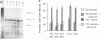Abstract
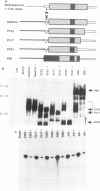
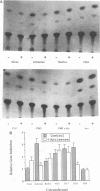
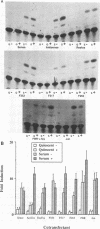
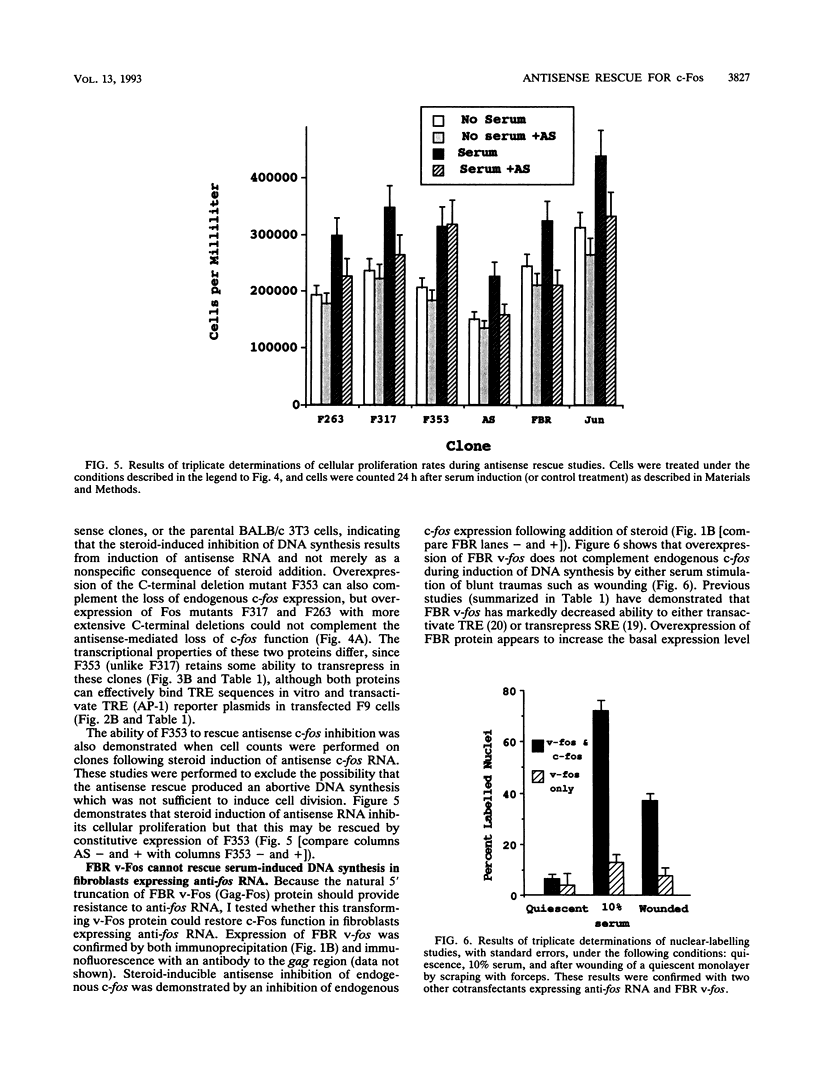
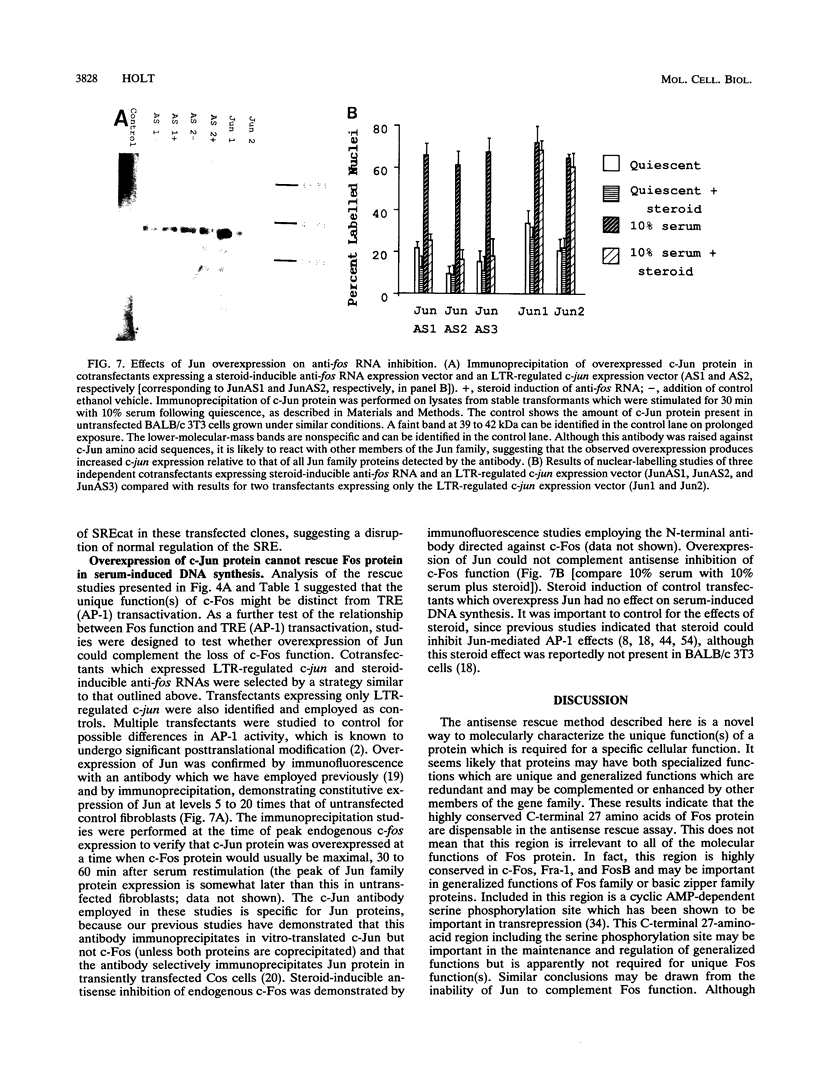
Serum induces the expression of a number of proteins with similar transcriptional properties, including those encoded by the proto-oncogenes c-fos and c-jun. This study employs a novel antisense rescue method to determine whether antisense-resistant genes (constructed by deletion of antisense RNA target sequences) can replace c-fos expression during serum-induced DNA synthesis. Immunoprecipitation studies and nuclease protection assays demonstrated that anti-fos RNA inhibited endogenous c-fos expression but did not inhibit expression of transfected antisense-resistant mutant c-fos genes. The results of nuclear-labelling and cellular-proliferation studies indicated that C terminally truncated Fos mutants, including FBR v-fos, could not rescue endogenous Fos, although full-length and minimally truncated c-fos expression vectors could restore serum-induced DNA synthesis in cells expressing anti-fos RNA. Overexpression of c-Jun protein (Jun) could not restore serum-induced DNA synthesis to cells expressing inducible anti-fos RNA despite equivalent transactivation of an AP-1 target gene. Thus, the antisense rescue method defines a specialized function for c-Fos protein which is distinct from the function(s) of Jun and/or transforming FBR v-Fos proteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. Transcriptional regulation by Fos and Jun in vitro: interaction among multiple activator and regulatory domains. Mol Cell Biol. 1991 Jul;11(7):3624–3632. doi: 10.1128/mcb.11.7.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Ballard D. W., Walker W. H., Doerre S., Sista P., Molitor J. A., Dixon E. P., Peffer N. J., Hannink M., Greene W. C. The v-rel oncogene encodes a kappa B enhancer binding protein that inhibits NF-kappa B function. Cell. 1990 Nov 16;63(4):803–814. doi: 10.1016/0092-8674(90)90146-6. [DOI] [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Field S. J., Johnson R. S., Mortensen R. M., Papaioannou V. E., Spiegelman B. M., Greenberg M. E. Growth and differentiation of embryonic stem cells that lack an intact c-fos gene. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9306–9310. doi: 10.1073/pnas.89.19.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franza B. R., Jr, Rauscher F. J., 3rd, Josephs S. F., Curran T. The Fos complex and Fos-related antigens recognize sequence elements that contain AP-1 binding sites. Science. 1988 Mar 4;239(4844):1150–1153. doi: 10.1126/science.2964084. [DOI] [PubMed] [Google Scholar]
- Gentz R., Rauscher F. J., 3rd, Abate C., Curran T. Parallel association of Fos and Jun leucine zippers juxtaposes DNA binding domains. Science. 1989 Mar 31;243(4899):1695–1699. doi: 10.1126/science.2494702. [DOI] [PubMed] [Google Scholar]
- Gius D., Cao X. M., Rauscher F. J., 3rd, Cohen D. R., Curran T., Sukhatme V. P. Transcriptional activation and repression by Fos are independent functions: the C terminus represses immediate-early gene expression via CArG elements. Mol Cell Biol. 1990 Aug;10(8):4243–4255. doi: 10.1128/mcb.10.8.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T., Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
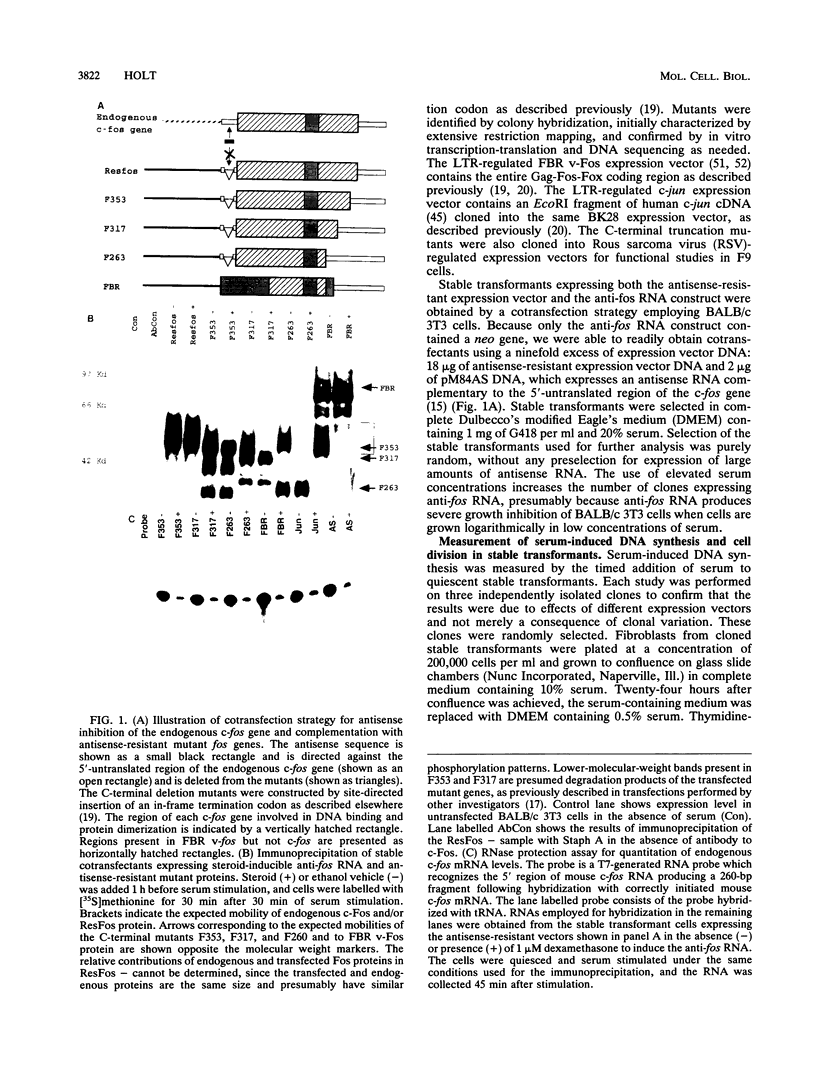
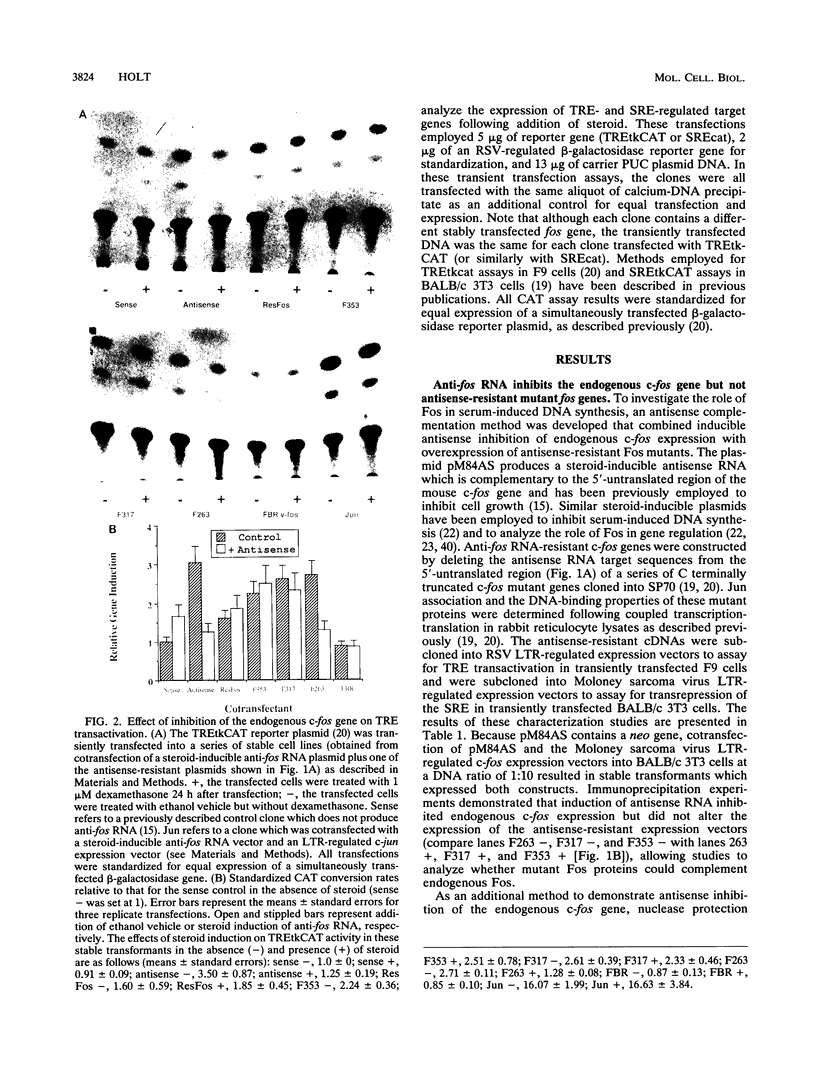
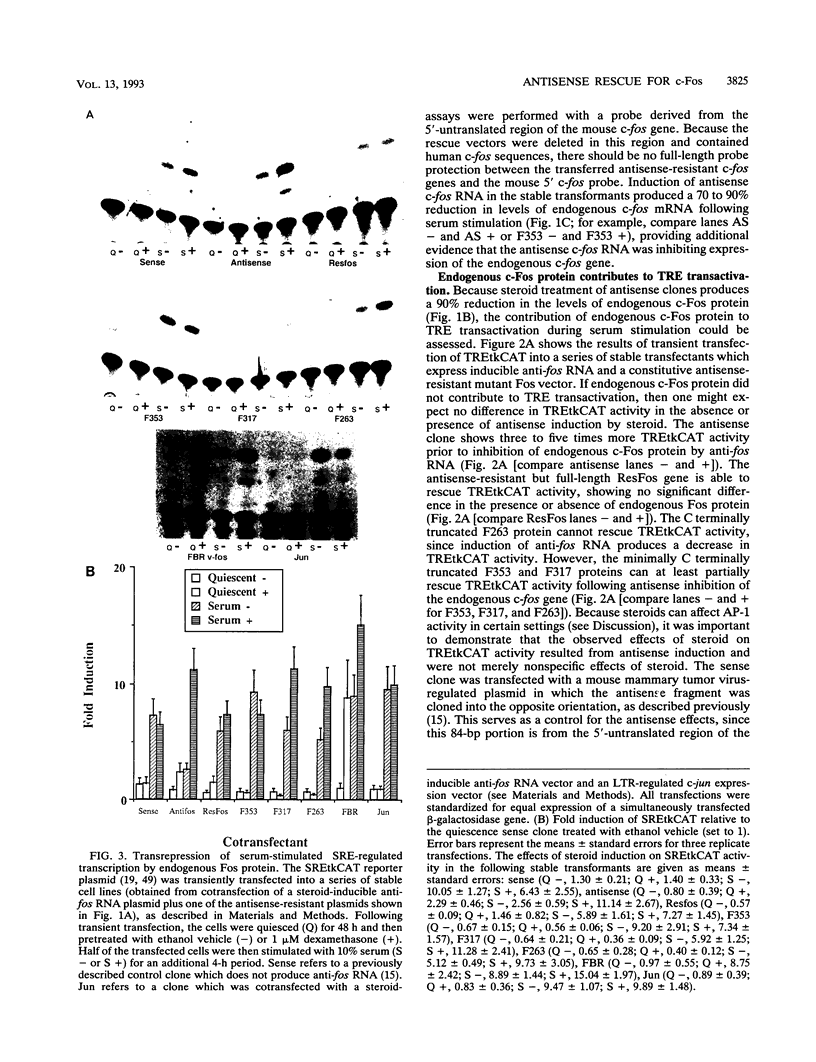
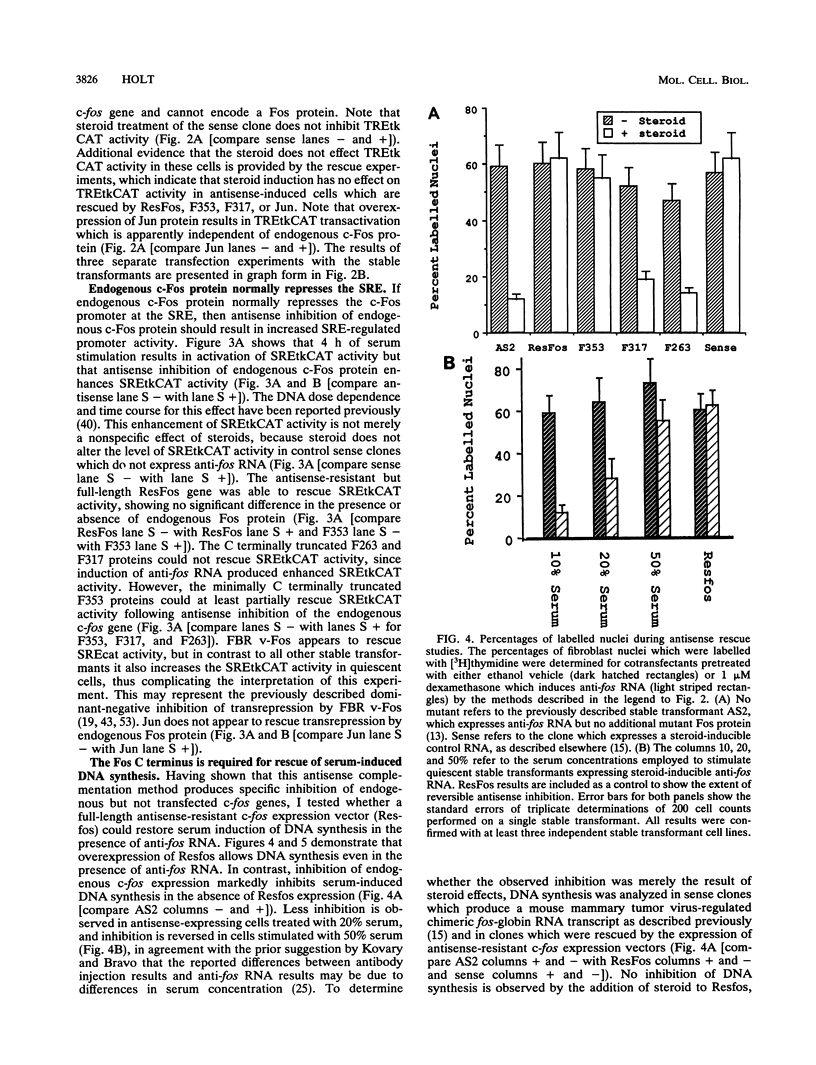
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue J., Kerr L. D., Ransone L. J., Bengal E., Hunter T., Verma I. M. c-rel activates but v-rel suppresses transcription from kappa B sites. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3715–3719. doi: 10.1073/pnas.88.9.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T., Müller R. Structure-function analysis of fos protein: a single amino acid change activates the immortalizing potential of v-fos. Cell. 1987 Feb 27;48(4):647–657. doi: 10.1016/0092-8674(87)90243-1. [DOI] [PubMed] [Google Scholar]
- Johnson R. S., Spiegelman B. M., Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992 Nov 13;71(4):577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kamata N., Jotte R. M., Holt J. T. Myristylation alters DNA-binding activity and transactivation of FBR (gag-fos) protein. Mol Cell Biol. 1991 Feb;11(2):765–772. doi: 10.1128/mcb.11.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerppola T. K., Curran T. Fos-Jun heterodimers and Jun homodimers bend DNA in opposite orientations: implications for transcription factor cooperativity. Cell. 1991 Jul 26;66(2):317–326. doi: 10.1016/0092-8674(91)90621-5. [DOI] [PubMed] [Google Scholar]
- Kerr L. D., Holt J. T., Matrisian L. M. Growth factors regulate transin gene expression by c-fos-dependent and c-fos-independent pathways. Science. 1988 Dec 9;242(4884):1424–1427. doi: 10.1126/science.2462278. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Denhez F., Kim K. Y., Holt J. T., Sporn M. B., Roberts A. B. Activation of the second promoter of the transforming growth factor-beta 1 gene by transforming growth factor-beta 1 and phorbol ester occurs through the same target sequences. J Biol Chem. 1989 Nov 15;264(32):19373–19378. [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf U., Büscher M., Schönthal A., Rahmsdorf H. J., Herrlich P. Autoregulation of fos: the dyad symmetry element as the major target of repression. EMBO J. 1989 Sep;8(9):2559–2566. doi: 10.1002/j.1460-2075.1989.tb08394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Macgregor P. F., Abate C., Curran T. Direct cloning of leucine zipper proteins: Jun binds cooperatively to the CRE with CRE-BP1. Oncogene. 1990 Apr;5(4):451–458. [PubMed] [Google Scholar]
- Meijlink F., Curran T., Miller A. D., Verma I. M. Removal of a 67-base-pair sequence in the noncoding region of protooncogene fos converts it to a transforming gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4987–4991. doi: 10.1073/pnas.82.15.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakabeppu Y., Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991 Feb 22;64(4):751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988 Dec 2;55(5):907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizawa M., Goto N., Kawai S. An avian transforming retrovirus isolated from a nephroblastoma that carries the fos gene as the oncogene. J Virol. 1987 Dec;61(12):3733–3740. doi: 10.1128/jvi.61.12.3733-3740.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Kim P. S. Evidence that the leucine zipper is a coiled coil. Science. 1989 Jan 27;243(4890):538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- Ofir R., Dwarki V. J., Rashid D., Verma I. M. Phosphorylation of the C terminus of Fos protein is required for transcriptional transrepression of the c-fos promoter. Nature. 1990 Nov 1;348(6296):80–82. doi: 10.1038/348080a0. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Sambucetti L. C., Curran T., Distel R. J., Spiegelman B. M. Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell. 1988 Feb 12;52(3):471–480. doi: 10.1016/s0092-8674(88)80039-4. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Voulalas P. J., Franza B. R., Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988 Dec;2(12B):1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
- Riabowol K. T., Vosatka R. J., Ziff E. B., Lamb N. J., Feramisco J. R. Microinjection of fos-specific antibodies blocks DNA synthesis in fibroblast cells. Mol Cell Biol. 1988 Apr;8(4):1670–1676. doi: 10.1128/mcb.8.4.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M., Sheng M., Greenberg M. E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990 Feb;4(2):255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Robinson-Benion C., Kamata N., Holt J. T. Antisense mapping of the c-fos promoter: role of the serum response element. Antisense Res Dev. 1991 Spring;1(1):21–33. doi: 10.1089/ard.1991.1.21. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Lamph W. W., Kamps M., Verma I. M. fos-associated cellular p39 is related to nuclear transcription factor AP-1. Cell. 1988 Aug 12;54(4):553–560. doi: 10.1016/0092-8674(88)90077-3. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Herrlich P., Rahmsdorf H. J., Ponta H. Requirement for fos gene expression in the transcriptional activation of collagenase by other oncogenes and phorbol esters. Cell. 1988 Jul 29;54(3):325–334. doi: 10.1016/0092-8674(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Schütte J., Minna J. D., Birrer M. J. Deregulated expression of human c-jun transforms primary rat embryo cells in cooperation with an activated c-Ha-ras gene and transforms rat-1a cells as a single gene. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2257–2261. doi: 10.1073/pnas.86.7.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. E., Frasch S., Nordheim A. Repression of c-fos transcription is mediated through p67SRF bound to the SRE. EMBO J. 1989 Sep;8(9):2567–2574. doi: 10.1002/j.1460-2075.1989.tb08395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. E., Schröter H., Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Rauscher F. J., 3rd, Morgan J. I., Curran T. Regulation of proenkephalin by Fos and Jun. Science. 1989 Dec 22;246(4937):1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- Subramaniam M., Schmidt L. J., Crutchfield C. E., 3rd, Getz M. J. Negative regulation of serum-responsive enhancer elements. Nature. 1989 Jul 6;340(6228):64–66. doi: 10.1038/340064a0. [DOI] [PubMed] [Google Scholar]
- Turner R., Tjian R. Leucine repeats and an adjacent DNA binding domain mediate the formation of functional cFos-cJun heterodimers. Science. 1989 Mar 31;243(4899):1689–1694. doi: 10.1126/science.2494701. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Enami S., Curran T., Verma I. M. FBR murine osteosarcoma virus. II. Nucleotide sequence of the provirus reveals that the genome contains sequences acquired from two cellular genes. Virology. 1984 May;135(1):229–243. doi: 10.1016/0042-6822(84)90133-8. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Fos C-terminal mutations block down-regulation of c-fos transcription following serum stimulation. EMBO J. 1988 Dec 20;7(13):4193–4202. doi: 10.1002/j.1460-2075.1988.tb03316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yen J., Wisdom R. M., Tratner I., Verma I. M. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5077–5081. doi: 10.1073/pnas.88.12.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- Zerial M., Toschi L., Ryseck R. P., Schuermann M., Müller R., Bravo R. The product of a novel growth factor activated gene, fos B, interacts with JUN proteins enhancing their DNA binding activity. EMBO J. 1989 Mar;8(3):805–813. doi: 10.1002/j.1460-2075.1989.tb03441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]