Abstract
Background
A large number of licensed elderly drivers are demented or are likely to become demented. On-road driving tests, a method often used to assess driver competency, are likely anxiety-provoking for elderly individuals. This article examines the relationship between anxiety and driving performance in a mildly demented and elderly control (EC) sample.
Methods
Anxiety ratings of fear and tension, as assessed by visual analog scales, of 84 patients clinically diagnosed with mild Alzheimer’s disease (AD) (68 safe/marginal and 16 unsafe drivers) were compared with those of 44 age- and education-equated safe/marginal EC participants, both before and after a standardized on-road driving test.
Results
Analyses revealed significant positive correlations between AD patients’ pre–road test and post–road test tension and post–road test fear ratings and total road test score. Subsequent analyses of variance showed no significant pre–road test differences in fear ratings between the three groups but significantly higher levels of tension among the unsafe AD participants. After adjusting for baseline group differences, unsafe AD drivers experienced stable or higher anxiety levels after road test, whereas both the EC and safe/marginal AD drivers endorsed a significant reduction in anxiety.
Discussion
Unlike their safe EC and safe AD driver counterparts, unsafe AD patients reported continued elevated levels of fear and tension after the road test. Given these findings, we suggest that the most appropriate time for driving instructors to counsel patients regarding their driving skills might be directly after the road test.
Keywords: Alzheimer’s disease, Driving, Road test, Anxiety, Visual analog mood scales
1. Introduction
A large number of licensed elderly drivers in North America are demented or are likely to become demented [1]. It is now well-documented that not all persons with dementia are incompetent drivers, particularly during the very early stages [2–6]. Moreover, the consequences of driving cessation in the elderly include difficulties with adequate transportation, disruption of caregivers’ lives, and depressive symptomatology [7–11]. Accordingly, increased emphasis has been placed on identifying and differentiating safe from unsafe demented drivers.
It should be noted that in Rhode Island it is not routine for patients with Alzheimer’s disease (AD) to be referred for an on-road driving test to assess driving competency. Individual physicians can request a road test from the Department of Motor Vehicles (DMV) if they are concerned about a patient’s driving competence. Alternatively, police officers or family members might make a referral to the DMV for an evaluation of a patient’s driving competency if concerned [12,13].
Standardized on-road driving assessments are viewed as the gold standard method being used to evaluate driving competence in elderly demented persons [3,14,15]. Despite the face validity of this approach, it is not without problems that might limit validity. For example, having to undergo an evaluation of one’s driving abilities is likely anxiety-provoking. In addition to the prospect of losing one’s license after failing the driving test, the on-road test requires the patient to drive a new car (driving instructor’s car) on an unfamiliar route. Very few studies have examined the relationship between on-road driving test performance and anxiety in elderly or younger persons. Rather, the majority of studies in the literature regarding anxiety and driving relate to how anxiolytics and benzodiazepines affect driving performance [16–19] or anxiety disorders resulting from motor vehicle accidents [20,21]. We were able to find one tangentially related study that examined the effects of state and trait anxiety on driving performance in two cohorts of subjects 48 years of age and younger. This study found a negative effect of anxiety on driving performance, and that women had greater levels of anxiety during the driving test than men [22].
The purpose of this study was to examine the relationship between self-rated anxiety levels and on-road driving performance in an elderly demented (AD) population and an elderly comparison group (EC). Specifically, we assessed before and after driving test self-ratings of fear and tension on standardized visual analog mood scales in safe EC, safe AD, and unsafe AD drivers (with driving safety rated by a professional driving instructor). We hypothesized that the three groups would have elevated self-ratings of fear and tension before the on-road driving test because of the novel test situation. However, after the road test, we predicted that although all three groups would experience a reduction in fear and tension, the unsafe AD drivers’ ratings would remain higher than the EC and safe AD drivers.
2. Methods and design
2.1. Participants
Participants included 128 individuals (44 healthy EC subjects and 84 individuals with mild AD) enrolled in a longitudinal study of driving and dementia. All participants in this study were clinical patients recruited from the Alzheimer’s Disease and Memory Disorders Center at Memorial Hospital of Rhode Island. Patients were not recruited because of specific concerns regarding their driving abilities. On the basis of overall road test performance, all subjects were classified as safe, marginal, or unsafe by a professional driving instructor. The final sample consisted of 44 safe/marginal EC (35 safe, 9 marginal), 68 safe/marginal AD (34 safe, 34 marginal), and 16 unsafe AD drivers.
All participants were aged 46 to 91 years, English speaking, currently driving at least one trip per week, had a valid driver’s license, and had a family member willing to participate as an informant. Among the participants with AD, 52 were rated as Clinical Dementia Rating scale (CDR) [23] 0.5 (very mild AD), and 32 were rated as CDR 1 (mild AD), on the basis of a complete diagnostic evaluation by a neurologist (B.R.O.). Twenty-three met criteria for possible AD and 61 for probable AD, on the basis of National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association [24] guidelines. The healthy EC group were family members of dementia patients with no history of dementia (all had CDR = 0) and Mini Mental Status Examination (MMSE) [25] scores greater than 26.
Exclusion criteria for both patients and comparison subjects included reversible causes of dementia and physical, ophthalmologic, or neurological disorders other than dementia that might impair driving abilities. Corrected visual acuity was better than 20/50 on eye chart testing, and visual fields were normal on confrontation testing for all participants. Certain psychiatric disorders were also excluded, including mental retardation, schizophrenia, bipolar disorder, or history of alcohol/substance abuse within the past year. Depression was not excluded if it was controlled with medications. Symptomatic anti-dementia drugs (eg, cholinesterase inhibitors, ginkgo, vitamin E, estrogens, nonsteroidal anti-inflammatory drugs) as well as antipsychotic and anxiolytic medications were permitted, but dosages were required to be stable for at least 6 weeks before entry into the study.
Of those patients approached who met inclusion criteria, 52.6% agreed to participate in the study. Of note, likelihood of participation was not correlated with MMSE score, education, gender, age, or a clinician-rated measure of insight.
Ethical approval was obtained from the institutional review board of Memorial Hospital of Rhode Island, Pawtucket, RI. Written informed consent to participate in the study was obtained from each participant before study entry. During the consent process, participants were explicitly made aware that they would be advised to discontinue driving if their performance was classified as unsafe by the professional driving instructor.
2.2. Procedures
Detailed descriptions of general procedures for participants and caregivers, including measures administered and data collection methods before the standardized on-road driving test, have been published elsewhere [26,27]. Briefly, all participants were first seen by a research assistant, who collected various measures from participants and caregivers, including how often and how far the individual typically drives and a detailed history of driving violations. Participants who met criteria for study entry were then seen by a neurologist before their on-road driving test. This appointment consisted of a full dementia diagnostic work-up, including a physical, neurological, ophthalmologic, and neuropsychological exam.
Within 2 weeks of the clinical assessment, participants were administered an on-road driving test by a professional and experienced (6 years of licensed, full-time work as a licensed driving instructor and trained in the evaluation of neurologically disabled drivers) driving instructor during daylight hours under good conditions (no precipitation or wet roads). The same instructor tested all participants in the current study and was blinded to participant diagnosis. A 10- to 15 minute pre-test was completed in the parking lot before the actual road test to ensure that the test was safe to perform and to familiarize the participant with the car and the instructor. The driving test was based on a published and reliable driving test, the Washington University Road Test [28], adapted for comparable streets in Rhode Island. Although the streets were different, all the same maneuvers and identical scoring procedures were used to produce a comparable test procedure for Rhode Island. Participants received an on-road driving score based on safe completion of each of the required maneuvers, ranging from 0 (best score) to 108 (worst score).
The instructor also made a trichotomous global rating of the participant’s driving ability, “safe,” “marginal,” or “unsafe.” For the purpose of this study, drivers classified as safe and marginal in either the EC or AD groups were combined into one group (ie, safe/marginal EC drivers and safe/marginal AD drivers). The rationale for combining the safe and marginal drivers in both the EC and AD groups was that these drivers were not advised to stop driving. This is in contrast to participants who were rated as unsafe by the driving instructor. In this case, they were allowed to take the driving test again once. If they declined retest or failed the retest, they were advised to stop driving. All of the participants who received such advice followed it. Inter-rater reliability for 20 participants (rated by a second professional driving instructor in the back seat) yielded moderate to substantial agreement for the global rating (kappa = 0.65). Intraclass correlation coefficient between the two raters (r) for the total on-road driving score was 0.82.
Both before and after the on-road test, participants self-rated their fear and tension levels by using the Visual Analog Mood Scales (VAMS) [29]. The VAMS are reliable and valid visual analog measures of eight specific internal mood states (afraid, confused, sad, angry, energetic, tired, happy, and tense). They place minimal cognitive or linguistic demands on the respondent [30] and have been validated in many patient samples, including stroke [31], dementia [32], elderly controls [33], psychiatric inpatients and outpatients [29,34], and multiple sclerosis patients [35]. Each VAMS scale involves a 100-mm vertical line with a simple schematic face (eg, neutral face) at the top of the pole and another schematic at the end of the pole (eg, fearful face). Corresponding words (ie, “Neutral” and “Afraid”) are printed above and below the faces, respectively. Patients are instructed to mark—by drawing a single line across the vertical line—how they feel at the time [29]. Scales are scored from 0 to 100 on the basis of the distance in millimeters from the top, “neutral” pole. Two of these eight visual analog scales, the Afraid and Tense scales, were used in the current study to assess participant anxiety. We used these two VAMS scales to measure two key but distinct features of anxiety [36] (fear and tension).
As stated above, AD and EC participants were administered the Afraid and Tense VAMS subscales both before and after the standardized-on road driving assessment. The post–driving test assessment of fear and tension was administered before participants were given any feedback regarding their driving test performance. For the purpose of our analyses, participants’ raw scores were linearly transformed into age- and gender-corrected T scores to provide interpretation relative to the standardization sample [29].
We initially examined bivariate Pearson correlations for both AD and EC participants’ pre-test and post-test fear and tension scores and total on-road driving test score.
The remaining analyses examined group differences in fear and tension ratings according to the driving instructor’s global rating of participants’ driving ability (safe/marginal or unsafe). For each outcome (self-reported fear and tension), pre-test to post-test change-scores were first analyzed by using an analysis of covariance (ANCOVA) model that included main effects of group (safe/marginal EC, safe/marginal AD, unsafe AD), pre-test values of the outcome, as well as any measured covariate showing between-group differences at baseline. In addition, interaction terms were added between group membership and all other variables in the initial model and explored via a backwards elimination procedure. If the covariate effects did not differ significantly by group, the corresponding interaction term was dropped, and the ANCOVA model was re-estimated. Finally, between-group differences in change-scores were summarized graphically by using regression-adjusted means that depict the expected change in outcome for a “typical” subject with pre-test outcome levels equal to the overall mean of the study participants.
3. Results
The safe/marginal EC, safe/marginal AD, and unsafe AD participants did not differ significantly in age, years of education, driving experience, or race (all participants but six were white, two Asian American, two African American, one Native American, and one other) (Table 1). However, there was a significantly greater percentage of men in the safe/marginal AD group, compared with the other two groups. As expected, the EC participants had significantly higher MMSE scores than the AD patients. Gender and MMSE scores were not significantly correlated with any of our outcome variables and, as such, were not used as covariates in the final model.
Table 1.
Sample descriptive information: Mean (standard deviation)
| Safe/Marginal EC (N = 44) | Safe/Marginal AD (N = 68) | Unsafe AD (N = 16) | |||
|---|---|---|---|---|---|
| Demographic variables | |||||
| Age (y) | 73.55 (9.13) | 75.29 (7.24) | 77.25 (5.69) | 3.00 | |
| Education (y) | 15.18 (3.01) | 13.93 (3.24) | 13.50 (3.88) | 5.13 | |
| Driving experience (y) | 52.25 (11.52) | 56.44 (9.79) | 51.38 (13.51) | 5.33 | |
| % Female | 56.82 | 30.88 | 75.00 | 13.76* | |
| % White | 97.73 | 94.12 | 93.75 | —† | |
| Clinical variables | |||||
| CDR Scale | 0.0 = 44 | 0.0 = 0 | 0.0 = 0 | 172.02* | |
| 0.5 = 0 | 0.5 = 44 | 0.5 = 8 | |||
| 1.0 = 0 | 1.0 = 24 | 1.0 = 8 | |||
| MMSE | 29.09 (1.12) | 24.41 (3.50) | 22.63 (3.79) | 68.43* | |
| Global Driving Test score (range, 0–108)‡ | 5.98 (4.51) | 11.13 (6.56) | 23.50 (7.73) | 68.73* | |
| Pre–road test VAMS Fear | 52.84 (11.94) | 52.93 (13.93) | 56.63 (12.31) | 1.16 | |
| Pre–road test VAMS Tension | 51.41 (10.52) | 50.54 (12.03) | 58.94 (15.14) | 6.47§ | |
| Post–road test VAMS Fear | 46.45 (5.29) | 47.90 (8.66) | 56.00 (13.58) | 14.87* | |
| Post–road test VAMS Tension | 44.09 (5.20) | 44.72 (6.40) | 53.00 (13.05) | 18.90* |
P ≤ .0001.
Test statistic not available because of small cell counts.
Item reverse scored.
P ≤ .05.
3.1. Correlations between on-road driving test total score and pre-test and post-test fear and tension ratings
Before the on-road driving test, AD participants’ tension (r(80) = .24, P < .05) but not fear (r(80) = .08, P = .48) ratings were significantly correlated with total on-road test score. This was in contrast to EC participants, in whom total road test score was correlated with neither pre-test fear (r(42) = .24, P = .12) nor tension (r(42) = .28, P < .07).
After the on-road driving test, AD participants’ fear (r(80) = .24, P < .05) and tension (r(80) = .28, P < .05) ratings were significantly correlated with total on-road test score. Again, this was in contrast to EC participants, in whom total road test score was correlated with neither post-test fear (r(42) = .05, P = .75) nor tension (r(42) = .17, P = .27).
3.2. Road test performance
The road test was scored on a scale of 0 to 108, with lower scores indicating better overall performance. The global driving test score of the safe/marginal EC drivers was compared with the performance of both the safe/marginal AD and unsafe AD drivers by using one-way ANOVA. The three groups significantly differed from each other in road test performance (, P < .0001), with safe/marginal EC drivers outperforming safe/marginal AD drivers on the road test, who in turn outperformed unsafe AD drivers. Planned contrasts revealed that there were significant differences between safe/marginal and unsafe AD drivers (, P < .0001), and that safe/marginal EC subjects performed significantly better on the road test than did both safe/marginal (, P < .0001) and unsafe (, P < .0001) AD participants.
3.3. Pre– and post–driving test fear ratings
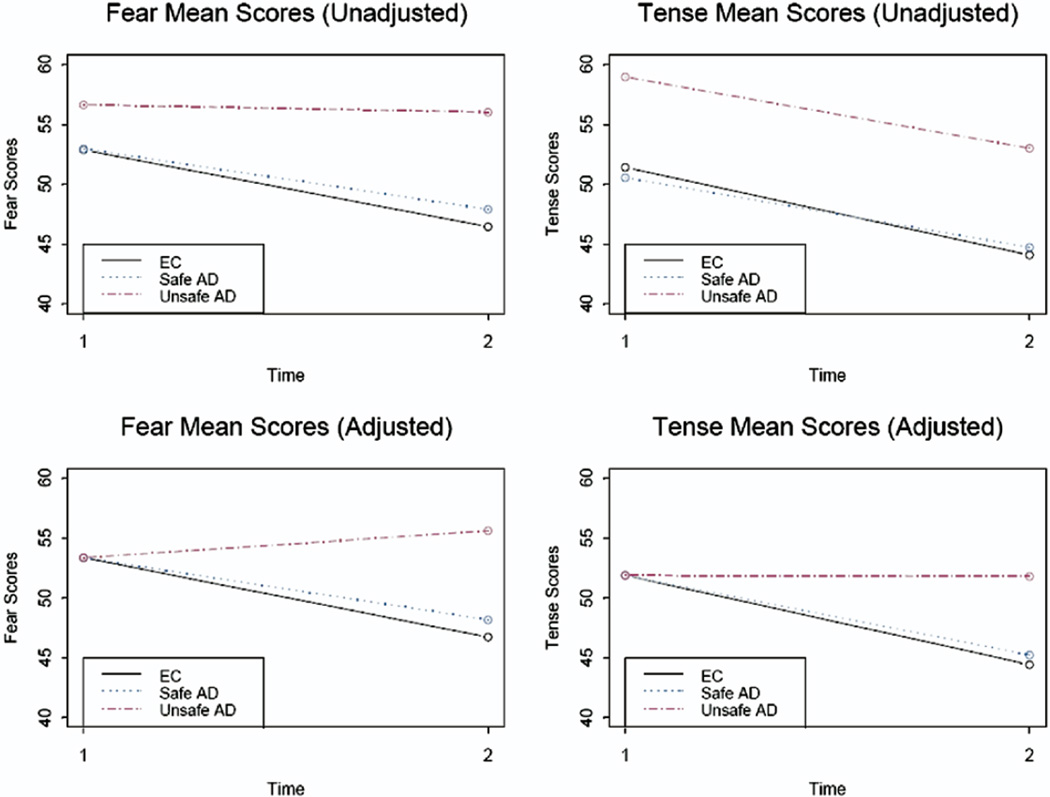
Before the road test, the average fear ratings for the unsafe AD drivers were higher than those for the safe/marginal AD and EC groups, which appeared to be almost identical (Table 1). However, these differences were not large enough to attain statistical significance (, P = .56). Next, we used an ANCOVA model to examine whether changes in pre–road test to post–road test fear depended on group, pre-test fear, or their interaction. The interaction test was not statistically significant, indicating that the effect of pre-test fear on the pre-post change-scores did not significantly differ by group (, P = .58). Dropping the interaction term and re-estimating the model showed statistically significant differences in change-scores as a result of both group (, P = .001) and baseline fear levels (, P < .0001). As seen in Table 1, both EC and AD safe/marginal drivers experienced a reduction in fear ratings after the road test, whereas unsafe AD drivers showed no change. Figure 1 displays crude sample means in the first row and adjusted means in the second row. The latter correct for any baseline differences in the outcome by predicting how a “typical” individual with average fear levels at baseline would behave in each study group. More specifically, after adjusting to a common baseline, the reduction in fear levels among safe/marginal AD drivers was seen to be smaller but not significantly different from that experienced by the safe/marginal ECs (, P = .36). However, unsafe AD drivers’ post-test fear ratings were significantly higher than those of both safe/marginal EC (, P < .0002) and safe/marginal AD drivers (, P < .0001). Indeed, they were the only group that showed increased fear levels after test (55.62), relative to the common baseline of 53.36.
Fig 1.
Raw and adjusted fear and tension mean scores before and after on-road test.
3.4. Pre–driving test and post–driving test tension ratings
Before the road test, there were significant group differences in tension between the safe/marginal EC, safe/marginal AD, and unsafe AD drivers (, P = .04). As seen in Table 1, unsafe AD drivers had higher average levels of tension at baseline than both safe/marginal EC (, P = .03) and safe/marginal AD drivers (, P = .01), with the latter two groups having almost identical means. An ANCOVA model that examined whether changes in pre–road test to-post–road test tension were all equally affected by pre-test tension levels showed a nonsignificant interaction (, P = .13). Accordingly, a main effects only model was fit, which adjusted group effects for a common effect of baseline tension levels. This showed significant main effects of group (, P = .001), with unsafe AD drivers experiencing higher post-test levels of tension than both safe/marginal EC (, P < .001) and safe/marginal AD drivers (, P < .001). The second column of Figure 1 displays both nonadjusted and adjusted means. Although all groups appear to have experienced an equal decrease in tension from pre-test to post-test on the basis of sample means, the unsafe AD drivers had much higher pre-test tension levels. Moreover, the dependence of the change-scores on baseline tension ratings was very highly significant (, P < .0001), with subjects high in pre-test tension more likely to experience a drop in post-test tension. Therefore, regression adjustment to a common baseline resulted in the observed reduction in tension levels ratings from 58.94 at pre-test to 53.00 at post-test, looking like an essentially flat longitudinal trajectory of 51.89 at pretest to 51.80 at post-test.
4. Discussion
Our examination of anxiety self-ratings both before and after a standardized on-road driving test revealed that broadly, total road test score was associated with pre-test tension and both post-test fear and tension ratings among AD patients, but neither pre-test nor post-test fear and tension ratings were associated with total road test score among EC drivers. More specific group level analyses in safe/marginal EC drivers, safe/marginal AD drivers, and unsafe AD drivers showed that (1) for the most part, self-ratings of fear and tension were greater before the driving test than after it, regardless of driving performance, in all three groups; and (2) adjusting for baseline group differences, safe/marginal EC and safe/marginal AD drivers experienced reductions in both fear and tension levels after the road test, unlike their unsafe AD counterparts, who failed to experience the large drop their high baseline values would have otherwise predicted.
To our knowledge, this is the first study to examine the relationship between anxiety ratings and on-road driving performance in an elderly demented population. Our findings of continued elevated levels of fear and tension, after adjusting for baseline levels, in unsafe AD drivers after the road test are particularly interesting in light of other recent findings. Specifically, the results of two recent studies demonstrated that both AD patients and their informants might overestimate the patient’s driving abilities before the on-road driving test [37,38]. There exists a discrepancy between AD patients’ and informants’ ratings of patient driving ability and actual performance as rated by a professional driving instructor on the standardized on-road driving test.
We can only speculate as to the reasons for our finding that unsafe AD drivers continue to exhibit elevated levels of anxiety, particularly fear, after the on-road driving test relative to safe/marginal EC and safe/marginal AD drivers. One hypothesis is that this persistent anxiety might be due to an increased awareness of their poor driving abilities. In other words, it is possible that unsafe AD drivers’ anxiety levels remain elevated because they realize that their driving abilities have diminished further than they initially anticipated before the on-road test. Unfortunately, we did not administer a measure of insight or awareness to test this hypothesis directly.
Although once again speculative, one practical implication of these findings is that the best time for the driving instructor to counsel unsafe AD drivers to stop driving might be immediately after the on-road driving test. At that point, unsafe drivers’ anxiety appears to remain elevated, and, potentially, they might have concurrent increased awareness of their poor driving abilities. With increased awareness and saliency of poor driving abilities, a joint discussion among unsafe driver and driving instructor (and preferably the caregiver) and subsequent recommendations to stop driving might be more readily accepted. Although the negative consequences of no longer being able to drive remain, being perceived as an active participant in the decision might possibly moderate the transition and, to a lesser extent, the affective response to driving cessation. This hypothesis could not be directly tested in the current study, because all participants received feedback regarding their performance immediately after the road test. Future studies should test this hypothesis by varying the time points at which the driving instructor provides feedback to participants regarding their road test performance.
A potential alternative outcome could be that heightened or persistent anxiety in unsafe AD drivers might confer a negative receptivity to counseling immediately after the road test, although this has not been our clinical experience.
Because our sample of unsafe AD drivers was relatively small, this study requires further replication with a larger sample before more confident conclusions can be put forth regarding the significance of the change in self-anxiety ratings after the road test.
In addition to using a larger sample, examining the relationship between anxiety levels and on-road driving test performance across subsequent on-road tests will be important for future study. Specifically, it is possible that both pre–driving test and post–driving test anxiety levels were related to the novelty of the first on-road driving test in both the EC and AD drivers. If this is the case, anxiety levels will be expected to decline as a function of multiple exposures to the test-taking situation over time. All of the participants in this study are being followed longitudinally, and future studies will examine how fear and tension scores vary with additional exposure to the on-road test. Examining this relationship in light of worsening driving abilities over time will be particularly interesting.
Last, the use of an awareness or insight measure after the road test would be valuable in future studies. Specifically, examining the relationship between total driving score and patients’ perceptions of their performance on the road test will allow us to directly address the question of whether post-test anxiety is associated with insight into deteriorated driving abilities in AD drivers.
In summary, the results from this preliminary study examining the relationship between self-rated anxiety levels and driving performance on a standardized on-road driving test in elderly demented patients revealed that unsafe AD drivers endorsed greater levels of anxiety following their road test compared to safe/marginal EC and AD drivers. Although not directly tested, increased awareness into poor driving abilities following the on-road test may account for these elevated anxiety ratings in unsafe AD drivers. If this is the case, the most appropriate time for driving instructors and unsafe drivers to mutually discuss driving performance and make recommendations for driving cessation may be immediately following the driving test.
Acknowledgments
Supported by grants RO1#AG16335 and P30-AG13846 from the National Institute on Aging. The authors would like to thank Dr John C. Morris for his helpful review of a previous version of this manuscript.
References
- 1.Brown LB, Ott BR. Driving and dementia: a review of the literature. J Geriatr Psychiatry Neurol. 2004;17:232–240. doi: 10.1177/0891988704269825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunt L, Morris JC, Edwards D, Wilson BS. Driving performance in persons with mild senile dementia of the Alzheimer type. J Am Geriatr Soc. 1993;41:747–753. doi: 10.1111/j.1532-5415.1993.tb07465.x. [DOI] [PubMed] [Google Scholar]
- 3.Hunt LA, Murphy CF, Carr D, Duchek JM, Buckles V, Morris JC. Reliability of the Washington University Road Test. Arch Neurol. 1997;54:707–712. doi: 10.1001/archneur.1997.00550180029008. [DOI] [PubMed] [Google Scholar]
- 4.Cox DJ, Quillian WC, Thorndike FP. Evaluating driving performance of outpatients with Alzheimer disease. J Am Board Fam Pract. 1998;11:264–271. doi: 10.3122/jabfm.11.4.264. [DOI] [PubMed] [Google Scholar]
- 5.Rebok GW, Keyl PM, Bylsma FW, Blaustein MJ, Tune L. The effects of Alzheimer’s disease on driving-related abilities. Alzheimer Dis Assoc Disord. 1994;4:228–240. doi: 10.1097/00002093-199408040-00002. [DOI] [PubMed] [Google Scholar]
- 6.Rizzo M, McGehee DV, Dawson JD, Anderson SN. Simulated car crashes at intersections in drivers with Alzheimer’s disease. Alzheimer Dis Assoc Disord. 2001;15:10–20. doi: 10.1097/00002093-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Post SG, Whitehouse P. Fairhill guidelines of the care of people with Alzheimer’ disease: a clinical summary. J Am Geriatr Soc. 1995;43:1423–1429. doi: 10.1111/j.1532-5415.1995.tb06625.x. [DOI] [PubMed] [Google Scholar]
- 8.Reuben DB, Silliman RA, Traines M. The aging driver: Medicine, policy, and ethics. J Am Geriatr Soc. 1988;36:1135–1142. doi: 10.1111/j.1532-5415.1988.tb04403.x. [DOI] [PubMed] [Google Scholar]
- 9.Persson D. The elderly driver: Deciding when to stop. The Gerontologist. 1993;33:88–91. doi: 10.1093/geront/33.1.88. [DOI] [PubMed] [Google Scholar]
- 10.Taylor BD, Tripodes S. The effects of driving cessation on the elderly with dementia and their informants. Accid Analy Prev. 2001;33:519–528. doi: 10.1016/s0001-4575(00)00065-8. [DOI] [PubMed] [Google Scholar]
- 11.Marottoli RA, Cooney LM, Wagner R, Doucette J, Tinetti ME. Predictors of automobile crashes and moving violations among elderly drivers. Ann Intern Med. 1994;121:842–846. doi: 10.7326/0003-4819-121-11-199412010-00003. [DOI] [PubMed] [Google Scholar]
- 12.Ott BR. Driving and dementia: the medical perspective. Med Health/RI. 1996;79:383–387. [PubMed] [Google Scholar]
- 13.Ott BR, Mernoff ST. Driving policy issues and the physician. Med Health/RI. 1999;82:428–431. [PubMed] [Google Scholar]
- 14.Fitten LJ, Perryman KM, Wilkinson C, Little RJ, Burns MM, Pachana N, et al. Alzheimer and vascular dementias and driving: a prospective road and laboratory study. JAMA. 1995;273:1360–1365. [PubMed] [Google Scholar]
- 15.Odenheimer GL, Beaudet M, Jette AM, Albert MS, Grande L, Minaker KL. Performance-based driving evaluation of the elderly driver: safety, reliability, and validity. J Gerontology. 1994;49:M153–M159. doi: 10.1093/geronj/49.4.m153. [DOI] [PubMed] [Google Scholar]
- 16.Clarkson JE, Gordon AM, Logan BK. Lorazepam and driving impairment. J Anal Toxicol. 2004;28:475–480. doi: 10.1093/jat/28.6.475. [DOI] [PubMed] [Google Scholar]
- 17.Iudice A, Bonanni E, Maestri M, Nucciarone B, Brotini S, Manca L, et al. Lormetazepam effects on daytime vigilance, psychomotor performance and simulated driving in young adult healthy volunteers. Int J Clin Pharmacol Ther. 2002;40:304–309. doi: 10.5414/cpp40304. [DOI] [PubMed] [Google Scholar]
- 18.Verster JC, Volkerts ER, Verbaten MN. Effects of alprazolam on driving ability, memory functioning and psychomotor performance: a randomized, placebo-controlled study. Neuropsychopharmacology. 2002;27:260–269. doi: 10.1016/S0893-133X(02)00310-X. [DOI] [PubMed] [Google Scholar]
- 19.O’Hanlon JF, Vermeeren A, Uiterwijk MM, van Veggel LM, Swijgman HF. Anxiolytics’ effects on the actual driving performance of patients and healthy volunteers in a standardized test An integration of three studies. Neuropsychobiology. 1995;31:81–88. doi: 10.1159/000119177. [DOI] [PubMed] [Google Scholar]
- 20.Mayou R, Bryant B. Consequences of road traffic accidents for different types of road user. Injury. 2003;34:197–202. doi: 10.1016/s0020-1383(02)00285-1. [DOI] [PubMed] [Google Scholar]
- 21.Bryant RA, Harvey AG. Initial posttraumatic stress responses following motor vehicle accidents. J Trauma Stress. 1996;9:223–224. doi: 10.1007/BF02110657. [DOI] [PubMed] [Google Scholar]
- 22.Lopes CG, Ventura JP. Anxiety and performance in a car driving test: influence and proximity relations. Psicolgia: teoria,-investigacao-e-practica. 2000;5:67–85. [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating: current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. Mini Mental State Examination (MMSE) Lutz, FL: Psychological Assessment Resources, Inc; 2002. [Google Scholar]
- 26.Ott BR, Anthony D, Papandonatos GD, D’Abreu A, Burock J, Curtin A, et al. Clinical assessment of the driving competence of patients with dementia. J Am Geriatr Soc. 2005;53:829–833. doi: 10.1111/j.1532-5415.2005.53265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown LB, Stern RA, Cahn-Weiner DA, Rogers B, Messer MA, Lannon MC, et al. Driving scenes test of the Neuropsychological Assessment Battery (NAB) and on-road driving performance in aging and very mild dementia. Arch Clin Neuropsych. 2005;20:209–215. doi: 10.1016/j.acn.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunt LA, Murphy CF, Carr D, Duchek JM, Buckles V, Morris JC. Reliability of the Washington University Road Test. Arch Neurol. 1997;54:707–712. doi: 10.1001/archneur.1997.00550180029008. [DOI] [PubMed] [Google Scholar]
- 29.Stern RA. Visual Analog Mood Scale (VAMS) Lutz, FL: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 30.Stern RA. Assessment of mood states in neurodegenerative disease: methodological and diagnostic recommendations. Semin Clin Neuropsychaitry. 1996;1:315–324. doi: 10.1053/SCNP00100315. [DOI] [PubMed] [Google Scholar]
- 31.Arruda JE, Stern RA, Somerville JA. Measurement of mood states in stroke patients: validation of the Visual Analog Mood Scales. Arch Phys Med and Rehabil. 1999;80:676–680. doi: 10.1016/s0003-9993(99)90171-5. [DOI] [PubMed] [Google Scholar]
- 32.Temple RO, Stern RA, Latham J, Ruffolo JS, Arruda JE, Tremont G. Assessment of mood state in dementia by the use of the visual analog mood scales (VAMS) Am J Geriatr Psychiatry. 2004;12:527–530. doi: 10.1176/appi.ajgp.12.5.527. [DOI] [PubMed] [Google Scholar]
- 33.Nyenhuis DL, Stern RA, Yamamoto C, Luchetta T, Arruda JE. Standardization and validation of the Visual Analog Mood Scales. The Clin Neuropsych. 1997;11:407–415. [Google Scholar]
- 34.Arruda JE, Stern RA, Legendre SA. Assessment of mood state in patients undergoing electroconvulsive therapy: the utility of Visual Analogue Mood Scales developed for cognitively-impaired patients. Convuls Ther. 1996;12:207–212. [PubMed] [Google Scholar]
- 35.Groom MJ, Lincoln NB, Francis VM, et al. Assessing mood in patients with multiple sclerosis. Clin Rehabil. 2003;17:847–857. doi: 10.1191/0269215503cr688oa. [DOI] [PubMed] [Google Scholar]
- 36.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV) 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 37.Wild K, Cotrell V. Identifying driving impairment in Alzheimer disease: a comparison of self and observer reports versus driving evaluation. Alzheimer Dis Assoc Disord. 2003;17:27–34. doi: 10.1097/00002093-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Brown LB, Ott BR, Papandonatos GD, Sui Y, Ready RE, Morris JC. Prediction of on-road driving performance in patients with early Alzheimer’s disease. J Am Geriatr Soc. 2005;53:94–98. doi: 10.1111/j.1532-5415.2005.53017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]