Abstract
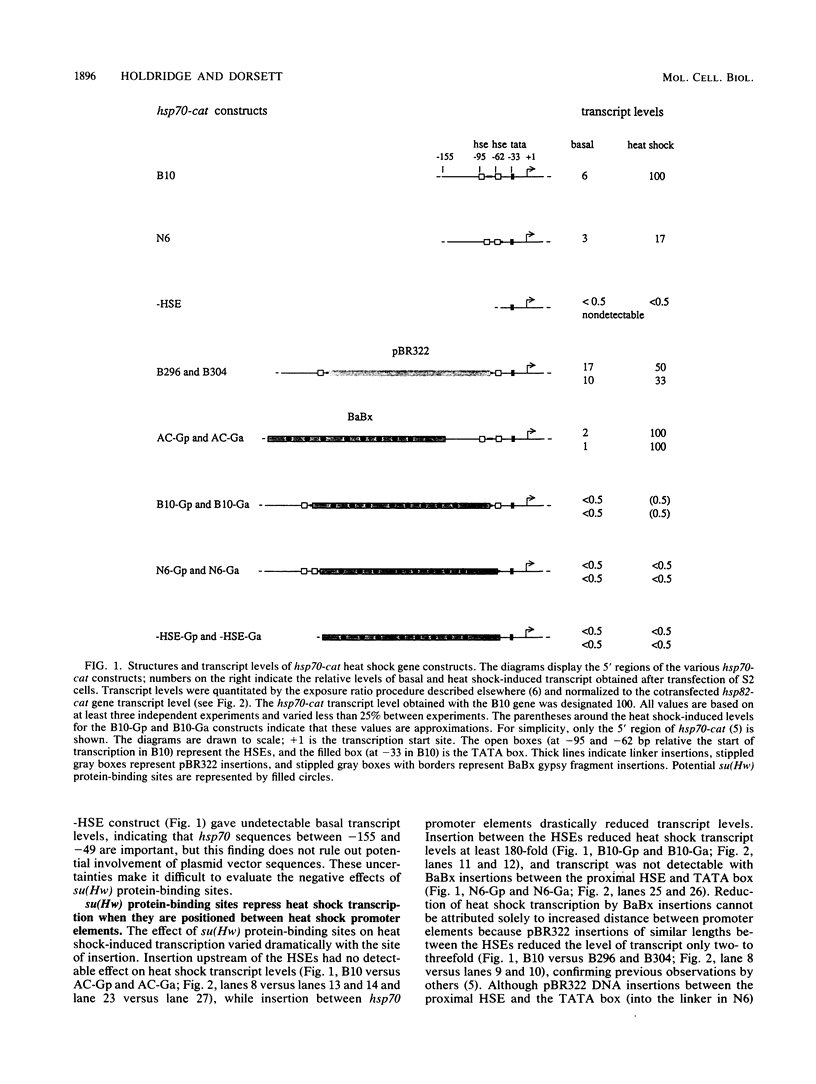
The suppressor of hairy-wing [su(Hw)] locus of Drosophila melanogaster encodes a zinc finger protein that binds a repeated motif in the gypsy retroposon. Mutations of su(Hw) suppress the phenotypes associated with mutations caused by gypsy insertions. To examine the mechanisms by which su(Hw) alters gene expression, a fragment of gypsy containing multiple su(Hw) protein-binding sites was inserted into various locations in the well-characterized Drosophila hsp70 heat shock gene promoter. We found no evidence for activation of basal hsp70 transcription by su(Hw) protein in cultured Drosophila cells but observed that it can repress heat shock-induced transcription. Repression occurred only when su(Hw) protein-binding sites were positioned between binding sites for proteins required for heat shock transcription. We propose that su(Hw) protein interferes nonspecifically with protein-protein interactions required for heat shock transcription, perhaps sterically, or by altering the ability of DNA to bend or twist.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin J., Mestril R., Lawson R., Klapper H., Voellmy R. The heat shock consensus sequence is not sufficient for hsp70 gene expression in Drosophila melanogaster. Mol Cell Biol. 1985 Jan;5(1):197–203. doi: 10.1128/mcb.5.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H. Molecular analysis of the yellow gene (y) region of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7369–7373. doi: 10.1073/pnas.82.21.7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campuzano S., Balcells L., Villares R., Carramolino L., García-Alonso L., Modolell J. Excess function hairy-wing mutations caused by gypsy and copia insertions within structural genes of the achaete-scute locus of Drosophila. Cell. 1986 Jan 31;44(2):303–312. doi: 10.1016/0092-8674(86)90764-6. [DOI] [PubMed] [Google Scholar]
- Cohen R. S., Meselson M. Inducible transcription and puffing in Drosophila melanogaster transformed with hsp70-phage lambda hybrid heat shock genes. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5509–5513. doi: 10.1073/pnas.81.17.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R. S., Meselson M. Periodic interactions of heat shock transcriptional elements. Nature. 1988 Apr 28;332(6167):856–858. doi: 10.1038/332856a0. [DOI] [PubMed] [Google Scholar]
- Dorsett D. Potentiation of a polyadenylylation site by a downstream protein-DNA interaction. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4373–4377. doi: 10.1073/pnas.87.11.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsett D., Viglianti G. A., Rutledge B. J., Meselson M. Alteration of hsp82 gene expression by the gypsy transposon and suppressor genes in Drosophila melanogaster. Genes Dev. 1989 Apr;3(4):454–468. doi: 10.1101/gad.3.4.454. [DOI] [PubMed] [Google Scholar]
- Dudler R., Travers A. A. Upstream elements necessary for optimal function of the hsp 70 promoter in transformed flies. Cell. 1984 Sep;38(2):391–398. doi: 10.1016/0092-8674(84)90494-x. [DOI] [PubMed] [Google Scholar]
- Dunn T. M., Hahn S., Ogden S., Schleif R. F. An operator at -280 base pairs that is required for repression of araBAD operon promoter: addition of DNA helical turns between the operator and promoter cyclically hinders repression. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5017–5020. doi: 10.1073/pnas.81.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J. A., Maniatis T. Drosophila Adh: a promoter element expands the tissue specificity of an enhancer. Cell. 1988 May 6;53(3):451–461. doi: 10.1016/0092-8674(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Garabedian M. J., Shepherd B. M., Wensink P. C. A tissue-specific transcription enhancer from the Drosophila yolk protein 1 gene. Cell. 1986 Jun 20;45(6):859–867. doi: 10.1016/0092-8674(86)90560-x. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Corces V. G. Separate regulatory elements are responsible for the complex pattern of tissue-specific and developmental transcription of the yellow locus in Drosophila melanogaster. Genes Dev. 1987 Nov;1(9):996–1004. doi: 10.1101/gad.1.9.996. [DOI] [PubMed] [Google Scholar]
- Geyer P. K., Green M. M., Corces V. G. Reversion of a gypsy-induced mutation at the yellow (y) locus of Drosophila melanogaster is associated with the insertion of a newly defined transposable element. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3938–3942. doi: 10.1073/pnas.85.11.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Green M. M., Corces V. G. Tissue-specific transcriptional enhancers may act in trans on the gene located in the homologous chromosome: the molecular basis of transvection in Drosophila. EMBO J. 1990 Jul;9(7):2247–2256. doi: 10.1002/j.1460-2075.1990.tb07395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer P. K., Spana C., Corces V. G. On the molecular mechanism of gypsy-induced mutations at the yellow locus of Drosophila melanogaster. EMBO J. 1986 Oct;5(10):2657–2662. doi: 10.1002/j.1460-2075.1986.tb04548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill G., Ptashne M. Negative effect of the transcriptional activator GAL4. Nature. 1988 Aug 25;334(6184):721–724. doi: 10.1038/334721a0. [DOI] [PubMed] [Google Scholar]
- Glaser R. L., Lis J. T. Multiple, compensatory regulatory elements specify spermatocyte-specific expression of the Drosophila melanogaster hsp26 gene. Mol Cell Biol. 1990 Jan;10(1):131–137. doi: 10.1128/mcb.10.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K., Levine M. S., Manley J. L. Synergistic activation and repression of transcription by Drosophila homeobox proteins. Cell. 1989 Feb 24;56(4):573–583. doi: 10.1016/0092-8674(89)90580-1. [DOI] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Hoch M., Schröder C., Seifert E., Jäckle H. cis-acting control elements for Krüppel expression in the Drosophila embryo. EMBO J. 1990 Aug;9(8):2587–2595. doi: 10.1002/j.1460-2075.1990.tb07440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack J. W. Molecular organization of the cut locus of Drosophila melanogaster. Cell. 1985 Oct;42(3):869–876. doi: 10.1016/0092-8674(85)90283-1. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., O'Farrell P. H. Activation and repression of transcription by homoeodomain-containing proteins that bind a common site. Nature. 1988 Dec 22;336(6201):744–749. doi: 10.1038/336744a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleher C. A., Goutte C., Johnson A. D. The yeast cell-type-specific repressor alpha 2 acts cooperatively with a non-cell-type-specific protein. Cell. 1988 Jun 17;53(6):927–936. doi: 10.1016/s0092-8674(88)90449-7. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Modolell J., Bender W., Meselson M. Drosophila melanogaster mutations suppressible by the suppressor of Hairy-wing are insertions of a 7.3-kilobase mobile element. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1678–1682. doi: 10.1073/pnas.80.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor binds to the regulatory site of an hsp 70 gene. Cell. 1984 May;37(1):273–283. doi: 10.1016/0092-8674(84)90323-4. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Forked, gypsys, and suppressors in Drosophila. Cell. 1985 Jun;41(2):429–437. doi: 10.1016/s0092-8674(85)80016-7. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Harrison D. A., Remington M. P., Spana C., Kelley R. L., Coyne R. S., Corces V. G. The Drosophila su(Hw) gene, which controls the phenotypic effect of the gypsy transposable element, encodes a putative DNA-binding protein. Genes Dev. 1988 Oct;2(10):1205–1215. doi: 10.1101/gad.2.10.1205. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Bienz M. A synthetic heat-shock promoter element confers heat-inducibility on the herpes simplex virus thymidine kinase gene. EMBO J. 1982;1(11):1473–1477. doi: 10.1002/j.1460-2075.1982.tb01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic O., Xiao H., Lis J. T. Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell. 1989 Dec 1;59(5):797–806. doi: 10.1016/0092-8674(89)90603-x. [DOI] [PubMed] [Google Scholar]
- Pick L., Schier A., Affolter M., Schmidt-Glenewinkel T., Gehring W. J. Analysis of the ftz upstream element: germ layer-specific enhancers are independently autoregulated. Genes Dev. 1990 Jul;4(7):1224–1239. doi: 10.1101/gad.4.7.1224. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J. Transcriptional interference and termination between duplicated alpha-globin gene constructs suggests a novel mechanism for gene regulation. Nature. 1986 Aug 7;322(6079):562–565. doi: 10.1038/322562a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Vectors for P element-mediated gene transfer in Drosophila. Nucleic Acids Res. 1983 Sep 24;11(18):6341–6351. doi: 10.1093/nar/11.18.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge B. J., Mortin M. A., Schwarz E., Thierry-Mieg D., Meselson M. Genetic interactions of modifier genes and modifiable alleles in Drosophila melanogaster. Genetics. 1988 Jun;119(2):391–397. doi: 10.1093/genetics/119.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R. T., Smith D. L., Johnson A. D. Flexibility of the yeast alpha 2 repressor enables it to occupy the ends of its operator, leaving the center free. Genes Dev. 1988 Jul;2(7):807–816. doi: 10.1101/gad.2.7.807. [DOI] [PubMed] [Google Scholar]
- Shuey D. J., Parker C. S. Binding of Drosophila heat-shock gene transcription factor to the hsp 70 promoter. Evidence for symmetric and dynamic interactions. J Biol Chem. 1986 Jun 15;261(17):7934–7940. [PubMed] [Google Scholar]
- Simon J. A., Lis J. T. A germline transformation analysis reveals flexibility in the organization of heat shock consensus elements. Nucleic Acids Res. 1987 Apr 10;15(7):2971–2988. doi: 10.1093/nar/15.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. A., Sutton C. A., Lobell R. B., Glaser R. L., Lis J. T. Determinants of heat shock-induced chromosome puffing. Cell. 1985 Apr;40(4):805–817. doi: 10.1016/0092-8674(85)90340-x. [DOI] [PubMed] [Google Scholar]
- Spana C., Harrison D. A., Corces V. G. The Drosophila melanogaster suppressor of Hairy-wing protein binds to specific sequences of the gypsy retrotransposon. Genes Dev. 1988 Nov;2(11):1414–1423. doi: 10.1101/gad.2.11.1414. [DOI] [PubMed] [Google Scholar]
- Topol J., Ruden D. M., Parker C. S. Sequences required for in vitro transcriptional activation of a Drosophila hsp 70 gene. Cell. 1985 Sep;42(2):527–537. doi: 10.1016/0092-8674(85)90110-2. [DOI] [PubMed] [Google Scholar]
- Vincent J. P., Kassis J. A., O'Farrell P. H. A synthetic homeodomain binding site acts as a cell type specific, promoter specific enhancer in Drosophila embryos. EMBO J. 1990 Aug;9(8):2573–2578. doi: 10.1002/j.1460-2075.1990.tb07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederrecht G., Shuey D. J., Kibbe W. A., Parker C. S. The Saccharomyces and Drosophila heat shock transcription factors are identical in size and DNA binding properties. Cell. 1987 Feb 13;48(3):507–515. doi: 10.1016/0092-8674(87)90201-7. [DOI] [PubMed] [Google Scholar]
- Wu C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature. 1984 May 17;309(5965):229–234. doi: 10.1038/309229a0. [DOI] [PubMed] [Google Scholar]
- Wu C., Wilson S., Walker B., Dawid I., Paisley T., Zimarino V., Ueda H. Purification and properties of Drosophila heat shock activator protein. Science. 1987 Nov 27;238(4831):1247–1253. doi: 10.1126/science.3685975. [DOI] [PubMed] [Google Scholar]
- Xiao H., Lis J. T. Germline transformation used to define key features of heat-shock response elements. Science. 1988 Mar 4;239(4844):1139–1142. doi: 10.1126/science.3125608. [DOI] [PubMed] [Google Scholar]