatal betamethasone decreases PVR and increases fetal pulmonary blood flow. Recent identification of genetic variations involving corticotropin-releasing hormone in patients with PPHN, as well as the effectiveness of hydrocortisone in improving oxygenation in lambs with PPHN, suggest that glucocorticoids may have a role in prevention and management of PPHN and HRF.37,38
Early versus delayed cord clamping
The current neonatal resuscitation guidelines recommend delayed umbilical cord clamping for at least 1 minute for newborn infants who do not require resuscitation at birth.39 Delayed cord clamping results in more stable blood pressures and improved iron status. Arcilla and colleagues40 evaluated the effect of late cord clamping on pulmonary hemodynamics in newborn infants by catheterizing the pulmonary artery. The mean ratio of pulmonary artery to systemic arterial pressure decreased to 0.7 by 2 hours and to 0.5 by 4 hours following early cord clamping. Following late cord clamping, pulmonary arterial pressures were almost 90% of systemic pressures by 9 hours. The investigators speculated that increased blood volume following late cord clamping results in distension of the pulmonary capillary and venous bed, resulting in increased pulmonary arterial pressure. Polycythemia with increased viscosity may contribute to high PVR. There are no reports of an increased incidence of PPHN associated with delayed cord clamping.
Temperature
Induction of severe hypothermia in lambs between 1 and 3 days old (decreasing temperature from 40°C to 30°C) increases mean pulmonary arterial pressure from 29 to 40 mm Hg.41 Perinatal asphyxia is a well-known predisposing factor for PPHN.42 There was considerable concern that therapeutic hypothermia in asphyxiated infants would increase the risk of PPHN. Pooled analysis of randomized trials has not shown an increased incidence of PPHN with hypothermia in this population.43 The type of cooling (selective head cooling vs whole body cooling) does not alter the incidence of PPHN.44
Asphyxia
Perinatal asphyxia interferes with the mechanisms of pulmonary transition at birth and modifies this complex adaptation impeding the decrease in PVR, and increasing the risk for PPHN.42 Multiple mechanisms cause respiratory failure and affect pulmonary circulation in asphyxia: fetal hypoxemia, ischemia, meconium aspiration, ventricular dysfunction, and acidosis can all increase PVR.42 Acute asphyxia is associated with reversible pulmonary vasoconstriction45 but chronic in utero asphyxia with or without meconium aspiration may be associated with vasoconstriction and vascular remodeling.46
Oxygen during neonatal resuscitation
Oxygen is a potent and specific pulmonary vasodilator. The use of 100% oxygen during initial ventilation of normal lambs at birth results in a small but significant decrease in PVR during the first few minutes of life compared with 21% or 50% oxygen.47 However, ventilation with 100% oxygen at birth impairs subsequent relaxation to iNO and acetylcholine, probably because of the formation of reactive oxygen species (ROS). Similar results were observed in lambs with pulmonary hypertension and a remodeled pulmonary vasculature.48 In lambs with asphyxia induced by umbilical cord occlusion, PVR was lower with 100% oxygen resuscitation compared with 21% oxygen at 1 minute of age but, by 2 minutes, PVR was similar in both groups. These findings suggest that optimal ventilation (and not hyperoxygenation) is the key to reducing PVR.49 Thirty minutes of resuscitation with 100% oxygen increased pulmonary arterial contractility and superoxide anion formation in pulmonary arteries. Using 100% oxygen therefore has transient advantages in rapidly reducing PVR but increases ROS formation, increases pulmonary arterial contractility, and impairs vasodilation to endothelium-dependent (acetylcholine) and endothelium-independent (iNO) agents. These findings support the neonatal resuscitation guidelines’ recommendations to use room air for initial resuscitation of term asphyxiated newborn infants.39
PULMONARY CIRCULATORY CHANGES IN HRF
Fig. 2 shows the 4 different patterns of pulmonary vascular changes in neonatal HRF. PPHN is characterized by increased ratio of pulmonary vascular resistance (PVR) to SVR resulting from (1) vasoconstriction; (2) structural remodeling of the pulmonary vasculature (Fig. 3); (3) intravascular obstruction from increased viscosity of blood, as in polycythemia; or (4) lung hypoplasia. This condition leads to right-to-left shunting of blood across the foramen ovale and ductus arteriosus, resulting in hypoxemia. Numerous disease states with diverse causes can result in a similar final pathophysiology. About 10% of cases with PPHN are idiopathic, with no associated pulmonary airspace disorder. However, PPHN is usually associated with other acute respiratory conditions, such as meconium aspiration syndrome (MAS), respiratory distress syndrome (RDS), pneumonia, or CDH. Hypoxemia in these conditions can be caused by parenchymal lung disease, surfactant deficiency (RDS) or inactivation (MAS, pneumonia), ventilation/perfusion (V/Q) mismatch, and intrapulmonary as well as extrapulmonary right-to-left shunting of blood (Fig. 4). In some newborns with HRF, a single mechanism predominates (eg, extrapulmonary right-to-left shunting in idiopathic PPHN). However, more commonly, several of these mechanisms contribute to hypoxemia. In MAS, obstruction of the airways by meconium results in decreasing V/Q ratios and increasing intrapulmonary right-to-left shunt. Other segments of the lungs may be overventilated relative to perfusion, causing increased physiologic dead space. The same patient may also have severe PPHN with extrapulmonary right-to-left shunting at the level of the ductus arteriosus and foramen ovale.
Fig. 2.
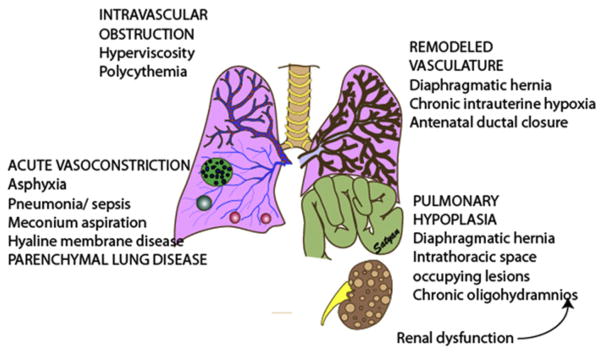
Pathologic changes in pulmonary circulation in neonatal HRF follows 4 patterns. Intravascular obstruction caused by increased viscosity as seen in polycythemia in the presence of normal pulmonary vasculature can cause PPHN. Asphyxia or parenchymal lung disease can lead to alveolar hypoxia and acute pulmonary vasoconstriction. Chronic pulmonary vascular remodeling can result from chronic intrauterine hypoxia, antenatal ductal closure, or CDH. Lung hypoplasia with paucity of pulmonary vasculature accompanies CDH; intrathoracic space occupying lesions, such as adenomatoid malformations; or chronic oligohydramnios syndromes, which could be secondary to chronic leakage of amniotic fluid or fetal oliguria from renal dysfunction. (Copyright © Satyan Lakshminrusimha.)
Fig. 3.
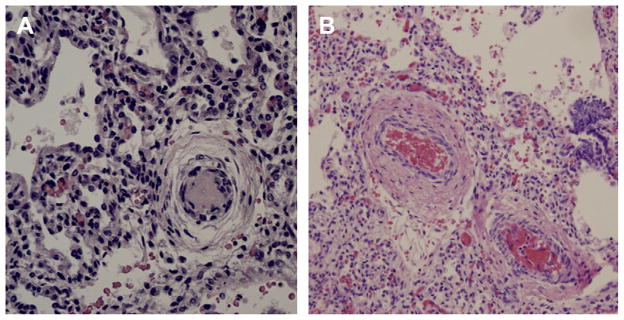
Pulmonary arterial remodeling in HRF. (A) Preterm infant with respiratory distress syndrome and PPHN; (B) Term infant with asphyxia and PPHN; note the smooth muscle cell layer thickening around pulmonary arteries.
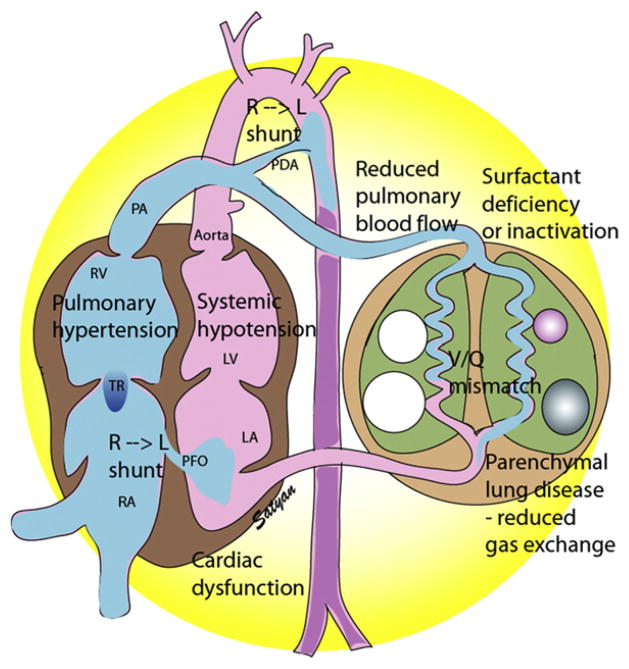
Fig. 4.
Hemodynamic changes in PPHN/HRF. Surfactant deficiency (RDS) or inactivation (MAS or pneumonia) results in parenchymal lung disease and ventilation-perfusion (V/Q) mismatch. Increased PVR results in reduced pulmonary blood flow and right-to-left shunt through the PDA and/or PFO. Pulmonary hypertension, often associated with systemic hypotension, results in septal deviation to the left. Cardiac dysfunction secondary to asphyxia, sepsis, or CDH may contribute to pulmonary venous hypertension and complicate HRF. LA, left atrium; LV, left ventricle; PA, pulmonary artery; PDA, patent ductus arteriosus; PFO, patent foramen ovale; RA, right atrium; RV, right ventricle; TR, tricuspid regurgitation. (Copyright © Satyan Lakshminrusimha.)
Pneumonia or meconium aspiration may release inflammatory mediators that induce vasoconstriction. Vasoconstrictors such as leukotrienes, platelet-activating factor, thromboxanes,50 and ET-151 have been found to be increased in PPHN. Chronic intra-uterine ETA receptor blockade following antenatal ductal ligation decreases pulmonary arterial pressure in utero, decreases right ventricular hypertrophy and distal muscularization of small pulmonary arteries, and further decreases the PVR at delivery in newborn lambs with PPHN.52 Thus ET-1 acting through ETA receptor stimulation might contribute to the pathogenesis and pathophysiology of PPHN. Derangements in the NO pathway of vasodilation can also result in the physiologic characteristics of PPHN. Pulmonary endothelial nitric oxide synthase (eNOS) gene and protein expression and enzyme activity are decreased in fetal lambs with PPHN induced by antenatal ductal ligation.53 In addition, the response to stimulators of eNOS is lost.54 In these lambs with PPHN, the vascular response to NO is also diminished,55 whereas the response to cyclic guanosine monophosphate-phosphodiesterase (cGMP) is normal. Thus, the decreased responsiveness seems to result from decreased vascular smooth muscle sensitivity to NO at the level of sGC. Because NO relaxes smooth muscle and inhibits vascular smooth muscle growth, diminished eNOS expression may contribute to both abnormal vasoreactivity and excessive muscularization of pulmonary vessels in PPHN.
Pulmonary hypertension sometimes occurs because of an abnormal pulmonary vascular bed despite the absence of alveolar hypoxia and hypercapnia and of lung inflammation. These infants can be grouped according to the degree of muscularization and the number of pulmonary arteries.56 In infants with hypoplastic lungs, as in CDH and oligohydramnios sequence (sometimes secondary to fetal renal dysfunction), PPHN may arise primarily as a consequence of a decreased number of vessels, causing decreased cross-sectional area of the pulmonary vascular bed, and leading to flow restriction. Patients with alveolar capillary dysplasia may have a similar vascular hypoplasia. These cases may be complicated by increased muscularization of the vessels.
Many infants who have HRF do not have right-to-left extracardiac shunting, and may have hypoxia because of intrapulmonary shunting or cardiac dysfunction (see Fig. 4). Determination of the hemodynamic profile of these babies using functional echocardiography is important to make the diagnosis, initiate therapy, and follow the changes with therapy.57 The gold standard in defining PPHN rests on the echocardiographic findings of right-to-left shunting of blood at the foramen ovale and/or the ductus arteriosus, as well as estimates of pulmonary arterial pressure using tricuspid regurgitation jet velocity. Using the modified Bernoulli equation, systolic right ventricular pressure (mm Hg) is estimated by 4v2 + right atrial pressure, where v is the maximal velocity of tricuspid regurgitation jet in meters per second on continuous-wave Doppler echocardiogram.58 The velocity of tricuspid regurgitation jet may also be influenced by right ventricular dysfunction, leading to underestimation of pulmonary arterial pressure. Right ventricular dysfunction caused by excessive afterload seems to be a major risk factor for poor outcome in HRF.42
Doppler measurements of atrial and ductal level shunts provide essential information to optimize management of a newborn with HRF. For example, left-to-right shunting at the foramen ovale and ductus arteriosus with marked hypoxemia suggests predominant intrapulmonary shunting, and interventions should be directed at optimizing lung inflation and recruitment. Increasing mean airway pressure and administering surfactant are likely to be more effective than iNO in improving oxygenation in babies with parenchymal lung disease and left-to-right shunt at patent ductus arteriosus (PDA) and patent foramen ovale (PFO).Presence of right-to-left shunting at the ductal level and left-to-right shunting at the atrial level similarly suggests PPHN with left ventricular dysfunction with some pulmonary venous hypertension (Table 1). This finding may be associated with the CDH59 and left ventricular dysfunction seen in sepsis and asphyxia.60 If right-to-left shunting is present at ductal and atrial levels and is associated with labile hypoxemia and tricuspid regurgitation, PPHN is the most likely diagnosis. However, patients with fixed hypoxemia with right-to-left shunting at ductal and atrial levels associated with a small left atrium without tricuspid regurgitation may have anomalous pulmonary venous return (see Table 1).
Table 1.
Differential diagnosis of hypoxemia in neonates based on the direction of shunt at atrial and ductal levels on echocardiography
| Diagnosis | Ductal Shunt | Atrial Shunt | Management |
|---|---|---|---|
| Parenchymal lung disease and V/Q mismatch and intrapulmonary shunt | L →R | L →R | Lung recruitment, specific therapy (antibiotics for pneumonia) NO may be beneficial |
| PPHN | R →L | R →L | Oxygenation, correction of acidosis and inhaled NO |
| Left ventricular dysfunction (common in diaphragmatic hernia, asphyxia, and sepsis)59,60 | R →L | L →R | Inotropes and vasodilators (Milrinone) |
| Tricuspid atresia/stenosis or pulmonic atresia/stenosis | L →R | R →L | Prostaglandin E1 + surgery |
| Total anomalous pulmonary venous return155 | R →L (large PA) | R →L (small LA and no tricuspid regurgitation) | Surgery |
From Lakshminrusimha S, Kumar VH. Diseases of pulmonary circulation. In: Fuhrman PP, Zimmerman JJ, editors. Pediatric critical care. Mosby; 2011. p. 641; with permission.
Pulmonary Circulatory Changes in Some Specific Conditions Resulting in Neonatal Respiratory Failure
Idiopathic PPHN
Idiopathic PPHN (also known as black-lung PPHN) is characterized by increase of PVR without a primary parenchymal lung disease. Autopsy studies of fatal idiopathic PPHN show severe hypertensive structural remodeling with vessel wall thickening and smooth muscle hyperplasia. The vascular smooth muscle extends to the level of intra-acinar arteries,61 resulting in increased PVR and failure to respond to birth-related stimuli, such as ventilation and oxygenation.62 A well-known cause of black-lung PPHN is exposure to indomethacin during the third trimester, resulting in closure of the ductus arteriosus in utero.24,63 A fetal lamb model of idiopathic PPHN is created by antenatal ductal ligation.64 This model shows the clinical and histopathologic features of PPHN.23 Abnormalities of the nitric oxide pathway (decreased eNOS,53 decreased sGC,55 and increased phosphodiesterase type 5 [PDE565]), superoxide anion pathway (increased superoxide66 and hydrogen peroxide67), and prostacyclin pathway (decreased prostacyclin synthase and prostacyclin IP receptor68) have been described in this model. Similar abnormalities in enzyme pathways may occur in human neonates with idiopathic PPHN.
CDH
CDH occurs in approximately 1 in 3000 births and is the most common cause of pulmonary hypoplasia in the neonate. Diaphragmatic hernia is associated with ipsilateral and contralateral lung hypoplasia, vascular paucity, and vascular remodeling. Most cases are diagnosed in the antenatal period. Initial delivery room management focuses on stabilization, gastrointestinal decompression, and immediate intubation. Bag-mask ventilation and introduction of more gas into the gastrointestinal tract should be avoided. Early corrective surgery is often associated with deterioration of respiratory function in the immediate postoperative period. There has been a paradigm shift focusing on cardiorespiratory stabilization and management of PPHN followed by surgery. There are 2 animal models of CDH: the rat model created by maternal ingestion of nitrofen, a herbicide, resulting in lung hypoplasia and a diaphragmatic defect; and a second model that is created by fetal surgery in lambs. Abnormalities in the nitric oxide synthase,69 sGC, and PDE5 function70 have been observed in these models. These abnormalities, associated with left ventricular hypoplasia, may contribute to poor response to iNO in CDH.
MAS
A combination of preexisting in utero hypoxia and meconium aspiration into the lungs with pulmonary hypertension often carries high morbidity. In the 1980s and 1990s, MAS was the most common cause of severe HRF and PPHN in neonates, but the incidence has decreased in recent years in the United States. A review of annual neonatal ECMO data from the Extracorporeal Life Support Organization (ELSO) registry (accessed in February 2012) shows that CDH accounts for more ECMO runs than MAS in recent years. This reduction is partly caused by reduction in postterm births in the United States in recent years, because MAS is more common in this population. Meconium aspiration with perinatal asphyxia leads to an immediate release of circulating vasoactive substances, which favor contraction and proliferation of smooth muscle fibers in the pulmonary circulation. Most cases of fatal MAS show evidence of smooth muscle hypertrophy in small pulmonary arteries.46 In addition, a decrease in the expression of eNOS was reported in umbilical venous endothelial cells isolated from human infants with MAS.71 In piglets, meconium instillation into the lungs increases PVR and asphyxia decreases SVR, and a combination of MAS and asphyxia worsen the ratio between PVR and SVR.72
Transient tachypnea of the newborn with HRF and PPHN (malignant transient tachypnea of the newborn)
Ramachandrappa and Jain27 reviewed the pathogenesis of respiratory morbidity following elective cesarean section. Many infants with hypoxemia following elective cesarean section are considered to have transient tachypnea of the newborn and wet lung syndrome and are placed on oxygen by hood or nasal cannula without positive pressure. Absorption atelectasis results in increasing oxygen requirements and progressive respiratory failure. It is possible that formation of ROS from high alveolar PaO2 may lead to increased pulmonary vascular reactivity and contribute to PPHN. Severe respiratory failure following elective cesarean section may occasionally require therapy with ECMO.73
Premature infant with bronchopulmonary dysplasia and pulmonary hypertension
Bronchopulmonary dysplasia (BPD) continues to be a major cause of morbidity and late mortality in extremely preterm infants. Pulmonary hypertension is observed in approximately 1 in 6 extremely low birth weight (ELBW) infants.74 BPD is associated with reduced cross-sectional perfusion area with decreased arterial density and abnormal muscularization of peripheral pulmonary arteries.75 Risk factors for developing pulmonary hypertension include low birth weight (small for GA), oligohydramnios,76 and prolonged mechanical ventilation. A recent prospective analysis showed that the onset of pulmonary hypertension in BPD is variable and can be as late as 3 to 4 months of age.74 A delay in diagnosis is associated with progressive pulmonary vascular disease, cor pulmonale, and high mortality. It is prudent to screen babies that are ventilated or require greater than 30% oxygen or have radiological evidence of BPD with an echocardiogram at 1 month of age and every 4 weeks until discharge to diagnose pulmonary hypertension early, leading to appropriate therapy. The optimal intervention strategies for reversing early pulmonary hypertension or treating established pulmonary hypertension are not clear. Multiple therapies, such as maintaining higher oxygen saturations, iNO, and sildenafil, are reported anecdotally to have been tried with mixed results.77–79
Air-leak syndromes
Air-leak syndromes such as pulmonary interstitial emphysema (PIE), pneumothorax, and pneumomediastinum are common complications of mechanical ventilation in preterm infants and are associated with respiratory failure. Among late preterm and term newborn infants, spontaneous pneumothorax is common and results in respiratory failure. Most of these infants improve spontaneously or require thoracocentesis or chest tube drainage with resolution of HRF. Smith and colleagues80 recently reported that almost half of late preterm/term infants with spontaneous, symptomatic pneumothorax that required needle or chest tube drainage developed PPHN. Acute increases in PVR with shunting secondary to hypoxemia or acidosis or caused by the primary lung disease must be considered in the differential diagnosis of persistent HRF in infants with pneumothorax.
PULMONARY HEMODYNAMIC CHANGES CAUSED BY THERAPY
A detailed review of inhaled NO, sildenafil, milrinone, and other pulmonary vasodilator agents is provided in the March 2012 issue of Clinics.81 This article focuses on the impact of various therapies in the NICU on the pulmonary circulation.
Mechanical Ventilation
Optimal lung recruitment during mechanical ventilation with appropriate use of positive end expiration pressure (PEEP) and/or mean airway pressure is a critical step during the management of HRF. When lungs are inflated at functional residual capacity (FRC), PVR is low. PVR is a combination of resistance offered by alveolar vessels and extra-alveolar vessels. When the lungs are underinflated or collapsed, the alveolar vessels are wide open but the extra-alveolar vessels are narrowed, resulting in increased PVR. When the alveoli are overinflated, the alveolar vessels are compressed, resulting in high PVR. Moreover, high PEEP or mean airway pressure may impair venous return and reduce cardiac output.82 An optimal balance is achieved when the lung expansion is at FRC. It is important to check frequent radiographs during the acute phase of PPHN to assess optimal lung expansion.
Many clinicians use high-frequency ventilation (HFV) to manage infants with PPHN. Considering the important role of parenchymal lung disease in specific disorders resulting in PPHN, adequate lung inflation and optimal ventilation are as essential as pharmacologic vasodilator therapy. In the case of inhaled vasodilators, optimal inflation and ventilation may be necessary for drug delivery.83 Infants with PPHN with a variety of causes have been successfully treated with HFV.84 High-frequency oscillatory ventilation (HFOV) decreases PaCO2 and increases oxygenation in infants with PPHN. HFOV may improve oxygenation through safer use of higher mean airway pressures to maintain lung volume and prevent atelectasis. Two studies have evaluated the effectiveness of HFV compared with conventional ventilation in rescuing infants with respiratory failure and PPHN from potential ECMO therapy.85,86 Neither mode of ventilation was more effective in preventing ECMO in these infants. In clinical pilot studies using iNO, a combination of HFOV and iNO resulted in the greatest improvement in oxygenation in some newborns who had severe PPHN complicated by diffuse parenchymal lung disease and underinflation.87 A randomized controlled trial showed that treatment with HFOV and iNO was often successful in patients who failed to respond to HFOV or iNO alone in severe PPHN, and the differences in responses were related to the specific disease associated with PPHN. Infants with RDS and MAS benefit most from a combination of HFOV and iNO therapy.88,89
Oxygen
Oxygen is a specific and potent pulmonary vasodilator and increased oxygen tension is an important mediator of reduction in PVR at birth. Alveolar hypoxia and hypoxemia increase PVR and contribute to the pathophysiology of PPHN. Avoiding hypoxemia by mechanical ventilation with high concentrations of oxygen used to be the mainstay of PPHN management. However, exposure to hyperoxia may result in formation of oxygen free radicals and lead to lung injury. As mentioned previously, brief exposure to 100% oxygen in newborn lambs increases contractility of the pulmonary arteries90 and formation of superoxide anions49 and reduces response to inhaled NO.47,48 Administration of intratracheal recombinant human superoxide dismutase (SOD; an antioxidant that breaks down superoxide anions) results in improved oxygenation in lambs with PPHN.91,92 Based on these studies, it seems that avoiding hyperoxia is as important as avoiding hypoxia in the management of PPHN.
The optimal PaO2 in the management of PPHN is not clear. Wung and colleagues93 suggested that gentle ventilation with avoidance of hyperoxia and hyperventilation results in good outcomes in neonates with respiratory failure. Decreasing PaO2 to less than 45 to 50 mm Hg results in increased PVR in newborn calves94 and lambs.48 In contrast, maintaining PaO2 at greater than 70 to 80 mm Hg does not result in additional decrease in PVR in both control lambs and lambs with PPHN. Maintaining preductal oxygen saturations in the 90% to 97% range seems to be associated with low PVR in the ductal ligation model of PPHN (Fig. 5). In animal studies, hypoxemia results in pulmonary vasoconstriction; normoxemia reduces PVR but hyperoxemia does not result in additional pulmonary vasodilation. To date, randomized studies comparing different PaO2 targets have not been conducted in infants with PPHN.
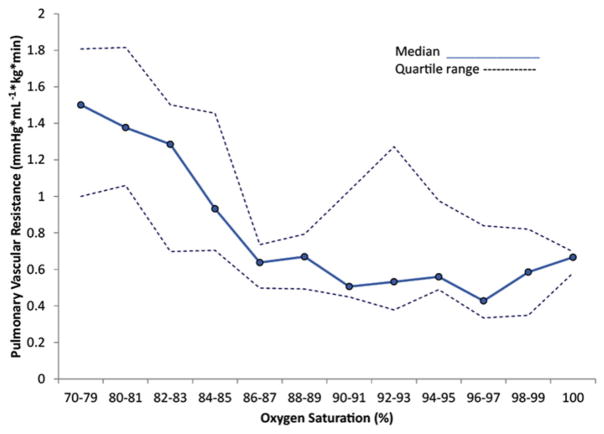
Fig. 5.
The effect of oxygen saturation on PVR in lambs with PPHN induced by antenatal ductal ligation: Median (solid line) and 25th and 75th percentile lines (dashed lines) are shown in the figure. Saturation range of 90% to 97% is associated with low PVR. (From Lakshminrusimha S, Swartz DD, Gugino SF, et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res 2009;66(5):542; with permission.)
Acidosis/Alkalosis
Acidosis (both metabolic and respiratory) constricts the pulmonary vasculature and increases PVR, whereas alkalosis selectively decreases PVR.94 Acidosis (pH<7.30) was associated with an exaggerated constrictor response to hypoxia. In 1978, Peckham and Fox95 published a study of 10 infants with significant PPHN who were treated with hyperventilation and showed significant improvement. Despite the small number of infants in this report, hyperventilation soon became a common therapy in the treatment of this disease and was effectively used as a strategy to improve PaO2.96 In 1985, Wung and colleagues93 challenged this practice. They managed 15 infants with severe PPHN using gentle ventilation maintaining PaO2 between 50 and 70 mm Hg and allowing PaCO2 to increase as high as 60 mm Hg. All infants survived, with only 1 developing chronic lung disease, thus questioning the strategy of hyperventilation. Moreover, studies in asphyxiated lambs showed that respiratory alkalosis reduced cerebral blood flow.97 Alkalosis achieved via ventilator-induced hypocarbia was subsequently shown to be associated with poor neurodevelopmental outcome and hearing loss.98,99 In a retrospective review of PPHN management at National Institute of Child Health and Human Development (NICHD) centers, Walsh-Sukys and colleagues100 reported that continuous alkali infusion was associated with increased use of ECMO and increased use of oxygen at 28 days of age. With the availability of selective pulmonary vasodilators, therapeutic alkalosis is no longer recommended in the management of PPHN. Based on animal data, avoiding acidosis (pH<7.30) may offer some protection against pulmonary vasoconstrictor response to hypoxia.94 However, this effect has not been systematically evaluated in human infants with PPHN.
Surfactant
Administration of intratracheal surfactant is a common practice in the presence of RDS, pneumonia, or MAS. In surfactant-depleted piglet models, instillation of surfactant is associated with a significant reduction in systemic and pulmonary arterial pressures.101,102 However, in human preterm infants, administration of surfactant is associated with selective reduction in pulmonary arterial pressure without any change in systemic pressure.103 Surfactant therapy has been shown to reduce the need for ECMO in term neonates with MAS.104–106 The effect of surfactant is probably a combination of its direct effect on compliance and recruitment and, when used in conjunction with iNO, an indirect effect through enhancing iNO delivery and V/Q matching.
iNO
The introduction of iNO, following its approval by the US Food and Drug Administration (FDA) in 1999 revolutionized the management of PPHN and HRF in the NICU. Large multicenter trials, the Neonatal Inhaled Nitric Oxide Study Group (NINOS) trial,107 the Clinical Inhaled Nitric Oxide Research Group (CINRGI) trial,108 and Roberts and colleagues’109 trial, showed that iNO reduced the need for ECMO. Treatment with iNO results in improved oxygenation and reduction in oxygenation index (OI; mean airway pressure in cm H2O × forced inspiratory oxygen [Fio2] × 100/PaO2 in mm Hg) in 50% to 60% of patients over a wide range of severity of HRF.110
Approximately two-thirds of neonates with parenchymal lung disease, such as MAS and RDS, and HRF respond well to iNO with improved oxygenation. The percentage of responders can be further enhanced with the use of HFOV, emphasizing the importance of lung recruitment during iNO therapy.89 A similar oxygenation response is observed in infants with idiopathic PPHN, but implementation of HFOV does not enhance this response. In contrast, HRF resulting from CDH responds poorly to both iNO and HFOV. Possible causal factors resulting in inadequate or ill-sustained response to iNO are discussed later (Fig. 6):
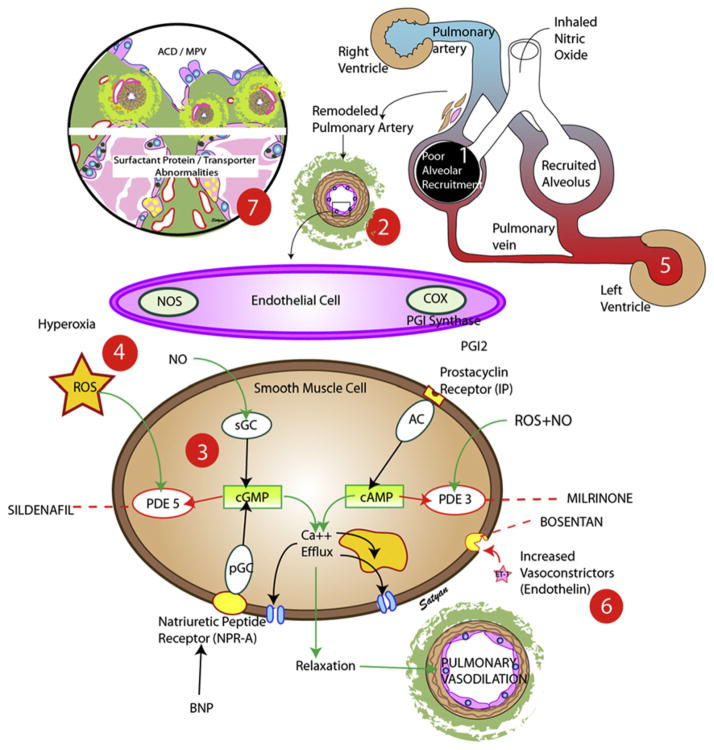
Fig. 6.
Common causes of failure to respond to iNO in neonatal HRF. (1) Failure to recruit alveoli before iNO administration prevents delivery of NO to its target organ, the resistance level pulmonary artery. (2) Remodeled pulmonary artery may have a fixed component of pulmonary vasoconstriction and not respond to vasodilators. (3) Enzyme abnormalities such as decreased sGC activity or increased PDE5 activity can decrease cGMP formation. (4) Increased formation of ROS such as superoxide anions can inactivate NO and stimulate PDE5. (5) Left ventricular failure results in pulmonary venous hypertension, and use of NO in this situation may worsen pulmonary edema and oxygenation. (6) High concentrations of vasoconstrictors, such as ET, may counteract the vasodilation induced by iNO. (7) Rare abnormalities such as alveolar capillary dysplasia with misaligned pulmonary veins (ACD/MPV) and surfactant protein-B deficiency or ATP binding cassette A3 (ABCA3) deficiency. AC, adenylate cyclase; BNP, B-type natriuretic peptide; COX, cyclooxygenase; NO, nitric oxide; NOS, nitric oxide synthase; PDE, phosphodiesterase; pGC, particulate guanylate cyclase; PGI, prostacyclin; ROS, reactive oxygen species; sGC, soluble guanylate cyclase. (Copyright © Satyan Lakshminrusimha.)
Poor alveolar recruitment
Inhaled NO has to reach its target organ, the resistance pulmonary arteriole, to induce pulmonary vasodilation. If there is parenchymal lung disease and/or atelectasis, iNO cannot reach alveoli and pulmonary vasculature. Appropriate alveolar recruitment with increased PEEP, mean airway pressure, and use of surfactant before initiation of iNO is likely to increase pulmonary vasodilation in response to iNO. Once iNO enters the pulmonary vasculature and interacts with hemoglobin in the red blood cells, methemoglobin (MHb) is formed. The increase in MHb following iNO therapy can be considered to reflect that iNO has reached the pulmonary vasculature. We have observed that MHb levels (corrected for NO dose) are significantly higher in neonates with a positive oxygenation response to iNO compared with neonates that do not respond to iNO.83 Better alveolar recruitment with HFV and surfactant is at least partly responsible for lower ECMO/death rates following iNO therapy in recent studies (19.5%)111 compared with the NINOS study (39%).107
Remodeled pulmonary vasculature
Chronic intrauterine pulmonary hypertension, such as is seen in CDH, and antenatal closure of the ductus arteriosus can result in thickening of the smooth muscle layer and adventitia with distal extension of musculature to normally nonmuscular arterioles. Remodeled vasculature tends to be associated with a fixed component of vasoconstriction and does not respond well to exogenous vasodilators. Endothelial dysfunction results in poor response to endothelium-dependent vasodilators, such as oxygen and acetylcholine. These abnormal vasodilator responses secondary to impaired sGC activity are well described in animal models of neonatal pulmonary hypertension55 and diaphragmatic hernia.70
Abnormalities of target enzymes
Nitric oxide stimulates sGC in the pulmonary arterial smooth muscle cell (PASMC) to produce cGMP. sGC is a heme-containing enzyme and can be inactivated by a variety of conditions. Animal models of PPHN have decreased sGC activity reducing cGMP production and relaxation to NO donors.55 More recently, specific activators of sGC have been shown to relax PASMC and may be potentially more effectively than iNO.112,113 An increase in PDE5 activity results in catabolism of cGMP and limitation of NO-induced vasodilation. Ventilation with high concentrations of inspired oxygen and exposure to ROS stimulates PDE5 activity114 and decreases cGMP levels. Inhibition of PDE5 with the use of sildenafil has been an effective strategy in the treatment of PPHN.115,116 Sildenafil, the first PDE5 inhibitor to be approved by the FDA for treatment of pulmonary hypertension in adults, is currently available for both oral and intravenous administration. Therapy with sildenafil has been studied in the acute phase of PPHN and in patients with chronic pulmonary hypertension. Sildenafil is currently not approved for use in neonates but has been used off label in the following circumstances: (1) management of PPHN in the acute phase in situations in which iNO and ECMO are not available,115 as in developing countries. In a recent pharmacokinetic study, intravenous sildenafil was shown to be effective in improving oxygenation as a primary agent (without the use of iNO).116 (2) To augment the effect of iNO in patients with partial or ill-sustained response to iNO. It may be particularly effective in patients following prolonged hyperoxic ventilation because ventilation with high oxygen concentrations and superoxide anions stimulates PDE5 activity.114 (3) To reduce the severity of, or to prevent rebound, pulmonary hypertension observed after weaning iNO.117 (4) Chronic oral therapy in infants with prolonged pulmonary hypertension, as in that associated with BPD118 or CDH. (5) Antenatal use of sildenafil was recently shown to decrease pulmonary hypertension in nitrofen-induced CDH in rat pups; there are no human studies to show the effect of antenatal sildenafil on fetal PVR. The primary concern with the use of intravenous or oral vasodilators, such as sildenafil, is the potential for a decrease in SVR with worsening of right-to-left shunt. The dose of sildenafil should be carefully adjusted to achieve pulmonary vasodilation without significant systemic vasodilation. The optimal dose of sildenafil in term neonates has been evaluated in a recent pharmacokinetic study.119 A slow load of 0.4 mg/kg over 3 hours results in early buildup of therapeutic plasma levels without significant reduction in systemic blood pressure. The dose of continuous infusion is 1.6 mg/kg/d. This intravenous dose (approximately 2 mg/kg/d) corresponds with the recommended oral dose of 4 to 8 mg/kg/d, assuming that oral bioavailability of sildenafil in neonates is similar to that in adults (40%).120–122 Hepatic immaturity or dysfunction and severe renal impairment can prolong the half-life of sildenafil121 and potentially increase in the risk of systemic hypotension.
ROS
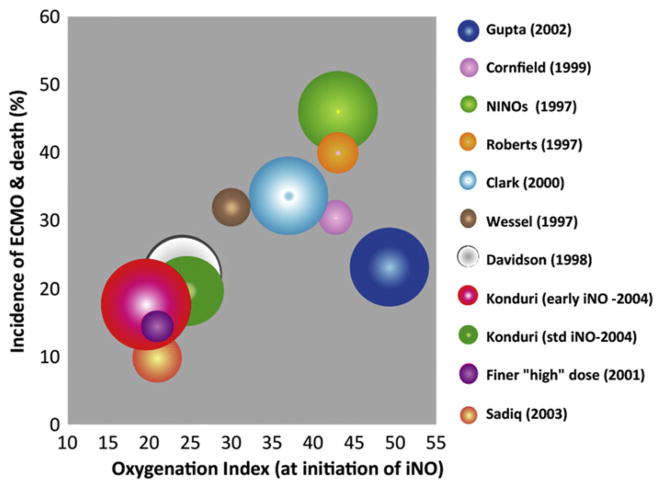
The primary determinant of the biologic half-life of endogenous NO is the local concentration of superoxide anions. The reaction between NO and superoxide anion yields toxic peroxynitrite with a second-order rate constant near the diffusion-controlled limit (K constant = 6.7 ± 0.9 × 109 M−1 s−1). This reaction constitutes an important sink for superoxide anions because it is about twice as fast as the maximum velocity of superoxide dismutase.123 In addition to direct inactivation of NO, ROS can decrease eNOS activity and sGC activity, and increase PDE5 activity, resulting in decreased cGMP levels. Increased ROS can be secondary to (1) ventilation or exposure to high oxygen49; (2) poor antioxidant defense mechanisms such as superoxide dismutase, catalase, and glutathione peroxidase levels124; and (3) increased production of superoxide anions from increased activity of enzymes such as nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase (Nox).66 The effect of prior oxygen exposure (in the form of OI) on response to iNO has been evaluated. Konduri and colleagues125 randomized near-term and term infants with HRF into early initiation of iNO (when OI is ≥15 but <25) or standard initiation (OI ≥25). There was no difference in the incidence of death (early iNO, 6.7% vs standard, 9.4%), ECMO (10.7% vs 12.1%), or death and ECMO combined (16.7% vs 19.5%). However, control infants receiving standard iNO deteriorated to OI greater than 40 more often than the early iNO group (14% vs 7%, P = .056). Based on this study, starting iNO at an OI less than 25 does not reduce the need for ECMO but may prevent progression of HRF and decrease exposure to high levels of oxygen in some neonates with HRF. Data from multiple trials of iNO in HRF are shown in Fig. 7. Based on these results, it seems that OI at initiation of iNO roughly corresponds with the frequency of ECMO/death in that cohort. The case series of gentle ventilation and iNO use from Columbia-Presbyterian hospital126 with lower target PaO2 and permissive hypercapnia was associated with a lower frequency of ECMO/death (28%) despite a high mean OI at initiation of iNO (46.8 ± 24.5). This association suggests that targeting lower PaO2 and limiting FiO2 (and possibly ROS generation) and barotrauma improves outcomes in PPHN.
Fig. 7.
The effect of OI at initiation of iNO on the incidence of ECMO and death in various trials: the size of the bubble is based on the number of infants enrolled in the iNO arm in that trial. The OI at initiation corresponds approximately with the incidence of ECMO or death. A case series from Columbia-Presbyterian Hospital using gentle ventilation is associated with lower incidence of ECMO/death despite high OI at initiation of iNO,126 suggesting that prior exposure to oxygen is a more important factor than precise OI at initiation of iNO.
Left ventricular dysfunction
Patients with HRF and PPHN typically have a right-to-left shunt at the level of ductus arteriosus and foramen ovale. In the presence of left ventricular dysfunction and/or hypoplasia, left atrial pressures are increased, resulting in a left-to-right shunt at the foramen ovale (see Table 1). Increased left atrial pressure results in pulmonary venous hypertension. Administration of iNO to a patient with pulmonary venous hypertension can result in potential flooding of the pulmonary capillary bed and worsening of pulmonary edema, resulting in clinical deterioration.59 Left ventricular hypoplasia associated with CDH127,128 may contribute to pulmonary venous hypertension and could be a potential explanation for impaired response to iNO in these patients.129 It has been suggested that an inodilator such as milrinone may be more effective than iNO in improving left ventricular function and reducing pulmonary venous hypertension.
Two case series report the effectiveness of milrinone in improving oxygenation in iNO-resistant PPHN.130,131 Unlike iNO, which acts through cGMP, milrinone inhibits phosphodiesterase 3A (PDE3A) enzyme in PASMCs and increases the level of a different second messenger, cAMP, resulting in pulmonary vasodilation. Pulmonary vasodilation in response to milrinone is proportional to PDE3A activity in PASMCs. Exposure to NO donors increases PDE3A expression in rat PASMCs.132 Ventilation of newborn lambs with oxygen and iNO increases PDE3A activity in resistance level pulmonary arteries compared with ventilation with oxygen alone.133 Pulmonary arterial rings isolated from lambs ventilated with iNO relax significantly better to milrinone compared with lambs ventilated with oxygen only. These studies suggest that exposure to iNO increases PDE3A activity and that milrinone may be uniquely effective in promoting pulmonary vasodilation and improving oxygenation in iNO-resistant PPHN,130,131 in addition to its cardiac inotropic effect.
Increased vasoconstrictor mediators
ET-1 is produced by the endothelium and exerts its powerful vasoconstrictor effect by acting on ET-A receptors on vascular smooth muscle cells.134 Increased levels of plasma immunoreactive ET-1 levels have been reported in neonates with PPHN, and these levels correlate with the severity of disease.135,136 Bosentan, an ET receptor antagonist, has been used in PPHN.137,138 Mohamed and colleagues137 recently reported a prospective randomized trial of bosentan versus placebo in PPHN. Oral bosentan (1 mg/kg twice a day) resulted in a significant improvement in OI compared with placebo. Close monitoring of liver function is important during bosentan therapy.
Rare causes of PPHN/HRF in term neonates
In some patients with PPHN/HRF resistant to all treatments including ECMO, lung biopsy may be required to confirm the diagnosis.139 Patients with alveolar capillary dysplasia and misalignment of pulmonary veins (ACD/MPV) typically present with HRF and PPHN shortly after birth. Lung histology shows simplification of alveolar architecture, widened and poorly developed septa, with a paucity of capillaries. Small pulmonary arteries are muscularized, accompanied by pulmonary veins within the same connective tissue sheath (see Fig. 6). Patients with surfactant protein-B (SPB) deficiency and ATP binding cassette A3 (ABCA3) transporter deficiencies present with intractable HRF. Infants with prolonged, severe HRF/PPHN out of proportion to their lung disease may require a lung biopsy or targeted genetic evaluation for definitive diagnosis.
Inotropes
PPHN is a syndrome associated with an increased PVR/SVR ratio. Systemic hypotension is a common feature of patients with PPHN and can be multifactorial. Common causes include (1) direct effect of the primary underlying disease such as sepsis or pneumonia, (2) myocardial dysfunction secondary to asphyxia or sepsis,60 (3) septal deviation to the left impinging on left ventricular end-diastolic volume and outflow tract, (4) ventilator therapies such as increased mean airway pressure reducing venous return,82 and (5) decreased pulmonary venous return caused by increased PVR reduces left ventricular preload.
It is a common practice in the NICU to obtain an echocardiogram to estimate systolic pulmonary arterial pressure and to increase systemic systolic pressure with an infusion of inotropes such as dopamine. Dopamine is a nonselective vasoconstrictor and can increase systemic arterial pressure as well as pulmonary arterial pressure in newborn goats140 and preterm human infants with PDA. Initiation of norepinephrine infusion (0.5–1 μg/kg/min) increased mean systemic arterial pressure from 39 ± 4 to 49 ± 4 mm Hg and increased mean pulmonary arterial pressure from 33 ± 4 to 42 ± 5 mm Hg, decreased pulmonary/systemic pressure ratio, and improved oxygenation in late preterm and term infants with PPHN.141 The investigators report echocardiographic findings that suggest increased pulmonary blood flow and speculate that norepinephrine may mediate an α2 receptor–mediated pulmonary vasodilation.
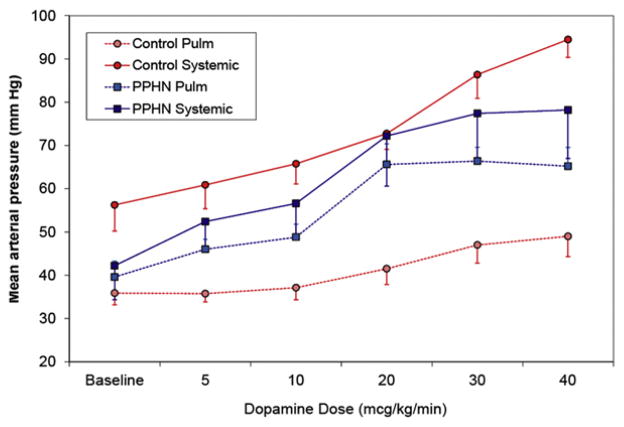
We recently evaluated the effect of dopamine on systemic arterial pressure and pulmonary arterial pressure in newborn lambs with PPHN induced by antenatal ductal ligation64 and their control twins. Control lambs without PPHN have significantly higher systemic blood pressure compared with pulmonary arterial pressure (Fig. 8). Administration of dopamine selectively increases systemic arterial pressure at a lower dose without significantly increasing pulmonary arterial pressure, and increases pulmonary blood flow in control lambs with normal pulmonary vasculature. In PPHN lambs with remodeled pulmonary arteries, pulmonary arterial pressure is at systemic levels and is more sensitive to vasoconstrictor effects of dopamine. Dopamine did not increase pulmonary blood flow in lambs with PPHN. These findings emphasize the need for frequent echocardiograms to evaluate pulmonary arterial pressure in patients with PPHN on high doses of dopamine and norepinephrine.
Fig. 8.
Effect of dopamine infusion on mean systemic arterial and mean pulmonary arterial pressure in normal newborn lambs and lambs with PPHN induced by antenatal ductal ligation. In newborn lambs with normal pulmonary vasculature, systemic blood pressure is significantly higher than pulmonary arterial pressure and increases relatively selectively in response to low doses of dopamine. In PPHN, systemic and pulmonary blood pressures are similar and increase in parallel in response to dopamine.
Partial Liquid Ventilation
Partial liquid ventilation (PLV) with perfluorocarbons has been studied in HRF in animal models142,143 and human infants.144 PLV has been shown to improve gas exchange and improve spatial distribution of pulmonary blood flow in models of lung injury.145 However, PLV does not prevent hypoxic pulmonary vasoconstriction in the absence of parenchymal lung injury.146 A combination of iNO and PLV improved oxygenation in a lamb model of CDH147 but did not decrease PVR in a piglet model of MAS142 with conventional ventilation. The use of high-frequency PLV results in a significant decrease in PVR and an improvement in pulmonary blood flow in a preterm lamb model of RDS.148 It is likely that PLV improves alveolar recruitment, compliance, and gas exchange, and its effect on pulmonary hemodynamics is secondary to these changes.
ECMO
ECMO refers to a life support technique designed to enhance gas exchange and provide pulmonary and/or cardiac support in severe HRF. ECMO requires diversion of blood from a major systemic vessel through a gas exchange device (membrane oxygenator) and back to a major vessel. The venoarterial approach (VA) has served as the primary mode of cannulation for both cardiac and respiratory failure in neonates and uses a central vein (usually jugular) for drainage and an artery (usually carotid) for return. As blood is diverted from the pulmonary circuit, immediate decompression of the right ventricle occurs in VA-ECMO. Venovenous (VV) cannulation is appropriate for patients with severe respiratory failure who do not require cardiac support and uses a major vein for blood drainage and a vein for return of oxygenated blood to the right heart.149,150 Pulmonary and right ventricular hemodynamics are not altered, although the blood entering the pulmonary artery has substantially higher PO2. The impact of such increased oxygen tension in the pulmonary circulation on PVR is not known. The presence of pulsatile flow in VV ECMO is associated with better cerebral hemodynamics but this could be a reflection of patient selection bias.151 Overall, no major differences have been reported in respiratory outcome between VA and VV ECMO.152,153
SUMMARY
Increased understanding of the pathophysiologic changes in the pulmonary circulation in neonatal HRF and PPHN in the last 2 decades has led to a substantial decrease in the number of neonatal respiratory patients requiring ECMO. Further clinical research into pulmonary vasodilator therapy has become more challenging because of a decreased number of patients and widespread availability of iNO, resulting in difficult study recruitment. Two unmet challenges remain in pulmonary circulatory disorders: CDH and premature infants with BPD and pulmonary hypertension.154 Multicenter trials to evaluate and develop appropriate strategies to ameliorate pulmonary vascular disease in these conditions are warranted.
KEY POINTS.
Pulmonary vascular resistance increases during late gestation and decreases at birth.
Pulmonary vascular transition at birth can be influenced by mode of delivery, asphyxia, body temperature, and oxygen concentration of the resuscitation gas.
Neonatal hypoxemic respiratory failure (HRF) is often secondary to parenchymal lung disease, ventilation-perfusion mismatch, or extrapulmonary right-to-left shunt.
Hypoxia causes pulmonary vasoconstriction, normoxia results in pulmonary vasodilation, but hyperoxia does not lead to additional vasodilation.
Inhaled nitric oxide (iNO) is a specific pulmonary vasodilator and is effective in 60% to 70% of late preterm and term neonates with HRF.
Inadequate or ill-sustained response to iNO may be secondary to poor alveolar recruitment, remodeled pulmonary vasculature, abnormalities of target enzymes, presence of reactive oxygen species, left ventricular dysfunction, or increased vasoconstrictive mediators.
Pulmonary hypertension associated with bronchopulmonary dysplasia and congenital diaphragmatic hernia is associated with high morbidity and mortality and its management is challenging.
Acknowledgments
I thank Drs Bobby Mathew, Veena Manja, and Corinne Leach for their critical review of this article.
Footnotes
Conflict of interest: Dr Lakshminrusimha is a member of the speaker’s bureau and a consultant for Ikaria LLC.
References
- 1.Lakshminrusimha S, Steinhorn RH. Pulmonary vascular biology during neonatal transition. Clin Perinatol. 1999;26(3):601–19. [PubMed] [Google Scholar]
- 2.Dawes GS. Pulmonary circulation in the foetus and new-born. Br Med Bull. 1966;22(1):61–5. doi: 10.1093/oxfordjournals.bmb.a070439. [DOI] [PubMed] [Google Scholar]
- 3.Ardran G, Dawes GS, Prichard MM, et al. The effect of ventilation of the foetal lungs upon the pulmonary circulation. J Physiol. 1952;118(1):12–22. doi: 10.1113/jphysiol.1952.sp004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasanen J, Wood DC, Weiner S, et al. Role of the pulmonary circulation in the distribution of human fetal cardiac output during the second half of pregnancy. Circulation. 1996;94(5):1068–73. doi: 10.1161/01.cir.94.5.1068. [DOI] [PubMed] [Google Scholar]
- 5.Rasanen J, Wood DC, Debbs RH, et al. Reactivity of the human fetal pulmonary circulation to maternal hyperoxygenation increases during the second half of pregnancy: a randomized study. Circulation. 1998;97(3):257–62. doi: 10.1161/01.cir.97.3.257. [DOI] [PubMed] [Google Scholar]
- 6.Heymann MA, Lewis AB, Rudolph AM. Pulmonary vascular responses during advancing gestation in fetal lambs in utero. Chest. 1977;71(Suppl 2):270–1. doi: 10.1378/chest.71.2_supplement.270. [DOI] [PubMed] [Google Scholar]
- 7.Lewis AB, Heymann MA, Rudolph AM. Gestational changes in pulmonary vascular responses in fetal lambs in utero. Circ Res. 1976;39(4):536–41. doi: 10.1161/01.res.39.4.536. [DOI] [PubMed] [Google Scholar]
- 8.Accurso FJ, Alpert B, Wilkening RB, et al. Time-dependent response of fetal pulmonary blood flow to an increase in fetal oxygen tension. Respir Physiol. 1986;63(1):43–52. doi: 10.1016/0034-5687(86)90029-0. [DOI] [PubMed] [Google Scholar]
- 9.Mensah E, Morin FC, 3rd, Russell JA, et al. Soluble guanylate cyclase mRNA expression change during ovine lung development. Pediatr Res. 1998;43:290. [Google Scholar]
- 10.Bloch KD, Filippov G, Sanchez LS, et al. Pulmonary soluble guanylate cyclase, a nitric oxide receptor, is increased during the perinatal period. Am J Physiol. 1997;272(3 Pt 1):L400–6. doi: 10.1152/ajplung.1997.272.3.L400. [DOI] [PubMed] [Google Scholar]
- 11.Kumar VH, Hutchison AA, Lakshminrusimha S, et al. Characteristics of pulmonary hypertension in preterm neonates. J Perinatol. 2007;27(4):214–9. doi: 10.1038/sj.jp.7211673. [DOI] [PubMed] [Google Scholar]
- 12.Luong C, Rey-Perra J, Vadivel A, et al. Antenatal sildenafil treatment attenuates pulmonary hypertension in experimental congenital diaphragmatic hernia. Circulation. 2011;123(19):2120–31. doi: 10.1161/CIRCULATIONAHA.108.845909. [DOI] [PubMed] [Google Scholar]
- 13.Chandrasekar I, Eis A, Konduri GG. Betamethasone attenuates oxidant stress in endothelial cells from fetal lambs with persistent pulmonary hypertension. Pediatr Res. 2008;63(1):67–72. doi: 10.1203/PDR.0b013e31815b43ee. [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Martinez R, Moreno-Alvarez O, Prat J, et al. Lung tissue blood perfusion changes induced by in utero tracheal occlusion in a rabbit model of congenital diaphragmatic hernia. Fetal Diagn Ther. 2009;26(3):137–42. doi: 10.1159/000254485. [DOI] [PubMed] [Google Scholar]
- 15.Jelin E, Lee H. Tracheal occlusion for fetal congenital diaphragmatic hernia: the US experience. Clin Perinatol. 2009;36(2):349–61. ix. doi: 10.1016/j.clp.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Bratu I, Flageole H, Laberge JM, et al. Pulmonary structural maturation and pulmonary artery remodeling after reversible fetal ovine tracheal occlusion in diaphragmatic hernia. J Pediatr Surg. 2001;36(5):739–44. doi: 10.1053/jpsu.2001.22950. [DOI] [PubMed] [Google Scholar]
- 17.Luks FI, Wild YK, Piasecki GJ, et al. Short-term tracheal occlusion corrects pulmonary vascular anomalies in the fetal lamb with diaphragmatic hernia. Surgery. 2000;128(2):266–72. doi: 10.1067/msy.2000.107373. [DOI] [PubMed] [Google Scholar]
- 18.Chambers CD, Hernandez-Diaz S, Van Marter LJ, et al. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. N Engl J Med. 2006;354(6):579–87. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- 19.Belik J. Fetal and neonatal effects of maternal drug treatment for depression. Semin Perinatol. 2008;32(5):350–4. doi: 10.1053/j.semperi.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Fornaro E, Li D, Pan J, et al. Prenatal exposure to fluoxetine induces fetal pulmonary hypertension in the rat. Am J Respir Crit Care Med. 2007;176(10):1035–40. doi: 10.1164/rccm.200701-163OC. [DOI] [PubMed] [Google Scholar]
- 21.Wilson KL, Zelig CM, Harvey JP, et al. Persistent pulmonary hypertension of the newborn is associated with mode of delivery and not with maternal use of selective serotonin reuptake inhibitors. Am J Perinatol. 2011;28(1):19–24. doi: 10.1055/s-0030-1262507. [DOI] [PubMed] [Google Scholar]
- 22.Talati AJ, Salim MA, Korones SB. Persistent pulmonary hypertension after maternal naproxen ingestion in a term newborn: a case report. Am J Perinatol. 2000;17(2):69–71. doi: 10.1055/s-2000-9271. [DOI] [PubMed] [Google Scholar]
- 23.Wild LM, Nickerson PA, Morin FC., 3rd Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res. 1989;25(3):251–7. doi: 10.1203/00006450-198903000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Alano MA, Ngougmna E, Ostrea EM, Jr, et al. Analysis of nonsteroidal antiinflammatory drugs in meconium and its relation to persistent pulmonary hypertension of the newborn. Pediatrics. 2001;107(3):519–23. doi: 10.1542/peds.107.3.519. [DOI] [PubMed] [Google Scholar]
- 25.Teitel DF, Iwamoto HS, Rudolph AM. Changes in the pulmonary circulation during birth-related events. Pediatr Res. 1990;27(4 Pt 1):372–8. doi: 10.1203/00006450-199004000-00010. [DOI] [PubMed] [Google Scholar]
- 26.Abman SH, Chatfield BA, Hall SL, et al. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990;259(6 Pt 2):H1921–7. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 27.Ramachandrappa A, Jain L. Elective cesarean section: its impact on neonatal respiratory outcome. Clin Perinatol. 2008;35(2):373–93. vii. doi: 10.1016/j.clp.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sulyok E, Csaba IF. Elective repeat cesarean delivery and persistent pulmonary hypertension of the newborn. Am J Obstet Gynecol. 1986;155(3):687–8. doi: 10.1016/0002-9378(86)90314-5. [DOI] [PubMed] [Google Scholar]
- 29.Hernandez-Diaz S, Van Marter LJ, Werler MM, et al. Risk factors for persistent pulmonary hypertension of the newborn. Pediatrics. 2007;120(2):e272–82. doi: 10.1542/peds.2006-3037. [DOI] [PubMed] [Google Scholar]
- 30.Ramachandrappa A, Jain L. Health issues of the late preterm infant. Pediatr Clin North Am. 2009;56(3):565–77. doi: 10.1016/j.pcl.2009.03.009. Table of Contents. [DOI] [PubMed] [Google Scholar]
- 31.Ramachandrappa A, Rosenberg ES, Wagoner S, et al. Morbidity and mortality in late preterm infants with severe hypoxic respiratory failure on extra-corporeal membrane oxygenation. J Pediatr. 2011;159(2):192–8.e3. doi: 10.1016/j.jpeds.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens TP, van Wijngaarden E, Ackerman KG, et al. Timing of delivery and survival rates for infants with prenatal diagnoses of congenital diaphragmatic hernia. Pediatrics. 2009;123(2):494–502. doi: 10.1542/peds.2008-0528. [DOI] [PubMed] [Google Scholar]
- 33.Hutcheon JA, Butler B, Lisonkova S, et al. Timing of delivery for pregnancies with congenital diaphragmatic hernia. BJOG. 2010;117(13):1658–62. doi: 10.1111/j.1471-0528.2010.02738.x. [DOI] [PubMed] [Google Scholar]
- 34.Sotiriadis A, Makrydimas G, Papatheodorou S, et al. Corticosteroids for preventing neonatal respiratory morbidity after elective caesarean section at term. Co-chrane Database Syst Rev. 2009;(4):CD006614. doi: 10.1002/14651858.CD006614.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Stutchfield P, Whitaker R, Russell I. Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: pragmatic randomised trial. BMJ. 2005;331(7518):662. doi: 10.1136/bmj.38547.416493.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guilherme R, Rotten D. Betamethasone before elective caesarean section at term: a survey of practice in France. Eur J Obstet Gynecol Reprod Biol. 2010;150(1):104. doi: 10.1016/j.ejogrb.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 37.Byers HM, Dagle JM, Klein JM, et al. Variations in CRHR1 are associated with persistent pulmonary hypertension of the newborn. Pediatr Res. 2012;71(2):162–7. doi: 10.1038/pr.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez M, Lakshminrusimha S, Wedgwood S, et al. Hydrocortisone normalizes oxygenation and cGMP regulation in lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2012;302(6):L595–603. doi: 10.1152/ajplung.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perlman JM, Wyllie J, Kattwinkel J, et al. Part 11: Neonatal resuscitation: 2010 International Consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2010;122(16 Suppl 2):S516–38. doi: 10.1161/CIRCULATIONAHA.110.971127. [DOI] [PubMed] [Google Scholar]
- 40.Arcilla RA, Oh W, Lind J, et al. Pulmonary arterial pressures of newborn infants born with early and late clamping of the cord. Acta Paediatr Scand. 1966;55(3):305–15. doi: 10.1111/j.1651-2227.1966.tb17659.x. [DOI] [PubMed] [Google Scholar]
- 41.Toubas PL, Hof RP, Heymann MA, et al. Effects of hypothermia and rewarming on the neonatal circulation. Arch Fr Pediatr. 1978;35(Suppl 10):84–92. [PubMed] [Google Scholar]
- 42.Lapointe A, Barrington KJ. Pulmonary hypertension and the asphyxiated newborn. J Pediatr. 2011;158(Suppl 2):e19–24. doi: 10.1016/j.jpeds.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 43.Thoresen M. Hypothermia after perinatal asphyxia: selection for treatment and cooling protocol. J Pediatr. 2011;158(Suppl 2):e45–9. doi: 10.1016/j.jpeds.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 44.Sarkar S, Barks JD, Bhagat I, et al. Pulmonary dysfunction and therapeutic hypothermia in asphyxiated newborns: whole body versus selective head cooling. Am J Perinatol. 2009;26(4):265–70. doi: 10.1055/s-0028-1103154. [DOI] [PubMed] [Google Scholar]
- 45.Cornish JD, Dreyer GL, Snyder GE, et al. Failure of acute perinatal asphyxia or meconium aspiration to produce persistent pulmonary hypertension in a neonatal baboon model. Am J Obstet Gynecol. 1994;171(1):43–9. doi: 10.1016/s0002-9378(94)70075-3. [DOI] [PubMed] [Google Scholar]
- 46.Murphy JD, Vawter GF, Reid LM. Pulmonary vascular disease in fatal meconium aspiration. J Pediatr. 1984;104(5):758–62. doi: 10.1016/s0022-3476(84)80962-2. [DOI] [PubMed] [Google Scholar]
- 47.Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res. 2007;62(3):313–8. doi: 10.1203/PDR.0b013e3180db29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lakshminrusimha S, Swartz DD, Gugino SF, et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res. 2009;66(5):539–44. doi: 10.1203/PDR.0b013e3181bab0c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lakshminrusimha S, Steinhorn RH, Wedgwood S, et al. Pulmonary hemodynamics and vascular reactivity in asphyxiated term lambs resuscitated with 21% and 100% oxygen. J Appl Physiol. 2011;111(5):1441–7. doi: 10.1152/japplphysiol.00711.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobyns EL, Wescott JY, Kennaugh JM, et al. Eicosanoids decrease with successful extracorporeal membrane oxygenation therapy in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 1994;149(4 Pt 1):873–80. doi: 10.1164/ajrccm.149.4.8143049. [DOI] [PubMed] [Google Scholar]
- 51.Langleben D, DeMarchie M, Laporta D, et al. Endothelin-1 in acute lung injury and the adult respiratory distress syndrome. Am Rev Respir Dis. 1993;148(6 Pt 1):1646–50. doi: 10.1164/ajrccm/148.6_Pt_1.1646. [DOI] [PubMed] [Google Scholar]
- 52.Ivy DD, Parker TA, Ziegler JW, et al. Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. J Clin Invest. 1997;99(6):1179–86. doi: 10.1172/JCI119274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shaul PW, Yuhanna IS, German Z, et al. Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol. 1997;272(5 Pt 1):L1005–12. doi: 10.1152/ajplung.1997.272.5.L1005. [DOI] [PubMed] [Google Scholar]
- 54.McQueston JA, Kinsella JP, Ivy DD, et al. Chronic pulmonary hypertension in utero impairs endothelium-dependent vasodilation. Am J Physiol. 1995;268(1 Pt 2):H288–94. doi: 10.1152/ajpheart.1995.268.1.H288. [DOI] [PubMed] [Google Scholar]
- 55.Steinhorn RH, Russell JA, Morin FC., 3rd Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol. 1995;268(4 Pt 2):H1483–9. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- 56.Geggel RL, Reid LM. The structural basis of PPHN. Clin Perinatol. 1984;11(3):525–49. [PubMed] [Google Scholar]
- 57.El-Khuffash AF, McNamara PJ. Neonatologist-performed functional echocardiography in the neonatal intensive care unit. Semin Fetal Neonatal Med. 2011;16(1):50–60. doi: 10.1016/j.siny.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 58.Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70(4):657–62. doi: 10.1161/01.cir.70.4.657. [DOI] [PubMed] [Google Scholar]
- 59.Kinsella JP. Inhaled nitric oxide in the term newborn. Early Hum Dev. 2008;84(11):709–16. doi: 10.1016/j.earlhumdev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Sehgal A, Athikarisamy SE, Adamopoulos M. Global myocardial function is compromised in infants with pulmonary hypertension. Acta Paediatr. 2012;101(4):410–3. doi: 10.1111/j.1651-2227.2011.02572.x. [DOI] [PubMed] [Google Scholar]
- 61.Murphy JD, Rabinovitch M, Goldstein JD, et al. The structural basis of persistent pulmonary hypertension of the newborn infant. J Pediatr. 1981;98(6):962–7. doi: 10.1016/s0022-3476(81)80605-1. [DOI] [PubMed] [Google Scholar]
- 62.Allen K, Haworth SG. Human postnatal pulmonary arterial remodeling. Ultrastructural studies of smooth muscle cell and connective tissue maturation. Lab Invest. 1988;59(5):702–9. [PubMed] [Google Scholar]
- 63.Manchester D, Margolis HS, Sheldon RE. Possible association between maternal indomethacin therapy and primary pulmonary hypertension of the newborn. Am J Obstet Gynecol. 1976;126(4):467–9. doi: 10.1016/0002-9378(76)90640-2. [DOI] [PubMed] [Google Scholar]
- 64.Morin FC., 3rd Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res. 1989;25(3):245–50. doi: 10.1203/00006450-198903000-00005. [DOI] [PubMed] [Google Scholar]
- 65.Hanson KA, Ziegler JW, Rybalkin SD, et al. Chronic pulmonary hypertension increases fetal lung cGMP phosphodiesterase activity. Am J Physiol. 1998;275(5 Pt 1):L931–41. doi: 10.1152/ajplung.1998.275.5.L931. [DOI] [PubMed] [Google Scholar]
- 66.Brennan LA, Steinhorn RH, Wedgwood S, et al. Increased superoxide generation is associated with pulmonary hypertension in fetal lambs: a role for NADPH oxidase. Circ Res. 2003;92(6):683–91. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 67.Wedgwood S, Steinhorn RH, Bunderson M, et al. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L660–6. doi: 10.1152/ajplung.00369.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lakshminrusimha S, Porta NF, Farrow KN, et al. Milrinone enhances relaxation to prostacyclin and iloprost in pulmonary arteries isolated from lambs with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. 2009;10(1):106–12. doi: 10.1097/PCC.0b013e3181936aee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karamanoukian HL, Peay T, Love JE, et al. Decreased pulmonary nitric oxide synthase activity in the rat model of congenital diaphragmatic hernia. J Pediatr Surg. 1996;31(8):1016–9. doi: 10.1016/s0022-3468(96)90076-7. [DOI] [PubMed] [Google Scholar]
- 70.de Buys Roessingh A, Fouquet V, Aigrain Y, et al. Nitric oxide activity through guanylate cyclase and phosphodiesterase modulation is impaired in fetal lambs with congenital diaphragmatic hernia. J Pediatr Surg. 2011;46(8):1516–22. doi: 10.1016/j.jpedsurg.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 71.Villanueva ME, Zaher FM, Svinarich DM, et al. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res. 1998;44(3):338–43. doi: 10.1203/00006450-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Aaltonen M, Soukka H, Halkola L, et al. Asphyxia aggravates systemic hypotension but not pulmonary hypertension in piglets with meconium aspiration. Pediatr Res. 2003;53(3):473–8. doi: 10.1203/01.PDR.0000049514.02607.03. [DOI] [PubMed] [Google Scholar]
- 73.Keszler M, Carbone MT, Cox C, et al. Severe respiratory failure after elective repeat cesarean delivery: a potentially preventable condition leading to extra-corporeal membrane oxygenation. Pediatrics. 1992;89(4 Pt 1):670–2. [PubMed] [Google Scholar]
- 74.Bhat R, Salas AA, Foster C, et al. Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics. 2012;129(3):e682–9. doi: 10.1542/peds.2011-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gorenflo M, Vogel M, Obladen M. Pulmonary vascular changes in bronchopulmonary dysplasia: a clinicopathologic correlation in short- and long-term survivors. Pediatr Pathol. 1991;11(6):851–66. doi: 10.3109/15513819109065482. [DOI] [PubMed] [Google Scholar]
- 76.Kim DH, Kim HS, Choi CW, et al. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012;101(1):40–6. doi: 10.1159/000327891. [DOI] [PubMed] [Google Scholar]
- 77.Nyp M, Sandritter T, Poppinga N, et al. Sildenafil citrate, bronchopulmonary dysplasia and disordered pulmonary gas exchange: any benefits? J Perinatol. 2012;32(1):64–9. doi: 10.1038/jp.2011.131. [DOI] [PubMed] [Google Scholar]
- 78.Banks BA, Seri I, Ischiropoulos H, et al. Changes in oxygenation with inhaled nitric oxide in severe bronchopulmonary dysplasia. Pediatrics. 1999;103(3):610–8. doi: 10.1542/peds.103.3.610. [DOI] [PubMed] [Google Scholar]
- 79.Mourani PM, Ivy DD, Gao D, et al. Pulmonary vascular effects of inhaled nitric oxide and oxygen tension in bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2004;170(9):1006–13. doi: 10.1164/rccm.200310-1483OC. [DOI] [PubMed] [Google Scholar]
- 80.Smith J, Schumacher RE, Donn SM, et al. Clinical course of symptomatic spontaneous pneumothorax in term and late preterm newborns: report from a large cohort. Am J Perinatol. 2011;28(2):163–8. doi: 10.1055/s-0030-1263300. [DOI] [PubMed] [Google Scholar]
- 81.Porta NF, Steinhorn RH. Pulmonary vasodilator therapy in the NICU: inhaled nitric oxide, sildenafil, and other pulmonary vasodilating agents. Clin Perinatol. 2012;39(1):149–64. doi: 10.1016/j.clp.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Linde LM, Simmons DH, Ellman EL. Pulmonary hemodynamics during positive-pressure breathing. J Appl Physiol. 1961;16:644–6. doi: 10.1152/jappl.1961.16.4.644. [DOI] [PubMed] [Google Scholar]
- 83.Pabalan MJ, Nayak SP, Ryan RM, et al. Methemoglobin to cumulative nitric oxide ratio and response to inhaled nitric oxide in PPHN. J Perinatol. 2009;29(10):698–701. doi: 10.1038/jp.2009.69. [DOI] [PubMed] [Google Scholar]
- 84.Carlo WA, Beoglos A, Chatburn RL, et al. High-frequency jet ventilation in neonatal pulmonary hypertension. Am J Dis Child. 1989;143(2):233–8. doi: 10.1001/archpedi.1989.02150140127034. [DOI] [PubMed] [Google Scholar]
- 85.Clark RH, Yoder BA, Sell MS. Prospective, randomized comparison of high-frequency oscillation and conventional ventilation in candidates for extracorporeal membrane oxygenation. Journal of Pediatrics. 1994;124(3):447–54. doi: 10.1016/s0022-3476(94)70374-4. [DOI] [PubMed] [Google Scholar]
- 86.Engle WA, Yoder MC, Andreoli SP, et al. Controlled prospective randomized comparison of high-frequency jet ventilation and conventional ventilation in neonates with respiratory failure and persistent pulmonary hypertension. J Perinatol. 1997;17(1):3–9. [PubMed] [Google Scholar]
- 87.Kinsella JP, Abman SH. Clinical approaches to the use of high-frequency oscillatory ventilation in neonatal respiratory failure. J Perinatol. 1996;16(2 Pt 2 Su):S52–5. [PubMed] [Google Scholar]
- 88.Kinsella JP, Abman SH. High-frequency oscillatory ventilation augments the response to inhaled nitric oxide in persistent pulmonary hypertension of the newborn: Nitric Oxide Study Group. Chest. 1998;114(Suppl 1):100S. doi: 10.1378/chest.114.1_supplement.100s. [DOI] [PubMed] [Google Scholar]
- 89.Kinsella JP, Truog WE, Walsh WF, et al. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. J Pediatr. 1997;131(1 Pt 1):55–62. doi: 10.1016/s0022-3476(97)70124-0. [DOI] [PubMed] [Google Scholar]
- 90.Lakshminrusimha S, Russell JA, Steinhorn RH, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59(1):137–41. doi: 10.1203/01.pdr.0000191136.69142.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lakshminrusimha S, Russell JA, Wedgwood S, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(12):1370–7. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinhorn RH, Albert G, Swartz DD, et al. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med. 2001;164(5):834–9. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 93.Wung JT, James LS, Kilchevsky E, et al. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985;76(4):488–94. [PubMed] [Google Scholar]
- 94.Rudolph AM, Yuan S. Response of the pulmonary vasculature to hypoxia and H+ ion concentration changes. J Clin Invest. 1966;45(3):399–411. doi: 10.1172/JCI105355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peckham GJ, Fox WW. Physiologic factors affecting pulmonary artery pressure in infants with persistent pulmonary hypertension. J Pediatr. 1978;93(6):1005–10. doi: 10.1016/s0022-3476(78)81239-6. [DOI] [PubMed] [Google Scholar]
- 96.Fox WW, Duara S. Persistent pulmonary hypertension in the neonate: diagnosis and management. J Pediatr. 1983;103(4):505–14. doi: 10.1016/s0022-3476(83)80573-3. [DOI] [PubMed] [Google Scholar]
- 97.Rosenberg AA. Response of the cerebral circulation to hypocarbia in postasphyxia newborn lambs. Pediatr Res. 1992;32(5):537–41. doi: 10.1203/00006450-199211000-00008. [DOI] [PubMed] [Google Scholar]
- 98.Bifano EM, Pfannenstiel A. Duration of hyperventilation and outcome in infants with persistent pulmonary hypertension. Pediatrics. 1988;81(5):657–61. [PubMed] [Google Scholar]
- 99.Hendricks-Munoz KD, Walton JP. Hearing loss in infants with persistent fetal circulation. Pediatrics. 1988;81(5):650–6. [PubMed] [Google Scholar]
- 100.Walsh-Sukys MC, Tyson JE, Wright LL, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105(1 Pt 1):14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 101.Yu XQ, Feet BA, Moen A, et al. Nitric oxide contributes to surfactant-induced vasodilation in surfactant-depleted newborn piglets. Pediatr Res. 1997;42(2):151–6. doi: 10.1203/00006450-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 102.Moen A, Yu XQ, Rootwelt T, et al. Acute effects on systemic and pulmonary hemodynamics of intratracheal instillation of porcine surfactant or saline in surfactant-depleted newborn piglets. Pediatr Res. 1997;41(4 Pt 1):486–92. doi: 10.1203/00006450-199704000-00006. [DOI] [PubMed] [Google Scholar]
- 103.Kaapa P, Seppanen M, Kero P, et al. Pulmonary hemodynamics after synthetic surfactant replacement in neonatal respiratory distress syndrome. J Pediatr. 1993;123(1):115–9. doi: 10.1016/s0022-3476(05)81553-7. [DOI] [PubMed] [Google Scholar]
- 104.Soll RF, Dargaville P. Surfactant for meconium aspiration syndrome in full term infants. Cochrane Database Syst Rev. 2000;(2):CD002054. doi: 10.1002/14651858.CD002054. [DOI] [PubMed] [Google Scholar]
- 105.Lotze A, Mitchell BR, Bulas DI, et al. Multicenter study of surfactant (beractant) use in the treatment of term infants with severe respiratory failure. Survanta in Term Infants Study Group. J Pediatr. 1998;132(1):40–7. doi: 10.1016/s0022-3476(98)70482-2. [DOI] [PubMed] [Google Scholar]
- 106.Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics. 1996;97(1):48–52. [PubMed] [Google Scholar]
- 107.Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. The Neonatal Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336(9):597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 108.Clark RH, Kueser TJ, Walker MW, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342(7):469–74. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 109.Roberts JD, Jr, Fineman JR, Morin FC, 3rd, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336(9):605–10. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 110.Golombek SG, Young JN. Efficacy of inhaled nitric oxide for hypoxic respiratory failure in term and late preterm infants by baseline severity of illness: a pooled analysis of three clinical trials. Clin Ther. 2010;32(5):939–48. doi: 10.1016/j.clinthera.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 111.Konduri GG. New approaches for persistent pulmonary hypertension of newborn. Clin Perinatol. 2004;31(3):591–611. doi: 10.1016/j.clp.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 112.Deruelle P, Balasubramaniam V, Kunig AM, et al. BAY 41-2272, a direct activator of soluble guanylate cyclase, reduces right ventricular hypertrophy and prevents pulmonary vascular remodeling during chronic hypoxia in neonatal rats. Biol Neonate. 2006;90(2):135–44. doi: 10.1159/000092518. [DOI] [PubMed] [Google Scholar]
- 113.Deruelle P, Grover TR, Storme L, et al. Effects of BAY 41-2272, a soluble guanylate cyclase activator, on pulmonary vascular reactivity in the ovine fetus. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L727–33. doi: 10.1152/ajplung.00409.2004. [DOI] [PubMed] [Google Scholar]
- 114.Farrow KN, Groh BS, Schumacker PT, et al. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res. 2008;102(2):226–33. doi: 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baquero H, Soliz A, Neira F, et al. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117(4):1077–83. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 116.Steinhorn RH, Kinsella JP, Pierce C, et al. Intravenous sildenafil in the treatment of neonates with persistent pulmonary hypertension. J Pediatr. 2009;155(6):841–847.e1. doi: 10.1016/j.jpeds.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 117.Atz AM, Wessel DL. Sildenafil ameliorates effects of inhaled nitric oxide withdrawal. Anesthesiology. 1999;91(1):307–10. doi: 10.1097/00000542-199907000-00041. [DOI] [PubMed] [Google Scholar]
- 118.Farrow KN, Steinhorn RH. Sildenafil therapy for bronchopulmonary dysplasia: not quite yet. J Perinatol. 2012;32(1):1–3. doi: 10.1038/jp.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mukherjee A, Dombi T, Wittke B, et al. Population pharmacokinetics of sildenafil in term neonates: evidence of rapid maturation of metabolic clearance in the early postnatal period. Clin Pharmacol Ther. 2009;85(1):56–63. doi: 10.1038/clpt.2008.177. [DOI] [PubMed] [Google Scholar]
- 120.Muirhead GJ, Rance DJ, Walker DK, et al. Comparative human pharmacokinetics and metabolism of single-dose oral and intravenous sildenafil. Br J Clin Pharmacol. 2002;53(Suppl 1):13S–20S. doi: 10.1046/j.0306-5251.2001.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muirhead GJ, Wilner K, Colburn W, et al. The effects of age and renal and hepatic impairment on the pharmacokinetics of sildenafil. Br J Clin Pharmacol. 2002;53(Suppl 1):21S–30S. doi: 10.1046/j.0306-5251.2001.00029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nichols DJ, Muirhead GJ, Harness JA. Pharmacokinetics of sildenafil after single oral doses in healthy male subjects: absolute bioavailability, food effects and dose proportionality. Br J Clin Pharmacol. 2002;53(Suppl 1):5S–12S. doi: 10.1046/j.0306-5251.2001.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huie RE, Padmaja S. The reaction of NO with superoxide. Free Radic Res Commun. 1993;18(4):195–9. doi: 10.3109/10715769309145868. [DOI] [PubMed] [Google Scholar]
- 124.Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009;66(2):121–7. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- 125.Konduri GG, Kim UO. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am. 2009;56(3):579–600. doi: 10.1016/j.pcl.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gupta A, Rastogi S, Sahni R, et al. Inhaled nitric oxide and gentle ventilation in the treatment of pulmonary hypertension of the newborn–a single-center, 5-year experience. J Perinatol. 2002;22(6):435–41. doi: 10.1038/sj.jp.7210761. [DOI] [PubMed] [Google Scholar]
- 127.Schwartz SM, Vermilion RP, Hirschl RB. Evaluation of left ventricular mass in children with left-sided congenital diaphragmatic hernia. J Pediatr. 1994;125(3):447–51. doi: 10.1016/s0022-3476(05)83293-7. [DOI] [PubMed] [Google Scholar]
- 128.Siebert JR, Haas JE, Beckwith JB. Left ventricular hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. 1984;19(5):567–71. doi: 10.1016/s0022-3468(84)80105-0. [DOI] [PubMed] [Google Scholar]
- 129.Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. The Neonatal Inhaled Nitric Oxide Study Group (NINOS) Pediatrics. 1997;99(6):838–45. doi: 10.1542/peds.99.6.838. [DOI] [PubMed] [Google Scholar]
- 130.Bassler D, Choong K, McNamara P, et al. Neonatal persistent pulmonary hypertension treated with milrinone: four case reports. Biol Neonate. 2006;89(1):1–5. doi: 10.1159/000088192. [DOI] [PubMed] [Google Scholar]
- 131.McNamara PJ, Laique F, Muang- S, et al. Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J Crit Care. 2006;21(2):217–22. doi: 10.1016/j.jcrc.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 132.Busch CJ, Graveline AR, Jiramongkolchai K, et al. Phosphodiesterase 3A expression is modulated by nitric oxide in rat pulmonary artery smooth muscle cells. J Physiol Pharmacol. 2010;61(6):663–9. [PMC free article] [PubMed] [Google Scholar]
- 133.Chen B, Lakshminrusimha S, Czech L, et al. Regulation of phosphodiesterase 3 in the pulmonary arteries during the perinatal period in sheep. Pediatr Res. 2009;66(6):682–7. doi: 10.1203/PDR.0b013e3181bce574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Steinhorn RH, Millard SL, Morin FC., 3rd Persistent pulmonary hypertension of the newborn. Role of nitric oxide and endothelin in pathophysiology and treatment. Clin Perinatol. 1995;22(2):405–28. [PubMed] [Google Scholar]
- 135.Kumar P, Kazzi NJ, Shankaran S. Plasma immunoreactive endothelin-1 concentrations in infants with persistent pulmonary hypertension of the newborn. Am J Perinatol. 1996;13(6):335–41. doi: 10.1055/s-2007-994352. [DOI] [PubMed] [Google Scholar]
- 136.Rosenberg AA, Kennaugh J, Koppenhafer SL, et al. Elevated immunoreactive endothelin-1 levels in newborn infants with persistent pulmonary hypertension. J Pediatr. 1993;123(1):109–14. doi: 10.1016/s0022-3476(05)81552-5. [DOI] [PubMed] [Google Scholar]
- 137.Mohamed WA, Ismail M. A randomized, double-blind, placebo-controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. J Perinatol. 2011 doi: 10.1038/jp.2011.157. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 138.Nakwan N, Choksuchat D, Saksawad R, et al. Successful treatment of persistent pulmonary hypertension of the newborn with bosentan. Acta Paediatr. 2009;98(10):1683–5. doi: 10.1111/j.1651-2227.2009.01386.x. [DOI] [PubMed] [Google Scholar]
- 139.Inwald D, Brown K, Gensini F, et al. Open lung biopsy in neonatal and paediatric patients referred for extracorporeal membrane oxygenation (ECMO) Thorax. 2004;59(4):328–33. doi: 10.1136/thx.2003.010793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sakamoto H, Takenoshita M, Asakura Y, et al. Comparison of circulatory effects between arginine vasopressin (AVP) and dopamine in conscious newborn goats. J Vet Med Sci. 1996;58(6):511–4. doi: 10.1292/jvms.58.511. [DOI] [PubMed] [Google Scholar]
- 141.Tourneux P, Rakza T, Bouissou A, et al. Pulmonary circulatory effects of norepinephrine in newborn infants with persistent pulmonary hypertension. J Pediatr. 2008;153(3):345–9. doi: 10.1016/j.jpeds.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 142.Barrington KJ, Singh AJ, Etches PC, et al. Partial liquid ventilation with and without inhaled nitric oxide in a newborn piglet model of meconium aspiration. Am J Respir Crit Care Med. 1999;160(6):1922–7. doi: 10.1164/ajrccm.160.6.9812068. [DOI] [PubMed] [Google Scholar]
- 143.Shaffer TH, Lowe CA, Bhutani VK, et al. Liquid ventilation: effects on pulmonary function in distressed meconium-stained lambs. Pediatr Res. 1984;18(1):47–52. [PubMed] [Google Scholar]
- 144.Leach CL, Greenspan JS, Rubenstein SD, et al. Partial liquid ventilation with perflubron in premature infants with severe respiratory distress syndrome. The LiquiVent Study Group. N Engl J Med. 1996;335(11):761–7. doi: 10.1056/NEJM199609123351101. [DOI] [PubMed] [Google Scholar]
- 145.Enrione MA, Papo MC, Leach CL, et al. Regional pulmonary blood flow during partial liquid ventilation in normal and acute oleic acid-induced lung-injured piglets. Crit Care Med. 1999;27(12):2716–23. doi: 10.1097/00003246-199912000-00019. [DOI] [PubMed] [Google Scholar]
- 146.Lueders M, Weiswasser J, Aly H, et al. Changes in pulmonary vascular resistance in response to partial liquid ventilation. J Pediatr Surg. 1998;33(1):85–90. doi: 10.1016/s0022-3468(98)90368-2. [DOI] [PubMed] [Google Scholar]
- 147.Wilcox DT, Glick PL, Karamanoukian HL, et al. Partial liquid ventilation and nitric oxide in congenital diaphragmatic hernia. J Pediatr Surg. 1997;32(8):1211–5. doi: 10.1016/s0022-3468(97)90684-9. [DOI] [PubMed] [Google Scholar]
- 148.Sukumar M, Bommaraju M, Fisher JE, et al. High-frequency partial liquid ventilation in respiratory distress syndrome: hemodynamics and gas exchange. J Appl Physiol. 1998;84(1):327–34. doi: 10.1152/jappl.1998.84.1.327. [DOI] [PubMed] [Google Scholar]
- 149.Klein MD, Andrews AF, Wesley JR, et al. Venovenous perfusion in ECMO for newborn respiratory insufficiency. A clinical comparison with venoarterial perfusion. Ann Surg. 1985;201(4):520–6. doi: 10.1097/00000658-198504000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Andrews AF, Klein MD, Toomasian JM, et al. Venovenous extracorporeal membrane oxygenation in neonates with respiratory failure. J Pediatr Surg. 1983;18(4):339–46. doi: 10.1016/s0022-3468(83)80178-x. [DOI] [PubMed] [Google Scholar]
- 151.Fukuda S, Aoyama M, Yamada Y, et al. Comparison of venoarterial versus veno-venous access in the cerebral circulation of newborns undergoing extracorporeal membrane oxygenation. Pediatr Surg Int. 1999;15(2):78–84. doi: 10.1007/s003830050521. [DOI] [PubMed] [Google Scholar]
- 152.Knight GR, Dudell GG, Evans ML, et al. A comparison of venovenous and venoarterial extracorporeal membrane oxygenation in the treatment of neonatal respiratory failure. Crit Care Med. 1996;24(10):1678–83. doi: 10.1097/00003246-199610000-00013. [DOI] [PubMed] [Google Scholar]
- 153.Anderson HL, 3rd, Snedecor SM, Otsu T, et al. Multicenter comparison of conventional venoarterial access versus venovenous double-lumen catheter access in newborn infants undergoing extracorporeal membrane oxygenation. J Pediatr Surg. 1993;28(4):530–4. doi: 10.1016/0022-3468(93)90611-n. [discussion 534–5] [DOI] [PubMed] [Google Scholar]
- 154.Aschner JL, Fike CD. New developments in the pathogenesis and management of neonatal pulmonary hypertension. In: Bancalari E, editor. The newborn lung. Philadelphia: Saunders Elsevier; 2008. pp. 241–99. [Google Scholar]
- 155.Lakshminrusimha S, Wynn RJ, Youssfi M, et al. Use of CT angiography in the diagnosis of total anomalous venous return. J Perinatol. 2009;29(6):458–61. doi: 10.1038/jp.2008.240. [DOI] [PubMed] [Google Scholar]