Abstract
Background
Adequate sedation is crucial to the management of children requiring assisted ventilation on Paediatric Intensive Care Units (PICU). The evidence-base of randomised controlled trials (RCTs) in this area is small and a trial was planned to compare midazolam and clonidine, two sedatives widely used within PICUs neither of which being licensed for that use. The application to obtain a Clinical Trials Authorisation from the Medicines and Healthcare products Regulatory Agency (MHRA) required a dossier summarising the safety profiles of each drug and the pharmacovigilance plan for the trial needed to be determined by this information. A systematic review was undertaken to identify reports relating to the safety of each drug.
Methodology/Principal Findings
The Summary of Product Characteristics (SmPC) were obtained for each sedative. The MHRA were requested to provide reports relating to the use of each drug as a sedative in children under the age of 16. Medline was searched to identify RCTs, controlled clinical trials, observational studies, case reports and series. 288 abstracts were identified for midazolam and 16 for clonidine with full texts obtained for 80 and 6 articles respectively. Thirty-three studies provided data for midazolam and two for clonidine. The majority of data has come from observational studies and case reports. The MHRA provided details of 10 and 3 reports of suspected adverse drug reactions.
Conclusions/Significance
No adverse reactions were identified in addition to those specified within the SmPC for the licensed use of the drugs. Based on this information and the wide spread use of both sedatives in routine practice the pharmacovigilance plan was restricted to adverse reactions. The Clinical Trials Authorisation was granted based on the data presented in the SmPC and the pharmacovigilance plan within the clinical trial protocol restricting collection and reporting to adverse reactions.
Introduction
Adequate sedation is crucial to the management of children requiring assisted ventilation on the Paediatric Intensive Care Unit (PICU). The level of sedation of post-operative infants has been shown to impact on subsequent morbidity, and even mortality [1], [2], [3]. Most critically ill children will require potent analgesic and sedative drugs to facilitate artificial ventilation, alleviate pain, prevent discomfort from procedures, and to prevent distress from the presence of unfamiliar personnel and from the high level of background noise, which can disturb natural sleeping patterns [4]. However, while under-sedation can result in significant morbidity, over- sedation can also be associated with distressing or dangerous adverse effects, which may be difficult to assess in critically ill children [5]. The SLEEPS trial (www.controlled-trials.com/ISRCTN02639863), a prospective multi-centre randomised, double-blind, equivalence study was designed to compare clonidine and midazolam as intravenous sedative agents in critically ill children requiring mechanical ventilation.
Midazolam, a benzodiazepine derivative, is the most commonly used sedative in critically ill children, both in the UK and abroad [6]. For sedation of the critically ill child it is usually given in combination with an opioid by intravenous infusion at doses between 50–300 micrograms/kg/hr [7]. It can also be administered via subcutaneous infusion, or via intravenous bolus. Acute effects of midazolam exposure through continuous infusion include adverse effects of the drug on the cardiovascular system [8], [9], while continuous exposure to midazolam over several days results in rapid development of tolerance and subsequent withdrawal phenomena [1].
Clonidine is an α-2 adrenergic agonist which has antihypertensive, analgesic and sedative effects. In recent years, the drug has become more frequently used as a sedative in critically ill children and is usually administered with an opioid by intravenous infusion or orally.
Midazolam and clonidine are used widely within paediatric intensive care but are not licensed for that use. As the conditions of use in the clinical trial differed from those licensed, the Clinical Trials Authorisation application to the Medicines and Healthcare products Regulatory Agency (MHRA) required that the Summary of Product Characteristics (SmPC) for its licensed use be complemented with a summary of relevant data supporting its use in the proposed clinical trial. The trial aimed to open to recruitment in 2009 therefore we aimed to include safety data up to 2008.
Within the application for funding for the SLEEPS trial the conclusions from a systematic review [10] were used to justify the need for the new trial. The need to use systematic reviews to avoid unnecessary new research is clear but research conducted on using systematic reviews in the design and planning of a new trial have indicated that their potential is not being optimised [11], [12], [13], [14].
The aim of this paper is to describe the systematic review of safety data included within the Clinical Trials Authorisation application associated with the continuous infusion of midazolam or clonidine as sedation for neonates, infants and older children requiring mechanical ventilation and to illustrate how this informed the pharmacovigilance plan for the SLEEPS trial.
Methods
All authors contributed to and agreed the protocol. Due to the variety of study designs, anticipated lack of direct comparisons, and required focus on safety profiles of each drug individually we did not plan to combine results within a meta-analysis or test for publication bias.
Criteria for considering studies for the review
We included Randomised Controlled Trials (RCTs), Controlled Clinical Trials (CCTs), observational studies and case reports or case series.
We included only studies assessing the safety of continuous intravenous infusion of midazolam or clonidine when used as sedation for mechanically ventilated children under the age of eighteen years. For the literature search the age limit was set to allow variability in definition of paediatric. Because this review targeted safety, we included all studies which described the active or passive monitoring of adverse effects in the methods and/or the absence or presence of adverse effects in the results. We included studies administering midazolam or clonidine either on their own or with a concomitant opioid, reflecting routine clinical practice. We excluded studies assessing the use of midazolam or clonidine via any route other than a continuous intravenous infusion. We excluded studies assessing the use of midazolam as sedation for procedures, or as an anticonvulsant.
Identification of studies
Prior to conducting the literature search to our knowledge there were no relevant published systematic reviews for clonidine and four relevant published systematic reviews [1], [10], [15], [16] for midazolam. Of these reviews, two related only to the use of midazolam in neonates [10], [15] and two were restricted to withdrawal symptoms [1], [16]. The Cochrane review assessing intravenous midazolam in neonates had been updated in 2002 [10].
MEDLINE was searched in September 2008. The search strategy is provided in Table 1. Two reviewers (IS and CS) independently screened each abstract identified and any queries were discussed with AW. Full texts of potentially eligible studies were obtained and their references screened to identify any additional eligible studies.
Table 1. Search Strategy.
| Database: Ovid MEDLINE(R) <1950 to August Week 4 2008> |
| Search Strategy: |
| 1 child$.mp. or child/(1500744) |
| 2 (paediatric$ or pediatr$).mp. (163973) |
| 3 infant/or infan$.mp. (864884) |
| 4 young$.mp. (304417) |
| 5 toddler.mp. (1117) |
| 6 bab$.mp. (54771) |
| 7 child,preschool/(625996) |
| 8 (preschool or pre-school).mp. (627837) |
| 9 adolesc$.mp. or adolescent/(1304128) |
| 10 teenage$.mp. (10507) |
| 11 youth$.mp. (21868) |
| 12 or/1–11 (2734500) |
| 13 (intensive care or critical care).mp. (90585) |
| 14 intensive care units, pediatric.mp. (2630) |
| 15 intensive care units, neonatal.mp. (6648) |
| 16 picu.mp. (929) |
| 17 p*ediatric intensive care.mp. (3149) |
| 18 “critically ill”.mp. (16329) |
| 19 ventilated.mp. (17171) |
| 20 ventilat$.mp. (106904) |
| 21 respiration, artificial.mp. (30372) |
| 22 positive pressure respiration.mp. (13060) |
| 23 intermittent positive pressure breathing.mp. (943) |
| 24 intermittent positive pressure ventilation.mp. (2397) |
| 25 (high frequency ventilation or high frequency oscillation).mp. (1912) |
| 26 high frequency positive pressure ventilation.mp. (127) |
| 27 (hfv or hfov).mp. (764) |
| 28 high frequency jet ventilation.mp. (1084) |
| 29 airway pressure release ventilation.mp. (78) |
| 30 aprv {No Related Terms} (56) |
| 31 continuous mandatory ventilation.mp. or continuous mandatory ventilation/(7) |
| 32 cmv.mp. (14725) |
| 33 intermittent mandatory ventilation.mp. (529) |
| 34 (synchron$ intermittent mandatory ventilation or simv).mp. (206) |
| 35 (pressure suppoprt ventilation or psv).mp. (1254) |
| 36 (extracorporeal membrane oxygenation or ecmo or oxygenation, extracorporeal membrane).mp. (4039) |
| 37 or/13–36 (223297) |
| 38 (intravenous or iv).mp. (467655) |
| 39 parenteral.mp. (63557) |
| 40 inject$.mp. (556405) |
| 41 infus$.mp. (224106) |
| 42 (infus$ or infusions, intravenous).mp. (224106) |
| 43 $venous.mp. (139547) |
| 44 or/38–43 (1144844) |
| 45 midazolam.mp. (7964) |
| 46 midazolam hydrochloride.mp. (64) |
| 47 midazolam maleate.mp. (26) |
| 48 (dormicum or flormidal or versed or hypnovel or dormonid).mp. (496) |
| 49 benzodiazepine.mp. or benzo$/(15817) |
| 50 or/45–49 (22992) |
| 51 (clonidine dihydrochloride or clonidine hydrochloride or clonidine monohydrobromide or clonidine monohydrochloride).mp. (200) |
| 52 (clofelin or clopheline or dixarit or gemiton or hemiton or catapres or catapres*an or clophazolin or colfenil or isoglaucon or klofelin or klonefil or m-5041t or st-155).mp. (464) |
| 53 (clonidine or clonidin$).mp. (15383) |
| 54 or/51–53 (15506) |
| 55 37 and 44 and 12 (8661) |
| 56 50 and 55 (288) |
| 57 53 and 55 (16) |
Authors of studies that did not report but may have collected relevant data were not contacted due to time constraints to submit the CTA application. An assessment was made about the likelihood of outcome reporting bias within those studies [17]. For studies that included data for adults and children, authors were contacted to obtain data for children alone.
The Summary of Product Characteristics (SmPC) were obtained (midazolam from F.Hoffmann-La Roche Ltd as midazolam was originally licensed by this company; clonidine from Boehringer Ingelheim UK sole UK manufacturer).
Information was requested from the MHRA under the Freedom of Information Act regarding reports of midazolam and clonidine being used as a sedative in children. The age limit was set to be 16 years, the upper age limit planned for the SLEEPS trial.
Data extraction
Data extraction was conducted by IS, CS, and CG. All extracted data were cross checked:
Study characteristics (design, age of participants, dose and duration of midazolam or clonidine therapy, concomitant sedative or opiate therapy);
Any data relating to the safety of these drugs, especially changes in blood pressure or heart rate on induction or cessation of midazolam or clonidine, withdrawal signs and symptoms, and negative effects on other organ function. The adverse effects were subsequently categorised into those relating to cardiovascular effects, withdrawal effects, and any other effects;
Assessment of methodological quality
For each RCT and observational study we evaluated how rigorously the adverse event data were ascertained by assessing whether adverse effects were actively or passively monitored. We also determined how well the methods were described and whether or not the adverse effects of interest were defined a priori. We examined each study to see if the authors of the study had assessed the likelihood that adverse events were related to the use of medication, in other words whether they were considered to be Adverse Drug Reactions (ADRs). If an assessment of causality was made, we examined whether this was done using an existing, validated tool, such as that described by Naranjo [18]. We also assessed whether authors distinguished between midazolam or clonidine and a concomitant medication as being the cause of a suspected ADR.
In addition, for each RCT we assessed the adequacy of randomisation, allocation concealment and masking of interventions, and in order to assess the quality of reporting we evaluated whether all children who had received midazolam or clonidine were included in the safety analysis. We made no assessment of the quality of case reports or case series, other than whether or not causality had been assessed.
Results
Results of the search
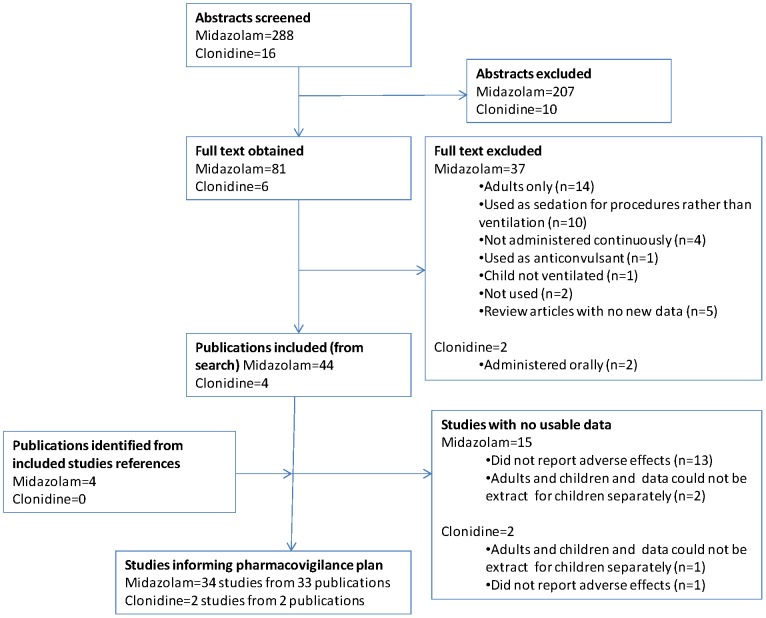
The results of the search are provided in Figure 1. Table 2 gives details of excluded studies for which full texts were obtained together with descriptions of studies that did not report but may have collected relevant data.
Figure 1. Study identification flow diagram.
Table 2. Details of excluded studies and included studies without usable data.
| Category | Study | Comments |
| Midazolam | ||
| Adults only | Aitkenhead 1989 [58] | |
| Bourgoin 2003 [59] | ||
| Cernaianu 1996 [60] | ||
| Chamorro 1996 [61] | ||
| Costa 1994 [62] | ||
| Deo 1994 [63] | ||
| Dirksen 1987 [64] | ||
| Flogel 1993 [65] | ||
| Lee 2007 [66] | ||
| Lescot 2007 [67] | ||
| Searle 1997 [68] | ||
| Sinclair 1988 [69] | ||
| Stewart 1999 [70] | ||
| Tanigami 1997 [71] | ||
| Used as sedation for a procedure rather than ventilation | Bitar 2003 [72] | This study assesses the use of sedation for plastic surgery procedures rather than as sedation on PICU |
| Djurberg 2002 [73] | The study assesses anesthesia for procedures rather than sedation for mechanical ventilation. | |
| Hertzog 1999 [74] | This study assesses anesthesia for procedures rather than sedation for mechanical ventilation | |
| Hickey 1992 [75] | Midazolam not administered as a sedative in PICU but for a procedure in this study | |
| Koroglu 2005 [76] | Dexmedetomidine and midazolam were administered for sedation during an MRI rather than as sedation on PICU | |
| Laussen 1995 [77] | Although midazolam infused alongside other medications, it is for a procedure rather than a continuous infusion | |
| Malagon 2005 [78] | Midazolam is used as procedural anaesthesia rather than sedation | |
| Somri 2007 [79] | Midazolam administered for a procedure rather than as sedation and children not ventilated. | |
| Wilson 2003 [80] | Midazolam is used as procedural anaesthesia rather than sedation | |
| Yldzdas 2004 [81] | Midazolam is used as procedural anaesthesia rather than sedation | |
| Not administered continuously | Gruber 2001 [82] | Midazolam was infused continuously during surgery but was only bolused once on PICU |
| Harte 1997 [8] | Midazolam was administered by bolus rather than continuous infusion in this study | |
| Van Straaten 1992 [9] | Midazolam administered as a bolus rather than a continuous infusion | |
| Hartvig 1993 [83] | The study assessed the suitability of constant rate infusions of ketamine as a sedative agent supplemented with intermittent doses of midazolam. Not administered continuously | |
| Used as anticonvulsant | Anand 1992 [3] | Midazolam not administered as a sedative but as a treatment for status epilepticus in this study. |
| Not ventilated | Prins 2005 [84] | Patient in the study were not ventilated |
| Not used | De Cosmo 2005 [85] | Assesses the role of propofol in Paediatric Intensive Care Unit (PICU) rather than midazolam. |
| Walker 2006 [86] | Study started after midazolam ceased. This study examined the use of dexmedetomidine after the failure of the standard regimen of opioids and benzodiazepines | |
| Review article | Aranda 2005 [15] | Review article |
| Ista 2007 [16] | Review article | |
| Ng 2003 [10] | Review article | |
| Notterman 1997 [87] | Review article | |
| Wolf 1994 [5] | This is a commentary | |
| No usable data | Al-Samsam 2005 [88] | This study examines the impact of environmental factors on PICU on quantity and architecture of sleep and did not assess the efficacy or safety of midazolam. 11 included patients were intubated, mechanically ventilated, and sedated with morphine and midazolam infusions. |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Ambrose 2000 [51] | The study assesses the adverse effects of clonidine. Ventilated children were given a background infusion of midazolam combined with a variable clonidine infusion. Reports adequacy of sedation and adverse effects on cardiovascular performance. Study is included within clonidine review but considered no usable data for midazolam. | |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Buck 2008 [89] | This study assesses the efficacy and safety of Dexmedetomidine rather than midazolam. Dexmedetomidine was started to minimize the use of midazolam before extubation or in patients who could not tolerate midazolam. | |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| DeBerry 2005 [90] | The study is a survey of pain and sedation medications in patients on extracorporeal membrane oxygenation. The survey did not ask questions relating to efficacy or safety of the drugs used. A 6 point likert scale ranging from not effective (1) to desired effect(6) was applied. This was a general score of clinician experience across patients and did not apply to individual patients. | |
| Midazolam was reported to be most effective drug administered. | ||
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Enomoto 2006 [91] | This was a case study involving prolonged use of dexmedetomidine on failure of midazolam with fentanyl to achieve adequate sedation and did not relate to adverse effects of midazolam. Study captured data after sedation with midazolam had ceased but midazolam reintroduced as an anti convulsant. | |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Jin 2007 [92] | This paper assesses the use of the COMFORT score. Midazolam was administered to 5 patients and inconjuction with fentanyl to 26 patients. Data on withdrawal symptoms were collected but reported for the comparison of use of the comfort or not rather than by sedatives. | |
| Likelihood of relevant safety is low and risk of outcome reporting bias low as sedative types were not the focus of the paper. | ||
| Kadilak 2004 [93] | The study assesses the safety of long-term intubation rather than the safety of drugs used for sedation by a retrospective 9-year review of children who required mechanical ventilatory support for at least 7 consecutive days. Of 98 children 76 (78%) were on midazolam infusions. Study aim did not require measurement of adverse event data. | |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Lugo 1999 [94] | The aim of the study was to assess costs in PICU and not to assess the adverse effects of midazolam. Midazolam infusion was continued until the hourly midazolam requirement was stable for at least 24 hrs. Thereafter, patients with a nasojejunal tube who were likely to require a minimum of three additional days of continuous sedation were transitioned from intravenous midazolam to enterally administered lorazepam. | |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Playfor 2000 [95] | This study did not assess the adverse effects of midazolam, it assessed the quality of sedation with continuous intravenous midazolam and morphine with additional oral sedation using chloral hydrate and antihistamines. | |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Rigby-Jones 2007 [96] | This study aimed to determine the pharmacokinetics of remifentanil in children requiring ventilation after cardiac surgery. Ventilated children were sedated with a fixed rate infusion of midazolam and a remifentanil infusion. | |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Saha 2006 [97] | The retrospective study aimed to describe which drugs were being used during transfer of critically ill children by retrieval teams. | |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Schmidt 2006 [98] | The study prospectively matched pairs of mechanically ventilated neonates under total parenteral nutrition and midazolam sedation. The aim was to evaluate fentanyl side effects on the neonatal bladder. All 40 patients received midazolam then one group received continuous fentanyl infusions aswell with the other group serving as controls. | |
| Reported use of the COMFOPRT and Hartwig scores indicates measurement of efficacy. Paper reports potential gastrointestinal side effects. Likelihood of relevant data for efficacy and safety is high but risk of outcome reporting bias low as use of fentanyl on the gall bladder and gastrointestinal side effects were aim of the paper. | ||
| Vernon 2000 [99] | This study assessed the effects of neuromuscular blockade drugs on oxygen consumption and energy expenditure. All patients were sedated using continuous infusions of midazolam and/or fentanyl. | |
| Likelihood of relevant safety data is low with low risk of outcome reporting bias. | ||
| Adults and children and data could not be extract for children separately | Prause 2000 [100] | Although children were included in this study, they were not analysed as a separate group therefore we are cannot determine if any adverse effects occurred in children. The authors were contacted to obtain paediatric specific data. No response received. |
| Shelly 1991 [101] | Although children were included in this study, they were not analysed as a separate group therefore we are cannot determine if any adverse effects occurred in children. The authors were contacted to obtain paediatric specific data. No response received. | |
| Clonidine | ||
| Administered orally | Artman 1983 [102] | Clonidine was administered orally rather than intravenously |
| Tanaka 1995 [103] | Clonidine was administered orally rather than intravenously | |
| Adults and children and data could not be extract for children separately | Prause 2000 [100] | Although children were included in this study, they were not analysed as a separate group therefore we are cannot determine if any adverse effects occurred in children. The authors were contacted to obtain paediatric specific data. No response received. |
| No usable data | Jenkins 2007 [6] | The aim of this study was to investigate the current practice of sedation, analgesia, and neuromuscular blockade in critically ill children on paediatric intensive care units. The study is included in the midazolam review but excluded for clonidine. It reports 12 patients received i.v. clonidine, and it is likely that withdrawal symptoms were recorded but not reported due to the small size of the clonidine sample. The paper reports on the use of clonidine to treat withdrawal symptoms. |
MIDAZOLAM
Thirty-four studies from 33 publications were eligible for inclusion and provided usable data [6], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50](See Figure 1).
The MHRA provided details of 10 reports of suspected ADRs.
CLONIDINE
Two studies from two publications were eligible for inclusion and provided usable data. [51], [52]
The MHRA provided details of three reports of suspected ADRs.
Characteristics of included studies
Summary details of eligible studies which did not report measuring adverse events are provided in Table 2.
RCTs and CCTs
No RCTs were identified for clonidine. Seven RCTs were identified for midazolam [19], [20], [21], [22], [23], [24], [25], of which 5 were set on Neonatal Intensive Care Units [19], [20], [22], [23], [25], and two on Paediatric Intensive Care Units [21], [24]. In all but two of the studies [19], [20] patients received midazolam and concomitant opiates, either as continuous infusion or on an intermittent basis. Table S1 describes the study characteristics.
One CCT of clonidine set on a Paediatric Intensive Care Unit was identified [51] and is summarised in Table S2.
Observational studies
Seventeen cohort studies, 13 prospective [6], [26], [27], [28], [29], [30], [33], [34], [36], [37], [39], [40], [50] and four retrospective [31], [32], [35], [38], were identified for midazolam. One study was based on a NICU [36] and the others were based on PICU. In all but three of these studies [32], [34], [37] midazolam was administered in conjunction with opiates and/or other sedative drugs. The characteristics are summarised in Table S1.
No observational studies were identified for clonidine.
Case series and case reports
Six case series [44], [45], [47], [48], [49] and 4 single case reports [41], [42], [43], [46] were identified for midazolam. Only two of the children described in all the case reports received midazolam without concomitant opiate sedative therapy [47], [48]. See Table S3.
One case report [52] was identified for clonidine and is described in Table S2. This case was reported as clonidine was being used for a new indication.
Information received from the MHRA
Five of the ten midazolam cases stated the duration of treatment which ranged from one day to 280 days but the information is unclear and suggests midazolam may have been administered as a bolus rather than a continuous infusion for some or all of the cases. In four of the cases, concomitant medications were administered alongside midazolam.
The following suspected adverse drug reactions were reported:
two convulsions,
two myoclonus,
one tremor and dyskinesia,
one hypoxia,
one psychomotor, withdrawal, confusional state and hallucination,
one withdrawal and dystonia,
one reported asthenia, muscle disorder and myoglobinuria and
one hepatic failure.
Three reports were received for clonidine. The duration of treatment was 1098 for the first case and one day for the following two cases. The infusion rate was unknown for the first case and 1 mcg/kg/hour and 0.7 ug/mg/hour in the other two cases. In one of the three cases, clonidine was administered with a concomitant muscle relaxant.
The following suspected adverse drug reactions were reported:
one hypertension,
one hypoventilation
one cardiac arrest and bradycardia.
The value of the data provided by the MHRA is limited as we do not have any information regarding the specific age groups involved, we do not know how many, if any, children were ventilated, and we do not know what duration this data has been collected over.
Methodological assessment of the studies
Risk of bias in RCTs
Table S4 provides details of the risk of bias assessment for each RCT. In summary across RCTs:
Quality of adverse effect monitoring
Table S4 provides details of the quality of adverse effect monitoring for each study with a summary across studies below.
Cardiovascular
Five RCTs actively monitored cardiovascular adverse effects of midazolam [19], [20], [21], [22], [25]. Of these two described their methods for monitoring haemodynamic parameters adequately [19], [25]. One study defined haemodynamic parameters a priori in physiological terms [19] and 2 defined hypotension in terms of whether patients required inotropic or volume support [22], [25]. Three of the studies reported all cardiovascular effects numerically by treatment group [19], [21], [25]. In one study [21] all patients who received midazolam were included in the safety analysis for cardiovascular adverse effects, in one study not all patients were included [19] and in the remaining studies this information was unclear.
Four prospective cohort studies actively monitored for cardiovascular adverse effects of midazolam, and the methods are clearly described [26], [33], [40], [50]. Of these, one [26] clearly defined tachycardia a priori but the other authors did not define abnormal haemodynamic parameters a priori.
Two retrospective studies assessed cardiovascular adverse events for midazolam, but it is neither clear how rigorously these were recorded in the medical case notes, nor how thoroughly they were sought by the investigators [35], [36]. A further two cohort studies, one prospective [30] and one retrospective [37], report on cardiovascular adverse events for midazolam but it is unclear whether these were monitored by active surveillance.
The CCT [51] actively monitored cardiovascular adverse effects of clonidine and described methods for haemodynamic assessment clearly. The study reported all cardiovascular effects numerically by treatment group but excluded two patients from one of the treatment groups as there was a failure to maintain adequate sedation.
Withdrawal
Of the 7 RCTs only 2 continued after the cessation of midazolam [23], [24] but did not actively monitor infants for signs of withdrawal. One [24] did not specify monitoring patients for signs of withdrawal, but did monitor for abnormal behaviour following cessation of the drug.
Five [6], [26], [27], [28], [34] prospective cohort studies actively monitored signs of withdrawal following cessation of midazolam. The methods used were clearly described, and three studies clearly defined the symptoms which would be considered suggestive of withdrawal [26], [28], [34].
Two other prospective cohort studies [29], [30] monitored for signs of withdrawal and three other studies [31], [37], [38] retrospectively assessed medical case notes for signs of withdrawal, but the methods were are not clearly described, and in the case of the retrospective studies it is unclear how thoroughly signs of withdrawal were documented in the notes in the first instance.
The identified CCT did not actively monitor infants for signs of withdrawal from clonidine infusion.
Neurological
Three RCTs actively monitored patients for neurological complications while receiving midazolam [22], [24], [25]. Of these only one study [24] described the methods used adequately, and this study was also the only one to define abnormal neurological findings a priori, describe neurological adverse effects numerically by treatment group, and specifically state that all patients who received midazolam were included in the safety analysis for neurological adverse effects.
Many studies that evaluated the risks of suffering withdrawal after midazolam infusions also assessed neurological symptoms as part of this assessment. Only two [35], [38] report on children with neurological symptoms not in the context of weaning or discontinuation of midazolam. Because these were retrospective case note reviews it is unclear how well the clinical data were recorded in the first instance. One neonatal study [23] assessed neurological outcomes in preterm infants, but it would appear that this assessment was related to the clinical efficacy of sedative regimes rather than direct consequences of using midazolam infusion.
The identified CCT [51] did not actively monitor patients for neurological complications while receiving clonidine infusion.
Prolonged sedation
One RCT [24] and two prospective cohort studies [34], [50] actively monitored children for prolonged sedation after discontinuation of midazolam infusion.
Metabolic/endocrine
One cohort study [40] prospectively assessed children receiving midazolam for altered hypothalamic-pituitary-adrenal axis function, and the authors clearly describe the cortisol stimulation test used. One study retrospectively assessed patients for metabolic acidosis and lipaemia [32]. This was done to assess the safety of propofol rather than midazolam.
Adverse events
Cardiovascular/respiratory
Two RCTs [19], [25] described some cardiovascular complications in children receiving midazolam infusion. Van Alfen van der velden [19] classed the cardiovascular complications as an ADR whereas Jacqz-Aigrain [25] did not attribute causality of the complications to the drug. One other RCT [20] also described some cardiovascular adverse effects, but classed them as not clinically significant and ‘transient’. Arya [22] and Tobias [21] did not observe adverse cardiovascular effects associated with midazolam infusion.
Van Alfen van der velden [19] described a decrease in cerebral blood flow after starting midazolam. Hypotension occurred in 7/21 preterm neonates who had received midazolam, and two of these infants required haemodynamic support. Jacqz-Aigrain [25] compared cardiovascular effects of midazolam compared with placebo in newborn infants and found that ‘heart rate and blood pressure were significantly lower in the midazolam group’, a difference which remained statistically significant until 48 hours. By day 5 the mean heart rate and blood pressure values were equal. 8 infants in the midazolam group required haemodynamic support [25]. The authors of this study did not classify these cardiovascular events as ADRs.
Three prospective cohort studies [26], [33], [36] describe some cardiovascular adverse effects of midazolam. Ista [26] states that hypotension was observed in “>13% of assessments” during weaning or after discontinuation of midazolam. Jacqz-Aigrain [36] describes hypotension in four newborn infants, in three of whom it occurred immediately after the initial bolus of midazolam, and in the fourth immediately after receiving a dose of fentanyl [36]. Shekerdemian [33] describes ‘transient’ haemodynamic changes in children receiving midazolam infusion [33]. One case report [46] describes a 40 month old child on continuous midazolam and opiate infusion who “experienced several episodes of hypotension” that required haemodynamic support.
One other case report [42] and two case series [44], [48] describe increase in heart rate and blood pressure associated with withdrawal from midazolam, rather than during the infusion. Biswas [43] reports a 6 month old child with investigations suggestive of myocardial ischaemia associated with tachycardia and hypertension from ‘Severe Benzodiazepine and Opioid Withdrawal’.
Of the seven studies that reported cardiovascular adverse effects, six of the studies appeared to classify the effects as being related to midazolam whereas one did not [25].
The CCT [51] did not observe any adverse cardiovascular effects associated with clonidine infusion and stated that “bradycardia and hypotension were not recorded in any patient”.
The case report [52] stated that “haemodynamic parameters were not adversely affected” by clonidine infusion.
Withdrawal and behavioural
Withdrawal from midazolam was not monitored or reported in any of the 7 RCTs.
Six prospective cohort studies report possible withdrawal symptoms after continuous infusion with midazolam [6], [26], [27], [28], [34] and all were considered to be ADRs. Ista [26] reports that, in 79 patients who received midazolam infusion as sedation and were observed a total of 2188 times, over 10% of the observations suggested some symptom associated with withdrawal. Jenkins [6] reported that 29/182 infants and children receiving midazolam suffered from withdrawal symptoms as judged by the attending clinicians. The symptoms were not described. It is unclear how many of the 27 children participating in the study of weaning protocols by Ducharme [27] suffered from withdrawal, but ‘many’ of the patients had ‘behavioural distress score’ of greater than zero while weaning from midazolam. Franck [28] reports that 13/15 patients exhibited some signs of withdrawal over three or more assessment periods, and the commonest symptoms were hyperpyrexia, ‘sleeplessness’, diarrhoea, dilated pupils and tremors. The commonest symptoms were those of agitation (57/79 patients), inconsolable crying (38/79), motor disturbance (43/79), sleep disturbance (73/79), and tachypnoea (72/79). It is unclear how many patients experienced no symptoms of withdrawal at all, nor how many of the observational periods were asymptomatic of withdrawal symptoms. Sheridan [30] reports that one child suffered withdrawal symptoms after discontinuation of midazolam. These symptoms consisted of vomiting, tremulousness and sweating. Hughes [34] reports that 8/53 children in their study had ‘abnormal behaviour’ after discontinuing midazolam therapy. Three patients had visual hallucinations (one of these also had auditory hallucinations), three were ‘clearly disorientated’ and two patients did not recognise their parents, had ‘puppet-like’ movements and ‘laughed inappropriately’.
Three retrospective cohort studies report possible withdrawal symptoms. Rosen [37] reported that 1/55 patients developed visual hallucinations and tremors after discontinuing midazolam but the authors did not class this as an ADR. Bergman [38] describes 3 children who, upon discontinuation of midazolam infusion, developed neurological symptoms. The authors state that these may have been a “toxic reaction or withdrawal reaction to prolonged intravenous infusion of midazolam”. Fonsmark [31] reported that 12/38 patients receiving midazolam were retrospectively judged to be suffering from withdrawal symptoms indicating that the authors suspect the adverse effects are related to the drug.
Four case series and five case reports described symptoms that were attributed by the authors to benzodiazepine withdrawal. The symptoms reported included irritability, agitation, restlessness or inconsolability [42], [43], [44], [46], [47], [48], [49], abnormal movements (Choreoathetoid or non purposeful [42], [46], [47], seizures [49], ‘moving limbs vigorously’[44], myoclonic jerks and orofacial abnormal movements [41]), hallucinations [41], [49], grimacing [44], [49], ‘jitteriness’[44], [45], clonus [42], disorientation or abnormal communicative skills [41], [44], [46], blindness [46], abnormal behaviour [41], hyperactivity and aggression [49] vomiting [44], [48], diarrhoea [43], poor feeding [44], hypertension and tachycardia [43], [44], [48], and yawning [43].
Of the 9 observational studies that reported withdrawal symptoms, 8 of these attribute the symptoms to the drug whereas one does not [37]. In addition there were 9 case series/case reports that reported withdrawal symptoms and all attribute the symptoms to the drug. None made formal causality assessments but all the symptoms reported as withdrawal are all expected side effects of midazolam. No author used methods to distinguish whether patients were suffering from Benzodiazepine Withdrawal Syndrome, or whether their clinical features reflected withdrawal from another drug.
Neither of the studies monitored or reported withdrawal from clonidine infusion.
Neurological
In two RCTs no neurological complications were reported [22], [24], and in one RCT [25] a child was withdrawn because of “major neurological disorders within 24 hours of inclusion, but the authors do not state that this was due to midazolam. One RCT [19] did not actively monitor participants for neurological complications, but reported that 5/11 patients treated with midazolam developed myoclonus.
Two retrospective cohort studies [35], [38] described neurological complications of midazolam infusion. Bergman [38] describes 3 children who developed abnormal movements after stopping midazolam (mentioned in section on withdrawal). One child became irritable and developed “arching of the back, stiff and abnormal movements, an inability to swallow, poor visual following, no social interaction, a stiff posture, and small amplitude choreic movements of the hands, feet and tongue”. Another child developed “choreoathetotic movements of the head, face, tongue and extremities”. The final child developed “frequent dyskinetic movements of the mouth”. The authors state that they suspected that these adverse effects “represented either a toxic reaction or a withdrawal reaction to prolonged intravenous infusion of midazolam”. Sheridan [35] reported that 2/24 children, upon extubation, developed persistent disconjugate gaze that lasted 5 days in one patient and 14 days in the other. The authors imply that they consider this to be an ADR.
Neither of the studies monitored or reported any neurological complications of clonidine infusion.
Prolonged sedation
One RCT [24] and two prospective cohort studies [34], [50] evaluated ‘prolonged sedation’ after midazolam. Parkinson [24] did not report any prolonged sedation after discontinuing midazolam, Hughes [34] reported that 4/53 patients took between 6 hours to 1 week to become fully alert, and Lloyd Thomas [50] reports that two children suffered from ‘prolonged sedation’, lasting 3.3 hours and one lasting 20.5 hours.
Endocrine/metabolic
One prospective cohort study (Booker 1986) assessed the effect of midazolam on the hypothalamic-pituitary-adrenal axis, by measuring response to synacthen stimulation. The authors conclude that “cortical secretion was not inhibited” by midazolam infusion [40].
One retrospective case-control study [32] compared the incidence of metabolic acidosis and lipaemia in children treated with midazolam with those treated with propofol. 17/92 patients on midazolam developed “clinically significant metabolic acidosis” and 1/92 had lipaemic serum. This primary aim of this study was to assess known side effects of propofol rather than midazolam. No other studies assessed these side effects in children treated with midazolam.
Discussion
We have systematically reviewed the adverse effects of midazolam and clonidine in children, when used for children requiring sedation while receiving mechanical assisted ventilation on PICU. To our knowledge this is the most comprehensive systematic review to date covering a broad range of adverse effects in all paediatric age groups.
Main findings of the review
The findings of this systematic review suggest that midazolam infusion may be associated with cardiovascular adverse effects. In neonates, midazolam has been associated with systemic and cerebral hypotension [19], . In older children an association between midazolam and haemodynamic side effects has also been suggested [46]. It would appear that discontinuation of midazolam can cause hypertension and tachycardia, which may be clinically significant [42], [43], [44], [48]. Discontinuation of midazolam has also been strongly associated with a variety of clinical features which could be suggestive of withdrawal. These symptoms can broadly be categorised as being related to irritability, neurological and behavioural abnormalities, gastro-intestinal dysfunction and autonomic dysfunction. [6], [26], [27], [28], [31], [34], [37], [38], [42], [43], [44], [45], [46], [47], [48], [49]. It would also appear, unsurprisingly, that these problems become more likely in children receiving higher doses of midazolam over longer periods [6], [31]. It is difficult to accurately estimate the frequency with which withdrawal symptoms occur, but in the prospective studies that we identified the reported incidence appears to range from 15% [6], [34]to 85%[28].
Midazolam infusion is also associated with neurological side effects [35], [38] and prolonged sedation [34], [40].
There are a limited number of studies published regarding the use of clonidine in all paediatric age groups. The studies we have reviewed would suggest that clonidine infusion does not have adverse cardiovascular effects [51], [52].
Robustness of this review
The scope of our review was focussed enough to enable us to concentrate on one specific indication of midazolam and clonidine, but broad enough for us to feel that we have included as much information about the side effects of these drugs when given in this situation as possible. Our review was conducted according to a predefined protocol, which was designed according to the guidelines suggested by the Cochrane Adverse Effects Methods Group [53]. In doing so we feel we have not only systematically identified all the relevant literature, but also rigorously appraised it.
Midazolam and clonidine are not only used for sedation on PICU. Clonidine is also administered orally for preoperative sedation in the paediatric population. Midazolam can also be administered as an intravenous bolus, as a subcutaneous infusion, intranasally or via the buccal route. The sedative properties of midazolam can be utilised during operative procedures, or for simple procedures on general paediatric wards, while its anticonvulsant properties render it a useful therapy for status epilepticus. These alternative indications and preparations of midazolam and clonidine may be associated with adverse effects specific to these settings and uses and warrant a separate systematic review of safety. It was felt that the safety of continuous infusion of midazolam and clonidine as sedation on PICU is a hugely important and distinct clinical question, which we feel justifies our exclusion of studies relating to other uses of these drugs.
Quality of the evidence
The studies we identified varied significantly in quality, and the data regarding adverse effects that we have identified must be interpreted in the context of a variety of limiting factors.
Much of the data in our review has come from observational studies and case reports. This is especially evident with regard to the paediatric (rather than neonatal) age group. For example, the only information relating to cardiac side effects during midazolam in children is from a case report [46]. With regard to the evaluation of withdrawal symptoms associated with midazolam, the data are all derived from observational studies in older children. One of the RCTs [24] we included was not adequately masked, and as the outcomes measured in this trial were subjective, this may have biased the results.
None of the studies included in this review used a causality tool to assess the likelihood that the adverse events identified were ADRs. However, all the adverse effects that we identified are already listed in the respective Summary of Product Characteristics and can therefore be considered “expected”.
Consensus guidelines regarding the conduct of clinical trials of sedation for neonates have been produced, but there is currently no consensus for the methodology, definitions, or outcomes that should be used in infants and older children on PICU [54], [55]. The studies we have identified vary in terms of the rigour with which adverse effects are sought, and the analysis and reporting of the results. The tools used to evaluate the presence of withdrawal, and the definitions of what constitutes benzodiazepine withdrawal itself, also vary between studies. Furthermore, the tools which are currently available have not been sufficiently validated, and this may explain the non-uniformity with which they are used in clinical research [56]. The clinical features of Benzodiazepine withdrawal are similar to those from opiate withdrawal, stress, delirium and inadequate pain management, and this already makes assessment of these symptoms within the context of the studies we have identified very difficult [5], [26]. Until a measure of withdrawal that has been properly designed and rigorously evaluated for measures of validity, reliability, responsiveness and ease of use in clinical and research situations the identification and quantification of benzodiazepine withdrawal in children will be compromised.
Implications for pharmacovigilance assessment in the SLEEPS trial
The decision about whether to collect all adverse event data or restrict collection should be determined by a risk assessment for the trial and consider how well established the risk/benefit profile of the medicines under study are, licensing status of the drugs and current level of clinical use. [57] The risk assessment for the SLEEPS trial was also informed by this systematic review which did not identify any additional safety concerns to those specified within the existing SmPCs therefore a decision was made to restrict data collection to adverse reactions. This decision was also influenced by the administrative burden at sites if research nurses were requested to collect all adverse events occurring in critically ill children, and that the volume of events unrelated to treatment would reduce the focus of pharmacovigilance monitoring. However given the low levels of data relating to clonidine we aimed to collect all adverse reactions rather than restrict to serious adverse reactions. In addition given the relatively sparse data on withdrawal symptoms this was added as a secondary outcome of the trial. This was considered particularly important as younger children may require substantially higher doses (mg/kg) than older children and adolescents and the risk of dependence is known to increase with dose and duration of sedation. Specifying withdrawal as an outcome meant that symptoms would be actively assessed in all children. The pharmacovigilance plan was specified within the trial protocol and reviewed by the MHRA during the CTA assessment.
In conclusion, our review has highlighted significant and potentially serious side effects associated when midazolam is administered according to usual PICU practice. Because children on PICU are already critically ill, side effects due to midazolam can easily be over-looked due to other pressing issues with their care or can be attributed to other causes of cardiovascular or neurological disturbance associated with their disease or treatment. This may explain the relatively low number of reports held by the MHRA. It is therefore crucial that clinicians are aware of the potential iatrogenic risks of midazolam and consider this drug as a potential cause of adverse events and actively report suspected reactions to the MHRA. Conversely the evidence regarding the safety of clonidine infusions as sedation for ventilated children on PICU is sparse. Critically ill children need adequate sedation to protect these vulnerable children from the known adverse effects of withholding sedation during their PICU admission. Identifying the specific features of drug side effects, finding an optimum drug therapy at the appropriate dose and achieving the right balance of sedation is a major challenge. Finding this balance requires high quality clinical research to improve what is currently a poor evidence-base regarding safety and optimal delivery.
Supporting Information
(PDF)
(PDF)
(PDF)
(PDF)
Funding Statement
The Medical Research Council funded NorthWest Hub for Trials Methodology Research supported contributions from Carrol Gamble, Ian Sinha, and Paula Williamson (grant number G0800792). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Tobias JD (2000) Tolerance, withdrawal, and physical dependency after long-term sedation and analgesia of children in the pediatric intensive care unit. Critical Care Medicine 28: 2122–2132. [DOI] [PubMed] [Google Scholar]
- 2. Anand KJ, Hansen DD, Hickey PR (1990) Hormonal-metabolic stress responses in neonates undergoing cardiac surgery. Anesthesiology 73: 661–670. [DOI] [PubMed] [Google Scholar]
- 3. Anand KJ, Hickey PR (1992) Halothane-morphine compared with high-dose sufentanil for anesthesia and postoperative analgesia in neonatal cardiac surgery.[see comment]. New England Journal of Medicine 326: 1–9. [DOI] [PubMed] [Google Scholar]
- 4. Tobias JD, Rasmussen GE (1994) Pain management and sedation in the pediatric intensive care unit. Pediatric Clinics of North America 41: 1269–1292. [DOI] [PubMed] [Google Scholar]
- 5. Wolf AR (1994) Neonatal sedation: more art than science.[comment]. Lancet 344: 628–629. [DOI] [PubMed] [Google Scholar]
- 6. Jenkins IA, Playfor SD, Bevan C, Davies G, Wolf AR (2007) Current United Kingdom sedation practice in pediatric intensive care. Paediatric Anaesthesia 17: 675–683. [DOI] [PubMed] [Google Scholar]
- 7. Playfor SD, Thomas DA, Choonara I (2003) Sedation and neuromuscular blockade in paediatric intensive care: a review of current practice in the UK. Paediatric Anaesthesia 13: 147–151. [DOI] [PubMed] [Google Scholar]
- 8. Harte GJ, Gray PH, Lee TC, Steer PA, Charles BG (1997) Haemodynamic responses and population pharmacokinetics of midazolam following administration to ventilated, preterm neonates. Journal of Paediatrics & Child Health 33: 335–338. [DOI] [PubMed] [Google Scholar]
- 9. van Straaten HL, Rademaker CM, de Vries LS (1992) Comparison of the effect of midazolam or vecuronium on blood pressure and cerebral blood flow velocity in the premature newborn. Developmental Pharmacology & Therapeutics 19: 191–195. [DOI] [PubMed] [Google Scholar]
- 10. Ng E, Taddio A, Ohlsson A (2003) Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit.[update of Cochrane Database Syst Rev. 2000;(2):CD002052; PMID: 10796280]. Cochrane Database of Systematic Reviews CD002052. [DOI] [PubMed] [Google Scholar]
- 11. Clarke L, Clarke M, Clarke T (2007) How useful are Cochrane reviews in identifying research needs? J Health Serv Res Policy 2007;12(2):101–3. J Health Serv Res Policy 12: 101–103. [DOI] [PubMed] [Google Scholar]
- 12. Goudie A, Sutton A, Jones D, Donald A (2010) Empirical assessment suggests that existing evidence could be used more fully in designing randomized controlled trials. Journal of Clinical Epidemiology 63: 983–991. [DOI] [PubMed] [Google Scholar]
- 13. Clarke M, Hopewell S, Chalmers I (2010) Clinical trials should begin and end with systematic reviews of relevant evidence: 12 years and waiting. Lancet 376: 20–21. [DOI] [PubMed] [Google Scholar]
- 14. Cooper N, Jones D, Sutton A (2005) The use of systematic reviews when designing studies. Clinical Trials 2: 260–264. [DOI] [PubMed] [Google Scholar]
- 15. Aranda JV, Carlo W, Hummel P, Thomas R, Lehr VT, et al. (2005) Analgesia and sedation during mechanical ventilation in neonates. Clinical Therapeutics 27: 877–899. [DOI] [PubMed] [Google Scholar]
- 16. Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M (2007) Withdrawal symptoms in children after long-term administration of sedatives and/or analgesics: a literature review. “Assessment remains troublesome”. Intensive Care Medicine 33: 1396–1406. [DOI] [PubMed] [Google Scholar]
- 17. Kirkham JJ, Dwan K, Dodd S, Altman DG, Smyth R, et al. (2010) The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 340: c365. [DOI] [PubMed] [Google Scholar]
- 18. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, et al. (1981) A method for estimating the probability of adverse drug reactions. Clinical Pharmacology & Therapeutics 30: 239–245. [DOI] [PubMed] [Google Scholar]
- 19. van Alfen-van der Velden AAEM, Hopman JCW, Klaessens JHGM, Feuth T, Sengers RCA, et al. (2006) Effects of midazolam and morphine on cerebral oxygenation and hemodynamics in ventilated premature infants. Biology of the Neonate 90: 197–202. [DOI] [PubMed] [Google Scholar]
- 20. Treluyer JM, Zohar S, Rey E, Hubert P, Iserin F, et al. (2005) Minimum effective dose of midazolam for sedation of mechanically ventilated neonates. Journal of Clinical Pharmacy & Therapeutics 30: 479–485. [DOI] [PubMed] [Google Scholar]
- 21. Tobias JD, Berkenbosch JW (2004) Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. Southern Medical Journal 97: 451–455. [DOI] [PubMed] [Google Scholar]
- 22. Arya V, Ramji S (2001) Midazolam sedation in mechanically ventilated newborns: a double blind randomized placebo controlled trial.[see comment]. Indian Pediatrics 38: 967–972. [PubMed] [Google Scholar]
- 23. Anand KJ, Barton BA, McIntosh N, Lagercrantz H, Pelausa E, et al. (1999) Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates.[erratum appears in Arch Pediatr Adolesc Med 1999 Aug;153(8):895]. Archives of Pediatrics & Adolescent Medicine 153: 331–338. [DOI] [PubMed] [Google Scholar]
- 24. Parkinson L, Hughes J, Gill A, Billingham I, Ratcliffe J, et al. (1997) A randomized controlled trial of sedation in the critically ill. Paediatric Anaesthesia 7: 405–410. [DOI] [PubMed] [Google Scholar]
- 25. Jacqz-Aigrain E, Daoud P, Burtin P, Desplanques L, Beaufils F (1994) Placebo-controlled trial of midazolam sedation in mechanically ventilated newborn babies.[see comment]. Lancet 344: 646–650. [DOI] [PubMed] [Google Scholar]
- 26. Ista E, van Dijk M, Gamel C, Tibboel D, de Hoog M (2008) Withdrawal symptoms in critically ill children after long-term administration of sedatives and/or analgesics: a first evaluation.[see comment]. Critical Care Medicine 36: 2427–2432. [DOI] [PubMed] [Google Scholar]
- 27. Ducharme C, Carnevale FA, Clermont M-S, Shea S (2005) A prospective study of adverse reactions to the weaning of opioids and benzodiazepines among critically ill children. Intensive & Critical Care Nursing 21: 179–186. [DOI] [PubMed] [Google Scholar]
- 28. Franck LS, Naughton I, Winter I (2004) Opioid and benzodiazepine withdrawal symptoms in paediatric intensive care patients. Intensive & Critical Care Nursing 20: 344–351. [DOI] [PubMed] [Google Scholar]
- 29. Sheridan RL, Keaney T, Stoddard F, Enfanto R, Kadillack P, et al. (2003) Short-term propofol infusion as an adjunct to extubation in burned children. Journal of Burn Care & Rehabilitation 24: 356–360. [DOI] [PubMed] [Google Scholar]
- 30. Sheridan R, Stoddard F, Querzoli E (2001) Management of background pain and anxiety in critically burned children requiring protracted mechanical ventilation. Journal of Burn Care & Rehabilitation 22: 150–153. [DOI] [PubMed] [Google Scholar]
- 31. Fonsmark L, Rasmussen YH, Carl P (1999) Occurrence of withdrawal in critically ill sedated children.[see comment]. Critical Care Medicine 27: 196–199. [DOI] [PubMed] [Google Scholar]
- 32. Pepperman ML, Macrae D (1997) A comparison of propofol and other sedative use in paediatric intensive care in the United Kingdom. Paediatric Anaesthesia 7: 143–153. [DOI] [PubMed] [Google Scholar]
- 33. Shekerdemian L, Bush A, Redington A (1997) Cardiovascular effects of intravenous midazolam after open heart surgery. Archives of Disease in Childhood 76: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hughes J, Gill A, Leach HJ, Nunn AJ, Billingham I, et al. (1994) A prospective study of the adverse effects of midazolam on withdrawal in critically ill children. Acta Paediatrica 83: 1194–1199. [DOI] [PubMed] [Google Scholar]
- 35. Sheridan RL, McEttrick M, Bacha G, Stoddard F, Tompkins RG (1994) Midazolam infusion in pediatric patients with burns who are undergoing mechanical ventilation. Journal of Burn Care & Rehabilitation 15: 515–518. [DOI] [PubMed] [Google Scholar]
- 36. Jacqz-Aigrain E, Daoud P, Burtin P, Maherzi S, Beaufils F (1992) Pharmacokinetics of midazolam during continuous infusion in critically ill neonates. European Journal of Clinical Pharmacology 42: 329–332. [DOI] [PubMed] [Google Scholar]
- 37. Rosen DA, Rosen KR (1991) Midazolam for sedation in the paediatric intensive care unit. Intensive Care Medicine 17 Suppl 1: S15–19. [DOI] [PubMed] [Google Scholar]
- 38. Bergman I, Steeves M, Burckart G, Thompson A (1991) Reversible neurologic abnormalities associated with prolonged intravenous midazolam and fentanyl administration.[see comment]. Journal of Pediatrics 119: 644–649. [DOI] [PubMed] [Google Scholar]
- 39. Hartwig S, Roth B, Theisohn M (1991) Clinical experience with continuous intravenous sedation using midazolam and fentanyl in the paediatric intensive care unit. European Journal of Pediatrics 150: 784–788. [DOI] [PubMed] [Google Scholar]
- 40. Booker PD, Beechey A, Lloyd-Thomas AR (1986) Sedation of children requiring artificial ventilation using an infusion of midazolam. British Journal of Anaesthesia 58: 1104–1108. [DOI] [PubMed] [Google Scholar]
- 41. Epstein D, Difazio M (2007) Orofacial automatisms induced by acute withdrawal from high-dose midazolam mimicking nonconvulsive status epilepticus in a child. Movement Disorders 22: 712–715. [DOI] [PubMed] [Google Scholar]
- 42. Cho HH, O'Connell JP, Cooney MF, Inchiosa MA Jr (2007) Minimizing tolerance and withdrawal to prolonged pediatric sedation: case report and review of the literature. Journal of Intensive Care Medicine 22: 173–179. [DOI] [PubMed] [Google Scholar]
- 43. Biswas AK, Feldman BL, Davis DH, Zintz EA (2005) Myocardial ischemia as a result of severe benzodiazepine and opioid withdrawal. Clinical Toxicology: The Official Journal of the American Academy of Clinical Toxicology & European Association of Poisons Centres & Clinical Toxicologists 43: 207–209. [PubMed] [Google Scholar]
- 44. Carnevale FA, Ducharme C (1997) Adverse reactions to the withdrawal of opioids and benzodiazepines in paediatric intensive care. Intensive & Critical Care Nursing 13: 181–188. [DOI] [PubMed] [Google Scholar]
- 45. Yaster M, Kost-Byerly S, Berde C, Billet C (1996) The management of opioid and benzodiazepine dependence in infants, children, and adolescents. Pediatrics 98: 135–140. [PubMed] [Google Scholar]
- 46. Ducharme MP, Munzenberger P (1995) Severe withdrawal syndrome possibly associated with cessation of a midazolam and fentanyl infusion. Pharmacotherapy 15: 665–668. [DOI] [PubMed] [Google Scholar]
- 47. Tobias JD, Deshpande JK, Gregory DF (1994) Outpatient therapy of iatrogenic drug dependency following prolonged sedation in the pediatric intensive care unit. Intensive Care Medicine 20: 504–507. [DOI] [PubMed] [Google Scholar]
- 48. van Engelen BG, Gimbrere JS, Booy LH (1993) Benzodiazepine withdrawal reaction in two children following discontinuation of sedation with midazolam. Annals of Pharmacotherapy 27: 579–581. [DOI] [PubMed] [Google Scholar]
- 49. Sury MR, Billingham I, Russell GN, Hopkins CS, Thornington R, et al. (1989) Acute benzodiazepine withdrawal syndrome after midazolam infusions in children.[see comment]. Critical Care Medicine 17: 301–302. [DOI] [PubMed] [Google Scholar]
- 50. Lloyd-Thomas AR, Booker PD (1986) Infusion of midazolam in paediatric patients after cardiac surgery. British Journal of Anaesthesia 58: 1109–1115. [DOI] [PubMed] [Google Scholar]
- 51. Ambrose C, Sale S, Howells R, Bevan C, Jenkins I, et al. (2000) Intravenous clonidine infusion in critically ill children: dose-dependent sedative effects and cardiovascular stability. British Journal of Anaesthesia 84: 794–796. [DOI] [PubMed] [Google Scholar]
- 52. Lyons B, Casey W, Doherty P, McHugh M, Moore K (1996) Pain relief with low-dose intravenous clonidine in a child with severe burns. Intensive Care Medicine 22: 249–251. [DOI] [PubMed] [Google Scholar]
- 53. Loke YK, Price D, Herxheimer A, Cochrane Adverse Effects Methods G (2007) Systematic reviews of adverse effects: framework for a structured approach. BMC Medical Research Methodology 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sinha I, Jones L, Smyth RL, Williamson PR (2008) A systematic review of studies that aim to determine which outcomes to measure in clinical trials in children. PLoS Medicine/Public Library of Science 5: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anand KJS, Aranda JV, Berde CB, Buckman S, Capparelli EV, et al. (2006) Summary proceedings from the neonatal pain-control group. Pediatrics 117: S9–S22. [DOI] [PubMed] [Google Scholar]
- 56. Easley RB, Nichols DG (2008) Withdrawal assessment in the pediatric intensive care unit: quantifying a morbidity of pain and sedation management in the critically ill child.[comment]. Critical Care Medicine 36: 2479–2480. [DOI] [PubMed] [Google Scholar]
- 57.MRC/DH joint project Clinical Trials Toolkit (2007) Work stream 6: Pharmacovigilance. Available: http://www.ct-toolkit.ac.uk/_db/_documents/Joint_Project_Guidance_on_Pharmacovigilance.pdf: Accessed: 12th December 2012.
- 58. Aitkenhead AR, Pepperman ML, Willatts SM, Coates PD, Park GR, et al. (1989) Comparison of propofol and midazolam for sedation in critically ill patients. Lancet 2: 704–709. [DOI] [PubMed] [Google Scholar]
- 59. Bourgoin A, Albanese J, Wereszczynski N, Charbit M, Vialet R, et al. (2003) Safety of sedation with ketamine in severe head injury patients: comparison with sufentanil. Critical Care Medicine 31: 711–717. [DOI] [PubMed] [Google Scholar]
- 60. Cernaianu AC, DelRossi AJ, Flum DR, Vassilidze TV, Ross SE, et al. (1996) Lorazepam and midazolam in the intensive care unit: a randomized, prospective, multicenter study of hemodynamics, oxygen transport, efficacy, and cost. Critical Care Medicine 24: 222–228. [DOI] [PubMed] [Google Scholar]
- 61. Chamorro C, de Latorre FJ, Montero A, Sanchez-Izquierdo JA, Jareno A, et al. (1996) Comparative study of propofol versus midazolam in the sedation of critically ill patients: results of a prospective, randomized, multicenter trial.[see comment]. Critical Care Medicine 24: 932–939. [DOI] [PubMed] [Google Scholar]
- 62. Costa J, Cabre L, Molina R, Carrasco G (1994) Cost of ICU sedation: comparison of empirical and controlled sedation methods. Clinical Intensive Care 5: 17–21. [PubMed] [Google Scholar]
- 63. Deo S, Knottenbelt JD (1994) The use of midazolam in trauma resuscitation. European Journal of Emergency Medicine 1: 111–114. [PubMed] [Google Scholar]
- 64. Dirksen MS, Vree TB, Driessen JJ (1987) Clinical pharmacokinetics of long-term infusion of midazolam in critically ill patients–preliminary results. Anaesthesia & Intensive Care 15: 440–444. [DOI] [PubMed] [Google Scholar]
- 65. Flogel CM, Ward DS, Wada DR, Ritter JW (1993) The effects of large-dose flumazenil on midazolam-induced ventilatory depression. Anesthesia & Analgesia 77: 1207–1214. [PubMed] [Google Scholar]
- 66. Lee Y, Wang JJ, Yang YL, Chen A, Lai HY (2007) Midazolam vs ondansetron for preventing postoperative nausea and vomiting: a randomised controlled trial.[see comment]. Anaesthesia 62: 18–22. [DOI] [PubMed] [Google Scholar]
- 67. Lescot T, Pereira AR, Abdennour L, Sanchez-Pena P, Naccache L, et al. (2007) Effect of loxapine on electrical brain activity, intracranial pressure, and middle cerebral artery flow velocity in traumatic brain-injured patients. Neurocritical Care 7: 124–127. [DOI] [PubMed] [Google Scholar]
- 68. Searle NR, Cote S, Taillefer J, Carrier M, Gagnon L, et al. (1997) Propofol or midazolam for sedation and early extubation following cardiac surgery. Canadian Journal of Anaesthesia 44: 629–635. [DOI] [PubMed] [Google Scholar]
- 69. Sinclair ME, Sear JW, Summerfield RJ, Fisher A (1988) Alfentanil infusions on the intensive therapy unit. Intensive Care Medicine 14: 55–59. [DOI] [PubMed] [Google Scholar]
- 70. Stewart L, Bullock R, Teasdale GM, Wagstaff A (1999) First observations of the safety and tolerability of a competitive antagonist to the glutamate NMDA receptor (CGS 19755) in patients with severe head injury. Journal of Neurotrauma 16: 843–850. [DOI] [PubMed] [Google Scholar]
- 71. Tanigami H, Yahagi N, Kumon K, Watanabe Y, Haruna M, et al. (1997) Long-term sedation with isoflurane in postoperative intensive care in cardiac surgery. Artificial Organs 21: 21–23. [DOI] [PubMed] [Google Scholar]
- 72. Bitar G, Mullis W, Jacobs W, Matthews D, Beasley M, et al. (2003) Safety and efficacy of office-based surgery with monitored anesthesia care/sedation in 4778 consecutive plastic surgery procedures.[see comment]. Plastic & Reconstructive Surgery 111: 150–156; discussion 157–158 [DOI] [PubMed] [Google Scholar]
- 73. Djurberg H, Pothmann Facharzt W, Joseph D, Tjan D, Zuleika M, et al. (2002) Anesthesia care for living-related liver transplantation for infants and children with end-stage liver disease: report of our initial experience. Journal of Clinical Anesthesia 14: 564–570. [DOI] [PubMed] [Google Scholar]
- 74. Hertzog JH, Campbell JK, Dalton HJ, Hauser GJ (1999) Propofol anesthesia for invasive procedures in ambulatory and hospitalized children: experience in the pediatric intensive care unit. Pediatrics 103: E30. [DOI] [PubMed] [Google Scholar]
- 75. Hickey PR, Wessel DL, Streitz SL, Fox ML, Kern FH, et al. (1992) Transcatheter closure of atrial septal defects: hemodynamic complications and anesthetic management. Anesthesia & Analgesia 74: 44–50. [DOI] [PubMed] [Google Scholar]
- 76. Koroglu A, Demirbilek S, Teksan H, Sagir O, But AK, et al. (2005) Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: preliminary results. British Journal of Anaesthesia 94: 821–824. [DOI] [PubMed] [Google Scholar]
- 77. Laussen PC, Hansen DD, Perry SB, Fox ML, Javorski JJ, et al. (1995) Transcatheter closure of ventricular septal defects: hemodynamic instability and anesthetic management. Anesthesia & Analgesia 80: 1076–1082. [DOI] [PubMed] [Google Scholar]
- 78. Malagon I, Hogenbirk K, van Pelt J, Hazekamp MG, Bovill JG (2005) Effect of three different anaesthetic agents on the postoperative production of cardiac troponin T in paediatric cardiac surgery. British Journal of Anaesthesia 94: 805–809. [DOI] [PubMed] [Google Scholar]
- 79. Somri M, Tome R, Yanovski B, Asfandiarov E, Carmi N, et al. (2007) Combined spinal-epidural anesthesia in major abdominal surgery in high-risk neonates and infants. Paediatric Anaesthesia 17: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 80. Wilson KE, Girdler NM, Welbury RR (2003) Randomized, controlled, cross-over clinical trial comparing intravenous midazolam sedation with nitrous oxide sedation in children undergoing dental extractions. British Journal of Anaesthesia 91: 850–856. [DOI] [PubMed] [Google Scholar]
- 81. Yldzdas D, Yapcoglu H, Ylmaz HL (2004) The value of capnography during sedation or sedation/analgesia in pediatric minor procedures. Pediatric Emergency Care 20: 162–165. [DOI] [PubMed] [Google Scholar]
- 82. Gruber EM, Laussen PC, Casta A, Zimmerman AA, Zurakowski D, et al. (2001) Stress response in infants undergoing cardiac surgery: a randomized study of fentanyl bolus, fentanyl infusion, and fentanyl-midazolam infusion.[see comment]. Anesthesia & Analgesia 92: 882–890. [DOI] [PubMed] [Google Scholar]
- 83. Hartvig P, Larsson E, Joachimsson PO (1993) Postoperative analgesia and sedation following pediatric cardiac surgery using a constant infusion of ketamine. Journal of Cardiothoracic & Vascular Anesthesia 7: 148–153. [DOI] [PubMed] [Google Scholar]
- 84. Prins SA, Peeters MYM, Houmes RJ, van Dijk M, Knibbe CAJ, et al. (2005) Propofol 6% as sedative in children under 2 years of age following major craniofacial surgery. British Journal of Anaesthesia 94: 630–635. [DOI] [PubMed] [Google Scholar]
- 85. De Cosmo G, Congedo E, Clemente A, Aceto P (2005) Sedation in PACU: the role of propofol. Current Drug Targets 6: 741–744. [DOI] [PubMed] [Google Scholar]
- 86. Walker J, Maccallum M, Fischer C, Kopcha R, Saylors R, et al. (2006) Sedation using dexmedetomidine in pediatric burn patients. Journal of Burn Care & Research 27: 206–210. [DOI] [PubMed] [Google Scholar]
- 87. Notterman DA (1997) Sedation with intravenous midazolam in the pediatric intensive care unit. Clinical Pediatrics 36: 449–454. [DOI] [PubMed] [Google Scholar]
- 88. Al-Samsam RH, Cullen P (2005) Sleep and adverse environmental factors in sedated mechanically ventilated pediatric intensive care patients. Pediatric Critical Care Medicine 6: 562–567. [DOI] [PubMed] [Google Scholar]
- 89. Buck ML, Willson DF (2008) Use of dexmedetomidine in the pediatric intensive care unit. Pharmacotherapy 28: 51–57. [DOI] [PubMed] [Google Scholar]
- 90. DeBerry BB, Lynch JE, Chernin JM, Zwischenberger JB, Chung DH (2005) A survey for pain and sedation medications in pediatric patients during extracorporeal membrane oxygenation. Perfusion 20: 139–143. [DOI] [PubMed] [Google Scholar]
- 91. Enomoto Y, Kudo T, Saito T, Hori T, Kaneko M, et al. (2006) Prolonged use of dexmedetomidine in an infant with respiratory failure following living donor liver transplantation. Paediatric Anaesthesia 16: 1285–1288. [DOI] [PubMed] [Google Scholar]
- 92. Jin HS, Yum MS, Kim SL, Shin HY, Lee EH, et al. (2007) The efficacy of the COMFORT scale in assessing optimal sedation in critically ill children requiring mechanical ventilation. Journal of Korean Medical Science 22: 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kadilak PR, Vanasse S, Sheridan RL (2004) Favorable short- and long-term outcomes of prolonged translaryngeal intubation in critically ill children. Journal of Burn Care & Rehabilitation 25: 262–265. [DOI] [PubMed] [Google Scholar]
- 94. Lugo RA, Chester EA, Cash J, Grant MJ, Vernon DD (1999) A cost analysis of enterally administered lorazepam in the pediatric intensive care unit.[see comment]. Critical Care Medicine 27: 417–421. [DOI] [PubMed] [Google Scholar]
- 95. Playfor SD, Thomas DA, Choonara I, Jarvis A (2000) Quality of sedation during mechanical ventilation. Paediatric Anaesthesia 10: 195–199. [DOI] [PubMed] [Google Scholar]
- 96. Rigby-Jones AE, Priston MJ, Sneyd JR, McCabe AP, Davis GI, et al. (2007) Remifentanil-midazolam sedation for paediatric patients receiving mechanical ventilation after cardiac surgery. British Journal of Anaesthesia 99: 252–261. [DOI] [PubMed] [Google Scholar]
- 97. Saha AS, Langridge P, Playfor SD (2006) The pattern of intravenous drug administration during the transfer of critically ill children by a specialist transport team. Paediatric Anaesthesia 16: 1063–1067. [DOI] [PubMed] [Google Scholar]
- 98. Schmidt B, Roth B, Stutzer H, Benz-Bohm G (2006) Prospective sonographic evaluation of fentanyl side effects on the neonatal gallbladder. European Journal of Clinical Pharmacology 62: 823–827. [DOI] [PubMed] [Google Scholar]
- 99. Vernon DD, Witte MK (2000) Effect of neuromuscular blockade on oxygen consumption and energy expenditure in sedated, mechanically ventilated children. Critical Care Medicine 28: 1569–1571. [DOI] [PubMed] [Google Scholar]
- 100. Prause A, Wappler F, Scholz J, Bause H, Schulte am Esch J (2000) Respiratory depression under long-term sedation with sufentanil, midazolam and clonidine has no clinical significance. Intensive Care Medicine 26: 1454–1461. [DOI] [PubMed] [Google Scholar]
- 101. Shelly MP, Sultan MA, Bodenham A, Park GR (1991) Midazolam infusions in critically ill patients. European Journal of Anaesthesiology 8: 21–27. [PubMed] [Google Scholar]
- 102. Artman M, Boerth R (1983) Clonidine Poisoning. A Complex Problem. American Journal of Diseases of Children 137: 171–174. [DOI] [PubMed] [Google Scholar]
- 103. Tanaka M, Nishikawa T (1995) Effects of clonidine premedication on the pressor response to α-adrenergic agonists. British Journal of Anaesthesia 75: 593–597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)