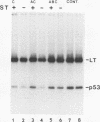
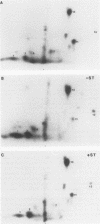
Abstract
Simian virus 40 (SV40) large-T antigen and the cellular protein p53 were phosphorylated in vivo by growing cells in the presence of 32Pi. The large-T/p53 complex was isolated by immunoprecipitation and used as a substrate for protein phosphatase 2A (PP2A) consisting of the catalytic subunit (C) and the two regulatory subunits, A and B. Three different purified forms of PP2A, including free C, the AC form, and the ABC form, could readily dephosphorylate both proteins. With both large-T and p53, the C subunit was most active, followed by the AC form, which was more active than the ABC form. The activity of all three forms of PP2A toward these proteins was strongly stimulated by manganese ions and to a lesser extent by magnesium ions. The presence of complexed p53 did not affect the dephosphorylation of large-T antigen by PP2A. The dephosphorylation of individual phosphorylation sites of large-T and p53 were determined by two-dimensional peptide mapping. Individual sites within large-T and p53 were dephosphorylated at different rates by all three forms of PP2A. The phosphates at Ser-120 and Ser-123 of large-T, which affect binding to the origin of SV40 DNA, were removed most rapidly. Three of the six major phosphopeptides of p53 were readily dephosphorylated, while the remaining three were relatively resistant to PP2A. Dephosphorylation of most of the sites in large-T and p53 by the AC form was inhibited by SV40 small-t antigen. The inhibition was most apparent for those sites which were preferentially dephosphorylated. Inhibition was specific for the AC form; no effect was observed on the dephosphorylation of either protein by the free C subunit or the ABC form. The inhibitory effect of small-t on dephosphorylation by PP2A could explain its role in transformation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Bauer M., Guhl E., Graessmann M., Graessmann A. Cellular mutation mediates T-antigen-positive revertant cells resistant to simian virus 40 transformation but not to retransformation by polyomavirus and adenovirus type 2. J Virol. 1987 Jun;61(6):1821–1827. doi: 10.1128/jvi.61.6.1821-1827.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikel I., Montano X., Agha M. E., Brown M., McCormack M., Boltax J., Livingston D. M. SV40 small t antigen enhances the transformation activity of limiting concentrations of SV40 large T antigen. Cell. 1987 Jan 30;48(2):321–330. doi: 10.1016/0092-8674(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Bischoff J. R., Friedman P. N., Marshak D. R., Prives C., Beach D. Human p53 is phosphorylated by p60-cdc2 and cyclin B-cdc2. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4766–4770. doi: 10.1073/pnas.87.12.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., Beales N., Shenk T., Berg P., di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci U S A. 1978 May;75(5):2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M., McCormack M., Zinn K. G., Farrell M. P., Bikel I., Livingston D. M. A recombinant murine retrovirus for simian virus 40 large T cDNA transforms mouse fibroblasts to anchorage-independent growth. J Virol. 1986 Oct;60(1):290–293. doi: 10.1128/jvi.60.1.290-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Chang L. S., Pater M. M., Hutchinson N. I., di Mayorca G. Transformation by purified early genes of simian virus 40. Virology. 1984 Mar;133(2):341–353. doi: 10.1016/0042-6822(84)90400-8. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Choi Y. W., Lee I. C., Ross S. R. Requirement for the simian virus 40 small tumor antigen in tumorigenesis in transgenic mice. Mol Cell Biol. 1988 Aug;8(8):3382–3390. doi: 10.1128/mcb.8.8.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- Cohen P. The structure and regulation of protein phosphatases. Annu Rev Biochem. 1989;58:453–508. doi: 10.1146/annurev.bi.58.070189.002321. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- Feunteun J., Kress M., Gardes M., Monier R. Viable deletion mutants in the simian virus 40 early region. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4455–4459. doi: 10.1073/pnas.75.9.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Graessmann A., Graessmann M., Tjian R., Topp W. C. Simian virus 40 small-t protein is required for loss of actin cable networks in rat cells. J Virol. 1980 Mar;33(3):1182–1191. doi: 10.1128/jvi.33.3.1182-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grässer F. A., Scheidtmann K. H., Tuazon P. T., Traugh J. A., Walter G. In vitro phosphorylation of SV40 large T antigen. Virology. 1988 Jul;165(1):13–22. doi: 10.1016/0042-6822(88)90653-8. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausing K., Scheidtmann K. H., Baumann E. A., Knippers R. Effects of in vitro dephosphorylation on DNA-binding and DNA helicase activities of simian virus 40 large tumor antigen. J Virol. 1988 Apr;62(4):1258–1265. doi: 10.1128/jvi.62.4.1258-1265.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M., Resche-Rigon M., Feunteun J. Phosphorylation pattern of large T antigens in mouse cells infected by simian virus 40 wild type or deletion mutants. J Virol. 1982 Sep;43(3):761–771. doi: 10.1128/jvi.43.3.761-771.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegler M., Perez C. F., Hardy C., Botchan M. Transformation mediated by the SV40 T antigens: separation of the overlapping SV40 early genes with a retroviral vector. Cell. 1984 Sep;38(2):483–491. doi: 10.1016/0092-8674(84)90503-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeken M., Bikel I., Livingston D. M., Brady J. trans-activation of RNA polymerase II and III promoters by SV40 small t antigen. Cell. 1988 Dec 23;55(6):1171–1177. doi: 10.1016/0092-8674(88)90261-9. [DOI] [PubMed] [Google Scholar]
- MacArthur H., Walter G. Monoclonal antibodies specific for the carboxy terminus of simian virus 40 large T antigen. J Virol. 1984 Nov;52(2):483–491. doi: 10.1128/jvi.52.2.483-491.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- McVey D., Brizuela L., Mohr I., Marshak D. R., Gluzman Y., Beach D. Phosphorylation of large tumour antigen by cdc2 stimulates SV40 DNA replication. Nature. 1989 Oct 12;341(6242):503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- Meek D. W., Eckhart W. Phosphorylation of p53 in normal and simian virus 40-transformed NIH 3T3 cells. Mol Cell Biol. 1988 Jan;8(1):461–465. doi: 10.1128/mcb.8.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara K., Cao X. R., Yen A., Chandler S., Driscoll B., Murphree A. L., T'Ang A., Fung Y. K. Cell cycle-dependent regulation of phosphorylation of the human retinoblastoma gene product. Science. 1989 Dec 8;246(4935):1300–1303. doi: 10.1126/science.2588006. [DOI] [PubMed] [Google Scholar]
- Mohr I. J., Stillman B., Gluzman Y. Regulation of SV40 DNA replication by phosphorylation of T antigen. EMBO J. 1987 Jan;6(1):153–160. doi: 10.1002/j.1460-2075.1987.tb04733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallas D. C., Shahrik L. K., Martin B. L., Jaspers S., Miller T. B., Brautigan D. L., Roberts T. M. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990 Jan 12;60(1):167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- Raycroft L., Wu H. Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Figge J., Bladon M. T., Chen L. B., Ellman M., Bikel I., Farrell M., Livingston D. M. Role of small t antigen in the acute transforming activity of SV40. Cell. 1982 Sep;30(2):469–480. doi: 10.1016/0092-8674(82)90244-6. [DOI] [PubMed] [Google Scholar]
- Rundell K. Complete interaction of cellular 56,000- and 32,000-Mr proteins with simian virus 40 small-t antigen in productively infected cells. J Virol. 1987 Apr;61(4):1240–1243. doi: 10.1128/jvi.61.4.1240-1243.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Cox J. Simian virus 40 t antigen affects the sensitivity of cellular DNA synthesis to theophylline. J Virol. 1979 Apr;30(1):394–396. doi: 10.1128/jvi.30.1.394-396.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad A., Anderson C. W., Carroll R. B. Mapping of phosphomonoester and apparent phosphodiester bonds of the oncogene product p53 from simian virus 40-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):897–901. doi: 10.1073/pnas.83.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Buck M., Schneider J., Kalderon D., Fanning E., Smith A. E. Biochemical characterization of phosphorylation site mutants of simian virus 40 large T antigen: evidence for interaction between amino- and carboxy-terminal domains. J Virol. 1991 Mar;65(3):1479–1490. doi: 10.1128/jvi.65.3.1479-1490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Echle B., Walter G. Simian virus 40 large T antigen is phosphorylated at multiple sites clustered in two separate regions. J Virol. 1982 Oct;44(1):116–133. doi: 10.1128/jvi.44.1.116-133.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Haber A. Simian virus 40 large T antigen induces or activates a protein kinase which phosphorylates the transformation-associated protein p53. J Virol. 1990 Feb;64(2):672–679. doi: 10.1128/jvi.64.2.672-679.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Hardung M., Echle B., Walter G. DNA-binding activity of simian virus 40 large T antigen correlates with a distinct phosphorylation state. J Virol. 1984 Apr;50(1):1–12. doi: 10.1128/jvi.50.1.1-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Simian virus 40 small t antigen is not required for the maintenance of transformation but may act as a promoter (cocarcinogen) during establishment of transformation in resting rat cells. J Virol. 1979 Dec;32(3):979–988. doi: 10.1128/jvi.32.3.979-988.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T. Stepwise phosphorylation of the NH2-terminal region of the simian virus 40 large T antigen. J Biol Chem. 1984 Jul 10;259(13):8633–8640. [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Sompayrac L., Danna K. J. A simian virus 40 dl884/tsA58 double mutant is temperature sensitive for abortive transformation. J Virol. 1983 May;46(2):620–625. doi: 10.1128/jvi.46.2.620-625.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürzbecher H. W., Maimets T., Chumakov P., Brain R., Addison C., Simanis V., Rudge K., Philp R., Grimaldi M., Court W. p53 interacts with p34cdc2 in mammalian cells: implications for cell cycle control and oncogenesis. Oncogene. 1990 Jun;5(6):795–781. [PubMed] [Google Scholar]
- Takahashi T., Nau M. M., Chiba I., Birrer M. J., Rosenberg R. K., Vinocour M., Levitt M., Pass H., Gazdar A. F., Minna J. D. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989 Oct 27;246(4929):491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Van Roy F., Fransen L., Fiers W. Metabolic turnover of phosphorylation sites in simian virus 40 large T antigen. J Virol. 1983 Jan;45(1):442–446. doi: 10.1128/jvi.45.1.442-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup D. M., Kauffman M. G., Kelly T. J. Activation of SV40 DNA replication in vitro by cellular protein phosphatase 2A. EMBO J. 1989 Dec 1;8(12):3891–3898. doi: 10.1002/j.1460-2075.1989.tb08568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter G., Ruediger R., Slaughter C., Mumby M. Association of protein phosphatase 2A with polyoma virus medium tumor antigen. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2521–2525. doi: 10.1073/pnas.87.7.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. I., Lickteig R. L., Estes R., Rundell K., Walter G., Mumby M. C. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991 Apr;11(4):1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]