Abstract
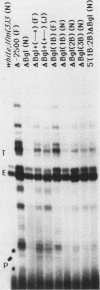
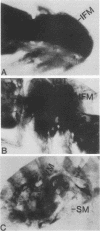
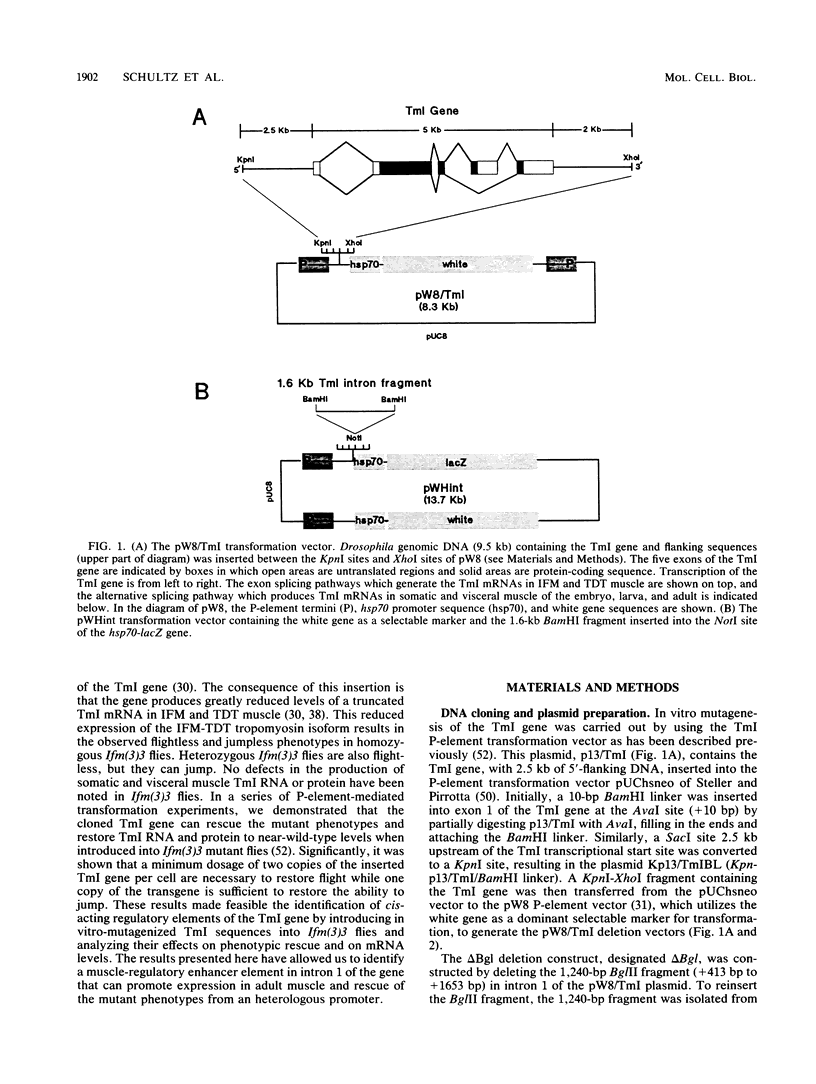
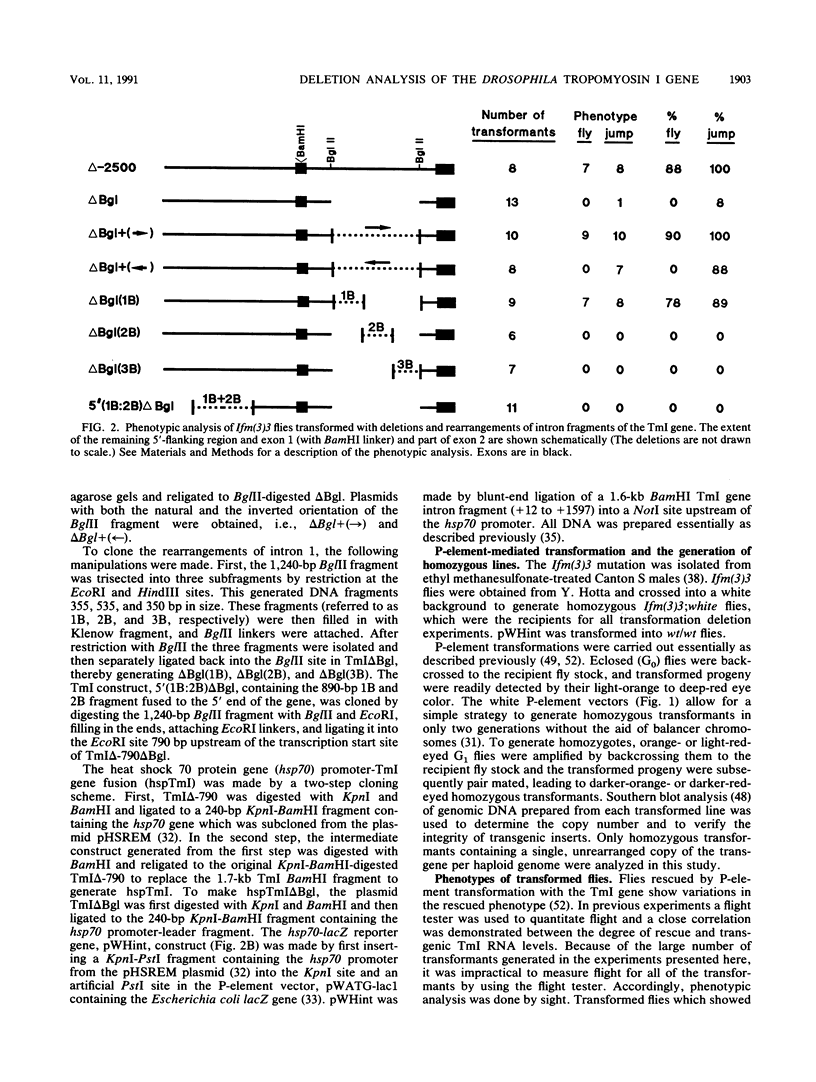
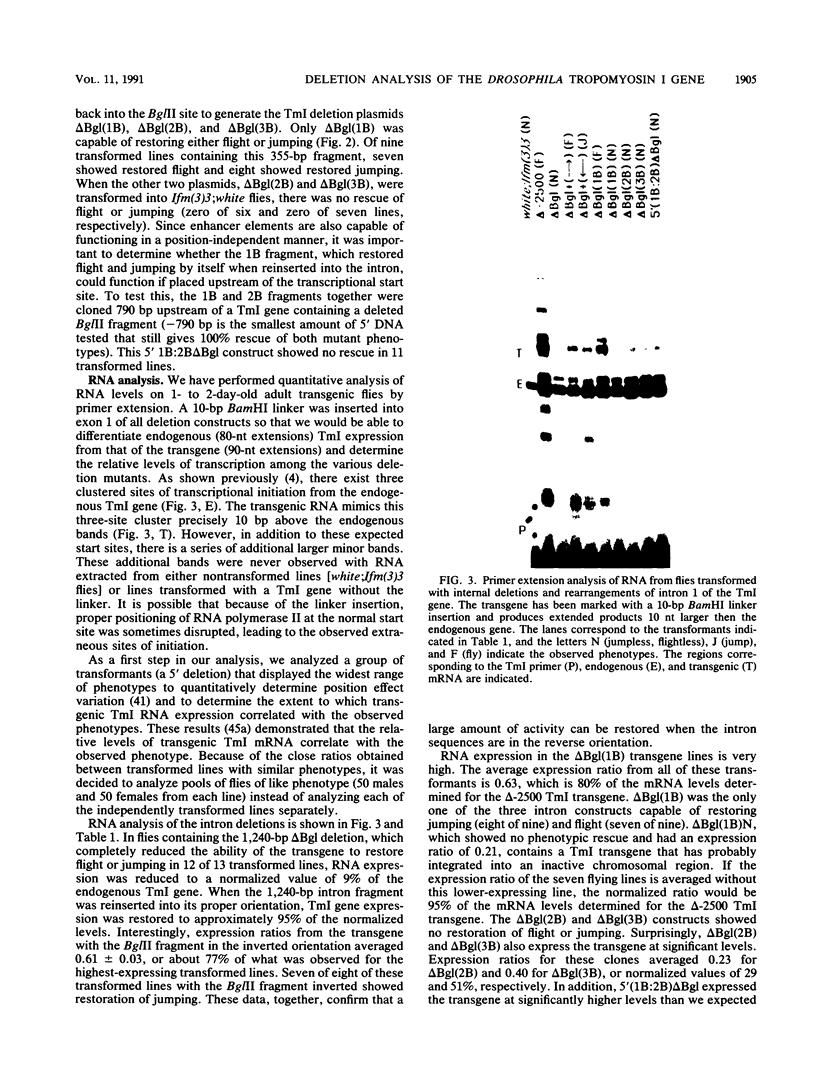
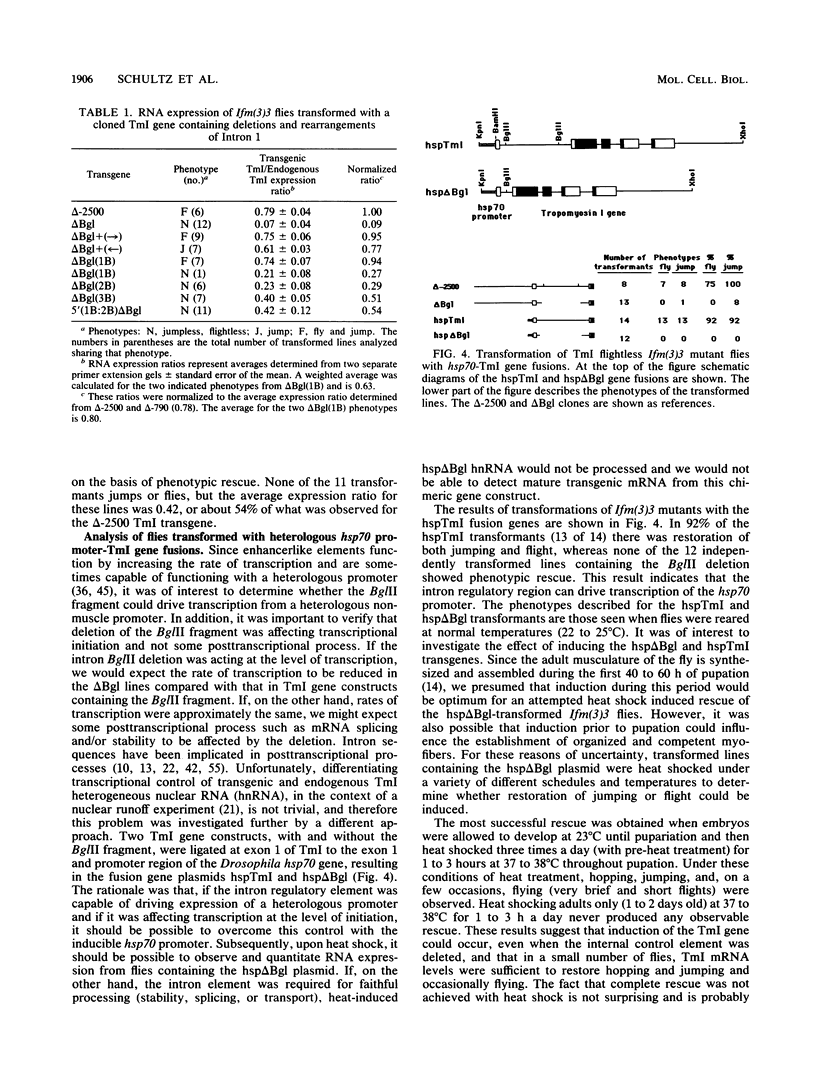
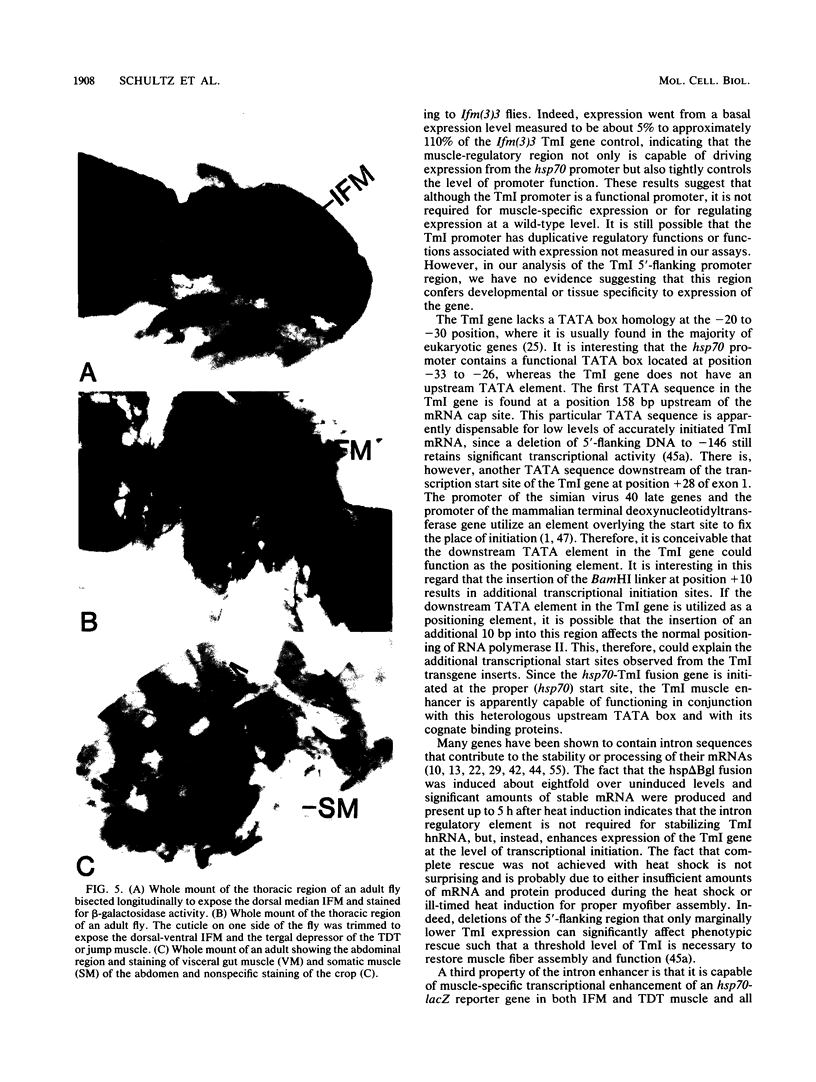
The control of expression of the Drosophila melanogaster tropomyosin I (TmI) gene has been investigated by P-element transformation and rescue of the flightless and jumpless TmI mutant strain, Ifm(3)3. To localize cis-acting DNA sequences that control TmI gene expression, Ifm(3)3 flies were transformed with P-element plasmids containing various deletions and rearrangements of the TmI gene. The effects of these mutations on TmI gene expression were studied by analyzing both the extent of rescue of the Ifm(3)3 mutant phenotypes and determining TmI RNA levels in the transformed flies by primer extension analysis. The results of our analysis indicate that a region located within intron 1 of the gene is necessary and sufficient for directing muscle-specific TmI expression in the adult fly. This intron region has characteristics of a muscle regulatory enhancer element that can function in conjunction with the heterologous nonmuscle hsp70 promoter to promote rescue of the mutant phenotypes and to direct expression of an hsp70-Escherichia coli lacZ reporter gene in adult muscle. The enhancer can be subdivided further into two domains of activity based on primer extension analysis of TmI mRNA levels and on the rescue of mutant phenotypes. One of the intron domains is required for expression in the indirect flight muscle of the adult. The function of the second domain is unknown, but it could regulate the level of expression or be required for expression in other muscle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayer D. E., Dynan W. S. Simian virus 40 major late promoter: a novel tripartite structure that includes intragenic sequences. Mol Cell Biol. 1988 May;8(5):2021–2033. doi: 10.1128/mcb.8.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Kittler E. L., Emerson C. P., Jr Structure, evolution, and regulation of a fast skeletal muscle troponin I gene. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8080–8084. doi: 10.1073/pnas.82.23.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi G. S., Boardman M., Storti R. V. Alternative splicing of a Drosophila tropomyosin gene generates muscle tropomyosin isoforms with different carboxy-terminal ends. Mol Cell Biol. 1984 Dec;4(12):2828–2836. doi: 10.1128/mcb.4.12.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi G. S., Storti R. V. Structure and DNA sequence of the tropomyosin I gene from Drosophila melanogaster. J Biol Chem. 1986 Jan 15;261(2):817–827. [PubMed] [Google Scholar]
- Bautch V. L., Storti R. V., Mischke D., Pardue M. L. Organization and expression of Drosophila tropomyosin genes. J Mol Biol. 1982 Dec 5;162(2):231–250. doi: 10.1016/0022-2836(82)90524-1. [DOI] [PubMed] [Google Scholar]
- Beall C. J., Sepanski M. A., Fyrberg E. A. Genetic dissection of Drosophila myofibril formation: effects of actin and myosin heavy chain null alleles. Genes Dev. 1989 Feb;3(2):131–140. doi: 10.1101/gad.3.2.131. [DOI] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Liska D. J., Apone S., Devarayalu S. Interactions between the promoter and first intron are involved in transcriptional control of alpha 1(I) collagen gene expression. Mol Cell Biol. 1988 Nov;8(11):4851–4857. doi: 10.1128/mcb.8.11.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein P., McKay J., Morishima J. K., Devarayalu S., Gelinas R. E. Regulatory elements in the first intron contribute to transcriptional control of the human alpha 1(I) collagen gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8869–8873. doi: 10.1073/pnas.84.24.8869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvagnet P. F., Strehler E. E., White G. E., Strehler-Page M. A., Nadal-Ginard B., Mahdavi V. Multiple positive and negative 5' regulatory elements control the cell-type-specific expression of the embryonic skeletal myosin heavy-chain gene. Mol Cell Biol. 1987 Dec;7(12):4377–4389. doi: 10.1128/mcb.7.12.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawerman G. Determinants of messenger RNA stability. Cell. 1987 Jan 16;48(1):5–6. doi: 10.1016/0092-8674(87)90346-1. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cohen J. B., Levinson A. D. A point mutation in the last intron responsible for increased expression and transforming activity of the c-Ha-ras oncogene. Nature. 1988 Jul 14;334(6178):119–124. doi: 10.1038/334119a0. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Deak I. I. Mutations of Drosophila melanogaster that affect muscles. J Embryol Exp Morphol. 1977 Aug;40:35–63. [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Fambrough D. M. Control of acetylcholine receptors in skeletal muscle. Physiol Rev. 1979 Jan;59(1):165–227. doi: 10.1152/physrev.1979.59.1.165. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Peachey L. D. Striated muscle-contractile and control mechanisms. J Cell Biol. 1981 Dec;91(3 Pt 2):166s–186s. doi: 10.1083/jcb.91.3.166s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A., Hinz U., Renkawitz-Pohl R. Intron and upstream sequences regulate expression of the Drosophila beta 3-tubulin gene in the visceral and somatic musculature, respectively. Proc Natl Acad Sci U S A. 1989 May;86(9):3215–3218. doi: 10.1073/pnas.86.9.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerster T., Picard D., Schaffner W. During B-cell differentiation enhancer activity and transcription rate of immunoglobulin heavy chain genes are high before mRNA accumulation. Cell. 1986 Apr 11;45(1):45–52. doi: 10.1016/0092-8674(86)90536-2. [DOI] [PubMed] [Google Scholar]
- Ghogawala Z., Choi E., Daly K. R., Blanco L. R., Griffith I. J., Glimcher L. H. An intronic 10-base-pair deletion in a class II A beta gene affects RNA processing. Mol Cell Biol. 1989 Oct;9(10):4402–4408. doi: 10.1128/mcb.9.10.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Reddy V. B., Swinscoe J., Lebowitz P., Weissman S. M. Heterogeneity and 5'-terminal structures of the late RNAs of simian virus 40. J Mol Biol. 1978 Dec 25;126(4):813–846. doi: 10.1016/0022-2836(78)90022-0. [DOI] [PubMed] [Google Scholar]
- Glaser R. L., Wolfner M. F., Lis J. T. Spatial and temporal pattern of hsp26 expression during normal development. EMBO J. 1986 Apr;5(4):747–754. doi: 10.1002/j.1460-2075.1986.tb04277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke P. D., Storti R. V. The Drosophila melanogaster tropomyosin II gene produces multiple proteins by use of alternative tissue-specific promoters and alternative splicing. Mol Cell Biol. 1988 Sep;8(9):3591–3602. doi: 10.1128/mcb.8.9.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward L. J., Schwartz R. J. Sequential expression of chicken actin genes during myogenesis. J Cell Biol. 1986 Apr;102(4):1485–1493. doi: 10.1083/jcb.102.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochschild A., Ptashne M. Interaction at a distance between lambda repressors disrupts gene activation. Nature. 1988 Nov 24;336(6197):353–357. doi: 10.1038/336353a0. [DOI] [PubMed] [Google Scholar]
- Jaynes J. B., Johnson J. E., Buskin J. N., Gartside C. L., Hauschka S. D. The muscle creatine kinase gene is regulated by multiple upstream elements, including a muscle-specific enhancer. Mol Cell Biol. 1988 Jan;8(1):62–70. doi: 10.1128/mcb.8.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlik C. C., Fyrberg E. A. An insertion within a variably spliced Drosophila tropomyosin gene blocks accumulation of only one encoded isoform. Cell. 1985 May;41(1):57–66. doi: 10.1016/0092-8674(85)90061-3. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Weber U., Gehring W. J. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 1987 May 26;15(10):3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipple D. C., Marsella-Herrick P. Versatile plasmid vectors for the construction, analysis and heat-inducible expression of hybrid genes in eukaryotic cells. Nucleic Acids Res. 1988 Aug 11;16(15):7748–7748. doi: 10.1093/nar/16.15.7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn R., Schäfer U., Schäfer M. pW-ATG-lac, P-element vectors for lacZ transcriptional gene fusions in Drosophila. Nucleic Acids Res. 1988 May 11;16(9):4163–4163. doi: 10.1093/nar/16.9.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. Y., Dechesne C. A., Eldridge J., Paterson B. M. An avian muscle factor related to MyoD1 activates muscle-specific promoters in nonmuscle cells of different germ-layer origin and in BrdU-treated myoblasts. Genes Dev. 1989 Jul;3(7):986–996. doi: 10.1101/gad.3.7.986. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Nadal-Ginard B. Transcriptional and cell cycle-mediated regulation of myosin heavy chain gene expression during muscle cell differentiation. J Biol Chem. 1983 Sep 25;258(18):11063–11073. [PubMed] [Google Scholar]
- Mogami K., Hotta Y. Isolation of Drosophila flightless mutants which affect myofibrillar proteins of indirect flight muscle. Mol Gen Genet. 1981;183(3):409–417. doi: 10.1007/BF00268758. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Bishop J. O. Changes in the mRNA population of chick myoblasts during myogenesis in vitro. Cell. 1977 Nov;12(3):751–765. doi: 10.1016/0092-8674(77)90275-6. [DOI] [PubMed] [Google Scholar]
- Peterson D. O., Beifuss K. K., Morley K. L. Context-dependent gene expression: cis-acting negative effects of specific procaryotic plasmid sequences on eucaryotic genes. Mol Cell Biol. 1987 Apr;7(4):1563–1567. doi: 10.1128/mcb.7.4.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piechaczyk M., Blanchard J. M., Marty L., Dani C., Panabieres F., El Sabouty S., Fort P., Jeanteur P. Post-transcriptional regulation of glyceraldehyde-3-phosphate-dehydrogenase gene expression in rat tissues. Nucleic Acids Res. 1984 Sep 25;12(18):6951–6963. doi: 10.1093/nar/12.18.6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Sanchez F., Tobin S. L., Rdest U., Zulauf E., McCarthy B. J. Two Drosophila actin genes in detail. Gene structure, protein structure and transcription during development. J Mol Biol. 1983 Feb 5;163(4):533–551. doi: 10.1016/0022-2836(83)90111-0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. J., Rothblum K. N. Gene switching in myogenesis: differential expression of the chicken actin multigene family. Biochemistry. 1981 Jul 7;20(14):4122–4129. doi: 10.1021/bi00517a027. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Transposition of cloned P elements into Drosophila germ line chromosomes. Science. 1982 Oct 22;218(4570):341–347. doi: 10.1126/science.6289435. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. A transposable P vector that confers selectable G418 resistance to Drosophila larvae. EMBO J. 1985 Jan;4(1):167–171. doi: 10.1002/j.1460-2075.1985.tb02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. A., Spizz G., Perry W. M., Vizard D., Weil T., Olson E. N. Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. Mol Cell Biol. 1988 Jul;8(7):2896–2909. doi: 10.1128/mcb.8.7.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansey T., Mikus M. D., Dumoulin M., Storti R. V. Transformation and rescue of a flightless Drosophila tropomyosin mutant. EMBO J. 1987 May;6(5):1375–1385. doi: 10.1002/j.1460-2075.1987.tb02378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yost H. J., Lindquist S. RNA splicing is interrupted by heat shock and is rescued by heat shock protein synthesis. Cell. 1986 Apr 25;45(2):185–193. doi: 10.1016/0092-8674(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Young H. A., Varesio L., Hwu P. Posttranscriptional control of human gamma interferon gene expression in transfected mouse fibroblasts. Mol Cell Biol. 1986 Jun;6(6):2253–2256. doi: 10.1128/mcb.6.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yutzey K. E., Kline R. L., Konieczny S. F. An internal regulatory element controls troponin I gene expression. Mol Cell Biol. 1989 Apr;9(4):1397–1405. doi: 10.1128/mcb.9.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]