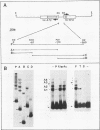
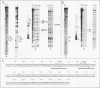
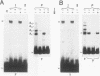
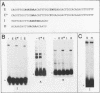
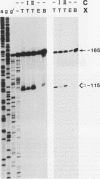
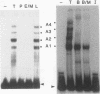
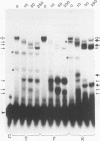
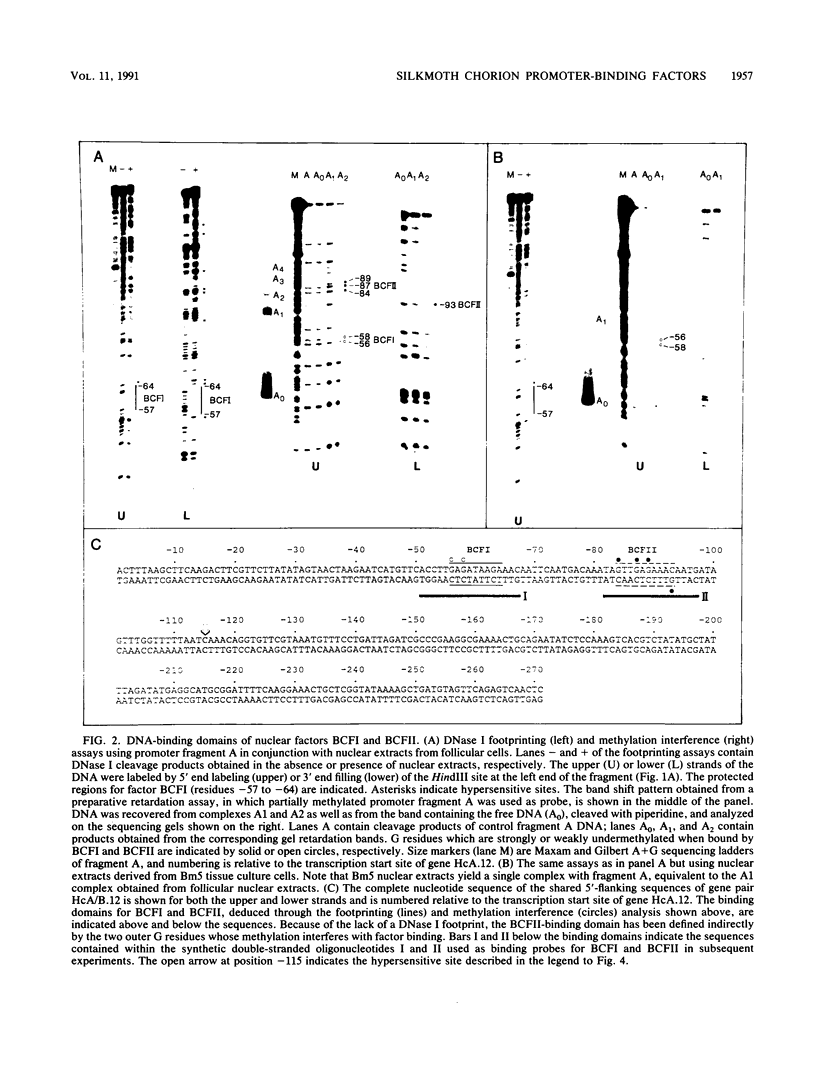
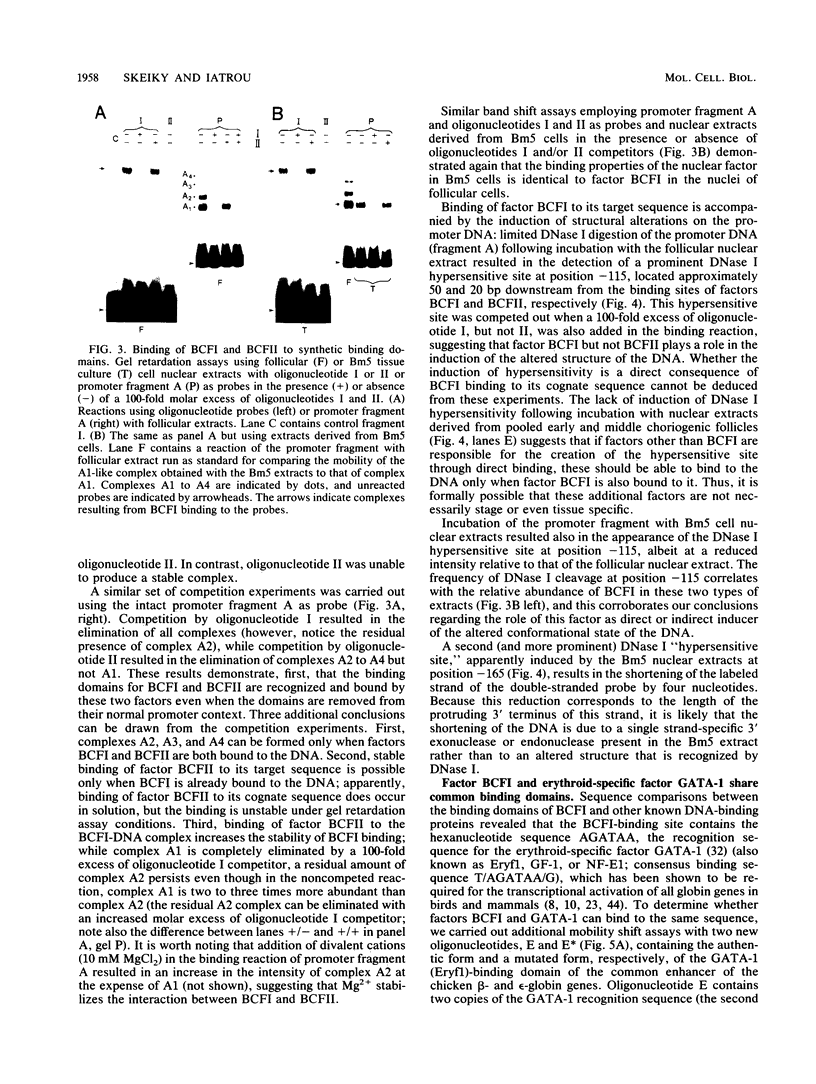
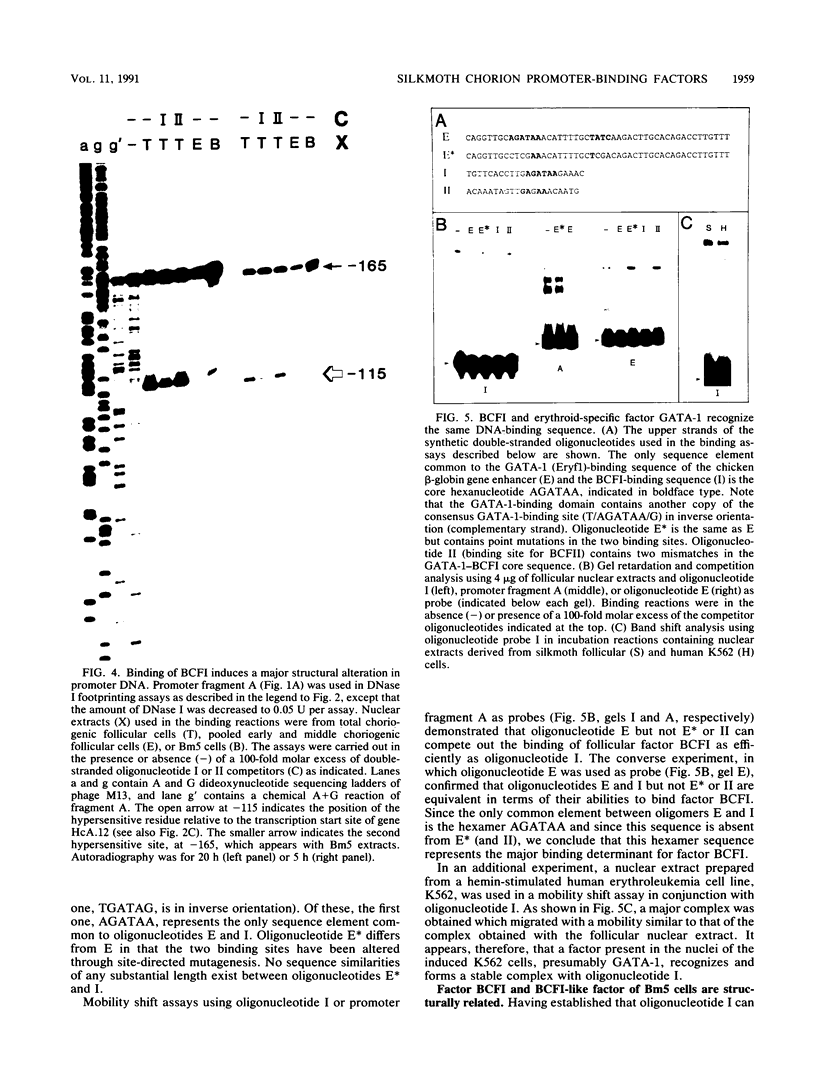
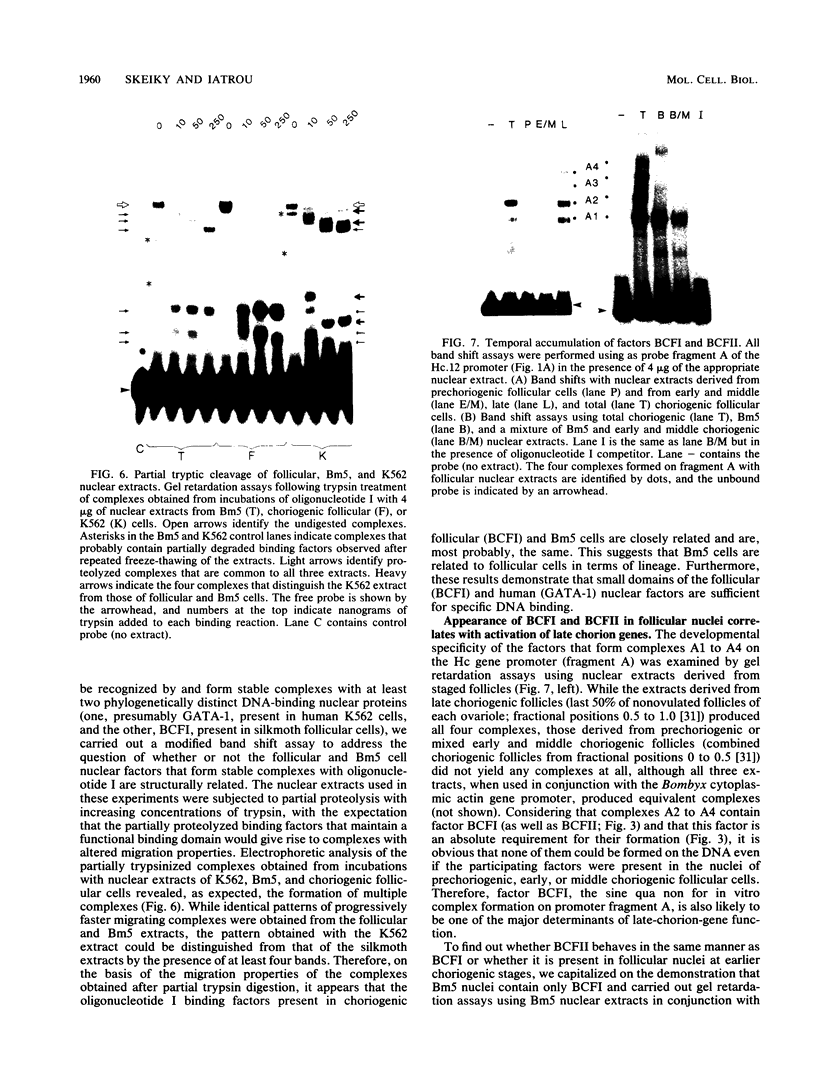
Abstract
Two DNA-binding proteins, BCFI and BCFII, that interact with defined promoter sequences of silkmoth chorion genes of late developmental specificity appear in the nuclei of follicular cells at a time that coincides with the transcriptional activation of the corresponding genes. BCFI prebinding is shown to be indispensable for stable binding of BCFII to its cognate sequence. BCFI and BCFII synergism requires a relatively stringent stereospecific alignment and is a prerequisite for the assembly of higher-order protein-promoter DNA complexes containing additional factors, which are neither gene (stage) nor class (chorion) specific. Binding of BCFI to its site correlates with the induction of DNA structural perturbations that may facilitate assembly of additional factors on the promoter. The BCFI-binding domain contains a core hexanucleotide sequence, AGATAA, which represents the major binding determinant of the erythroid-specific transcription factor GATA-1 of higher vertebrates. This sequence is shown to be necessary and sufficient for binding of BCFI, as it is for a factor that is present in induced K562 human erythroleukemic cells, presumably GATA-1. Comparative analyses of mobility shift patterns obtained with partially proteolyzed preparations of these two unrelated factors were used to confirm that a BCFI-like chorion promoter-binding protein, which is present in the nuclei of an established silkmoth cell line derived from ovarian tissue, is in fact BCFI. The transcriptional repression of endogenous chorion genes in this cell line coupled with the documented absence of factor BCFII suggests that the synergistic interactions between these two factors constitute a minimum requirement for late chorion gene expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Burke W. D., Eickbush T. H. The silkmoth late chorion locus. I. Variation within two paired multigene families. J Mol Biol. 1986 Aug 5;190(3):343–356. doi: 10.1016/0022-2836(86)90006-9. [DOI] [PubMed] [Google Scholar]
- Chodosh L. A., Olesen J., Hahn S., Baldwin A. S., Guarente L., Sharp P. A. A yeast and a human CCAAT-binding protein have heterologous subunits that are functionally interchangeable. Cell. 1988 Apr 8;53(1):25–35. doi: 10.1016/0092-8674(88)90484-9. [DOI] [PubMed] [Google Scholar]
- Eickbush T. H., Burke W. D. Silkmoth chorion gene families contain patchwork patterns of sequence homology. Proc Natl Acad Sci U S A. 1985 May;82(9):2814–2818. doi: 10.1073/pnas.82.9.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickbush T. H., Kafatos F. C. A walk in the chorion locus of Bombyx mori. Cell. 1982 Jun;29(2):633–643. doi: 10.1016/0092-8674(82)90179-9. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galson D. L., Housman D. E. Detection of two tissue-specific DNA-binding proteins with affinity for sites in the mouse beta-globin intervening sequence 2. Mol Cell Biol. 1988 Jan;8(1):381–392. doi: 10.1128/mcb.8.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M. R., Clermont-Rattner E. Organization of the chorion genes of Bombyx mori, a multigene family. III. Detailed marker composition of three gene clusters. Genetics. 1980 Sep;96(1):201–212. doi: 10.1093/genetics/96.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace T. D. Establishment of a line of cells from the silkworm Bombyx mori. Nature. 1967 Nov 11;216(5115):613–613. doi: 10.1038/216613a0. [DOI] [PubMed] [Google Scholar]
- Hibner B. L., Burke W. D., Lecanidou R., Rodakis G. C., Eickbush T. H. Organization and expression of three genes from the silkmoth early chorion locus. Dev Biol. 1988 Feb;125(2):423–431. doi: 10.1016/0012-1606(88)90223-0. [DOI] [PubMed] [Google Scholar]
- Iatrou K., Tsitilou S. G. Coordinately expressed chorion genes of Bombyx mori: is developmental specificity determined by secondary structure recognition? EMBO J. 1983;2(9):1431–1440. doi: 10.1002/j.1460-2075.1983.tb01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iatrou K., Tsitilou S. G., Kafatos F. C. DNA sequence transfer between two high-cysteine chorion gene families in the silkmoth Bombyx mori. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4452–4456. doi: 10.1073/pnas.81.14.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Kafatos F. C. Coordinately expressed members of two chorion multi-gene families are clustered, alternating and divergently orientated. Nature. 1980 Apr 17;284(5757):635–638. doi: 10.1038/284635a0. [DOI] [PubMed] [Google Scholar]
- Jones N. Transcriptional regulation by dimerization: two sides to an incestuous relationship. Cell. 1990 Apr 6;61(1):9–11. doi: 10.1016/0092-8674(90)90207-u. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Carner K. R., Masiarz F. R., Tjian R. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell. 1987 Dec 24;51(6):1079–1090. doi: 10.1016/0092-8674(87)90594-0. [DOI] [PubMed] [Google Scholar]
- Lecanidou R., Rodakis G. C., Eickbush T. H., Kafatos F. C. Evolution of the silk moth chorion gene superfamily: gene families CA and CB. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6514–6518. doi: 10.1073/pnas.83.17.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Mitsialis S. A., Kafatos F. C. Regulatory elements controlling chorion gene expression are conserved between flies and moths. Nature. 1985 Oct 3;317(6036):453–456. doi: 10.1038/317453a0. [DOI] [PubMed] [Google Scholar]
- Mitsialis S. A., Spoerel N., Leviten M., Kafatos F. C. A short 5'-flanking DNA region is sufficient for developmentally correct expression of moth chorion genes in Drosophila. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7987–7991. doi: 10.1073/pnas.84.22.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsialis S. A., Veletza S., Kafatos F. C. Transgenic regulation of moth chorion gene promoters in Drosophila: tissue, temporal, and quantitative control of four bidirectional promoters. J Mol Evol. 1989 Dec;29(6):486–495. doi: 10.1007/BF02602920. [DOI] [PubMed] [Google Scholar]
- Mounier N., Prudhomme J. C. Isolation of actin genes in Bombyx mori: the coding sequence of a cytoplasmic actin gene expressed in the silk gland is interrupted by a single intron in an unusual position. Biochimie. 1986 Sep;68(9):1053–1061. doi: 10.1016/s0300-9084(86)80179-1. [DOI] [PubMed] [Google Scholar]
- Nadel M. R., Kafatos F. C. Specific protein synthesis in cellular differentiation. IV. The chorion proteins of Bombyx mori and their program of synthesis. Dev Biol. 1980 Mar;75(1):26–40. doi: 10.1016/0012-1606(80)90141-4. [DOI] [PubMed] [Google Scholar]
- Nussinov R. Sequence signals in eukaryotic upstream regions. Crit Rev Biochem Mol Biol. 1990;25(3):185–224. doi: 10.3109/10409239009090609. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Peterson C. L., Calame K. L. Complex protein binding within the mouse immunoglobulin heavy-chain enhancer. Mol Cell Biol. 1987 Dec;7(12):4194–4203. doi: 10.1128/mcb.7.12.4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodakis G. C., Kafatos F. C. Origin of evolutionary novelty in proteins: how a high-cysteine chorion protein has evolved. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3551–3555. doi: 10.1073/pnas.79.11.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Identification of a novel lymphoid specific octamer binding protein (OTF-2B) by proteolytic clipping bandshift assay (PCBA). EMBO J. 1988 Dec 20;7(13):4221–4229. doi: 10.1002/j.1460-2075.1988.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea M. J., King D. L., Conboy M. J., Mariani B. D., Kafatos F. C. Proteins that bind to Drosophila chorion cis-regulatory elements: a new C2H2 zinc finger protein and a C2C2 steroid receptor-like component. Genes Dev. 1990 Jul;4(7):1128–1140. doi: 10.1101/gad.4.7.1128. [DOI] [PubMed] [Google Scholar]
- Skeiky Y. A., Iatrou K. Silkmoth chorion antisense RNA. Structural characterization, developmental regulation and evolutionary conservation. J Mol Biol. 1990 May 5;213(1):53–66. doi: 10.1016/S0022-2836(05)80121-4. [DOI] [PubMed] [Google Scholar]
- Spoerel N. A., Nguyen H. T., Eickbush T. H., Kafatos F. C. Gene evolution and regulation in the chorion complex of Bombyx mori. Hybridization and sequence analysis of multiple developmentally middle A/B chorion gene pairs. J Mol Biol. 1989 Sep 5;209(1):1–19. doi: 10.1016/0022-2836(89)90166-6. [DOI] [PubMed] [Google Scholar]
- Spoerel N., Nguyen H. T., Kafatos F. C. Gene regulation and evolution in the chorion locus of Bombyx mori. Structural and developmental characterization of four eggshell genes and their flanking DNA regions. J Mol Biol. 1986 Jul 5;190(1):23–35. doi: 10.1016/0022-2836(86)90072-0. [DOI] [PubMed] [Google Scholar]
- Struhl K. The DNA-binding domains of the jun oncoprotein and the yeast GCN4 transcriptional activator protein are functionally homologous. Cell. 1987 Sep 11;50(6):841–846. doi: 10.1016/0092-8674(87)90511-3. [DOI] [PubMed] [Google Scholar]
- Trainor C. D., Evans T., Felsenfeld G., Boguski M. S. Structure and evolution of a human erythroid transcription factor. Nature. 1990 Jan 4;343(6253):92–96. doi: 10.1038/343092a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Wall L., deBoer E., Grosveld F. The human beta-globin gene 3' enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988 Sep;2(9):1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- Zon L. I., Tsai S. F., Burgess S., Matsudaira P., Bruns G. A., Orkin S. H. The major human erythroid DNA-binding protein (GF-1): primary sequence and localization of the gene to the X chromosome. Proc Natl Acad Sci U S A. 1990 Jan;87(2):668–672. doi: 10.1073/pnas.87.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]