ABSTRACT
BACKGROUND
Since 2005, the Centers for Medicare and Medicaid Services (CMS) has required all Medicare Advantage (MA) plans to report prescribing rates of high risk medications (HRM).
OBJECTIVE
To determine predictors of receipt of HRMs, as defined by the National Committee for Quality Assurance’s “Drugs to Avoid in the Elderly” quality indicator, in a national sample of MA enrollees.
DESIGN AND PARTICIPANTS
Retrospective analysis of Healthcare Effectiveness Data and Information Set (HEDIS) data for 6,204,824 enrollees, aged 65 years or older, enrolled in 415 MA plans in 2009. To identify predictors of HRM use, we fit generalized linear models and modeled outcomes on the risk-difference scale.
MAIN OUTCOME MEASURES
Receipt or non-receipt of one or two HRMs.
KEY RESULTS
Approximately 21 % of MA enrollees received at least one HRM and 4.8 % received at least two. In fully adjusted models, females had a 10.6 (95 % CI: 10.0–11.2) higher percentage point rate of receipt than males, and residence in any of the Southern United States divisions was associated with a greater than 10 percentage point higher rate, as compared with the reference New England division. Higher rates were also observed among enrollees with low personal income (6.5 percentage points, 95 % CI: 5.5–7.5), relative to those without low income and those residing in areas in the lowest quintile of socioeconomic status (2.7 points, 95 % CI: 1.9–3.4) relative to persons residing in the highest quintile. Enrollees ≥ 85 years old, black enrollees, and other minority groups were less likely to receive these medications. Over 38 % of MA enrollees residing in the hospital referral region of Albany, Georgia received at least one HRM, a rate four times higher than the referral region with the lowest rate (Mason City, Iowa).
CONCLUSIONS
Use of HRMs among MA enrollees varies widely by geographic region. Persons living in the Southern region of the U.S., whites, women, and persons of low personal income and socioeconomic status are more likely to receive HRMs.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2244-9) contains supplementary material, which is available to authorized users.
KEY WORDS: high risk medications, geriatric prescribing, potentially inappropriate medication, quality prescribing, disparities
Adverse drug events resulting from poor quality prescribing are a significant public health problem.1 An increased burden of chronic diseases and age-related changes in drug metabolism predispose the elderly to adverse drug events precipitated by the use of high risk medications (HRM).2,3 HRM use in the geriatric population is associated with increases in morbidity, mortality, hospitalization, inpatient length of stays, and health care spending.4–6
HRMs in the elderly are broadly defined as medications that should be avoided among patients 65 years of age or older, because the associated adverse effects outweigh potential benefits or because safer alternatives are available,7 a principle codified most prominently by the Beers8 and Zhan9 criteria. To improve the quality of drug prescribing in the elderly, the Centers for Medicare and Medicaid Services (CMS) requires all Medicare Advantage (MA) health plans to report publicly on the prescribing rates of HRMs, as defined by the Healthcare Effectiveness Data and Information Set’s (HEDIS) “Drugs to Avoid in the Elderly” quality measure. The term HRM is often used interchangeably with ‘potentially inappropriate medication’,8,9 a phrase that acknowledges the complexity inherent in assessing the quality of patient-specific drug management decisions among heterogeneous patient populations. In this manuscript, we use ‘high risk,’ because that is the specification of the quality measure used in the analysis.
Successful efforts to reduce HRM use in the elderly require knowledge of how prescribing of these agents varies geographically and the factors that predict their use. However, there are few nationally representative studies of the prevalence and predictors of HRM use, particularly following the implementation of the Medicare prescription drug benefit in 2006. Medicare Part D extended prescription drug coverage to the majority of Medicare’s approximately 44 million beneficiaries, only one-third of whom had drug coverage previously. More universal access to drug coverage among the elderly has increased pharmaceutical use,10,11 and therefore, by extension, opportunities for receipt of HRMs. Further, there is limited evidence about HRM use in the MA program, which now comprises approximately 25 % of the Medicare population. Because such plans assume financial risk for paying for Medicare-covered services and integrate the financing and delivery of care, and because the MA population may be healthier, on average, and younger than traditional Medicare enrollees,12 the rate of HRM use in this population may differ from that observed in the traditional Medicare population.
In this study, we examined predictors of HRM use utilizing the HEDIS quality indicator in a national sample of over 6 million elderly MA enrollees. We also characterized geographic variation in HRM use with the aim of identifying subpopulations at increased risk of receiving these medications.
METHODS
Data Sources
We obtained individual-level Medicare HEDIS data for the 2009 calendar year from CMS. The National Committee for Quality Assurance (NCQA) developed HEDIS to measure and compare the quality of care delivered to enrollees in health plans. Since 1997, all Medicare managed care plans have been required to submit HEDIS data to the CMS. In 2005, NCQA developed two HEDIS quality indicators, both entitled “Drugs to Avoid in the Elderly,” to measure the percentage of Medicare enrollees in each plan that received at least one HRM or at least two different HRMs. Clinical comorbidity information and the names of specific medications are not available in the HEDIS data (refer to eTable 1 in the online appendix for a list of medications included in the measure).
Conceptually and operationally, the Beers and Zhan criteria for use of “potentially inappropriate medications in the elderly” form the backbone of the HEDIS measures. Both measures, Beers in particular, are widely used in the literature and have been accepted through various iterations as the best available clinical tools for the screening of HRMs. Research has shown a link between medications on the Beers list and serious adverse effects in the elderly, including falls, fractures, gastrointestinal bleeding, and delirium.13–15 The final list of 110 medications used to identify claims for HRMs borrows heavily from the Zhan and Beers criteria and was determined by NCQA through a modified Delphi consensus process that included a panel of clinical, methodological, and organizational science experts. A more detailed description of this process16 and comparison of the HEDIS measure with other criteria can be found elsewhere.17
Study Population
We matched 98 % of the HEDIS observations to the Medicare enrollment file from the corresponding year to determine enrollees’ demographic characteristics. We used the CMS Medicare Advantage Plan Directory to obtain information on health plan characteristics. If these data were missing, we contacted the health plan directly. Using the Dartmouth Atlas of Healthcare, we assigned each enrollee to a hospital-referral region (HRR) on the basis of zip code of residence. HRRs represent distinct regional health care markets within which residents seek the services of a tertiary hospital or major referral center.18 HRRs have been utilized widely to characterize geographic variation in healthcare quality and delivery.19–21
Among 6,514,278 enrollees eligible for the HEDIS measure, we excluded 86,131 who died during the measurement year, who were outside the U.S., or those enrolled in health plans outside the U.S. We further excluded 8,707 observations from six plans that erroneously reported a greater rate of enrollees receiving two or more HRMs than the rate of enrollees receiving one or more HRMs. After all exclusions, the final study sample consisted of 6,204,824 enrollees.
Outcome Variables
The two primary dependent variables of interest were: (1) receipt of at least one prescription for a HRM; and (2) receipt of at least two different HRMs during the measurement year as defined by the HEDIS “Drugs to Avoid in the Elderly” quality indicators. To be eligible for inclusion in the denominator for either indicator, enrollees must be 65 years of age or older, continuously enrolled in the managed care plan throughout the measurement year, and have prescription drug benefits for the duration of the measurement year. To be included in the numerator, enrollees were required to have a prescription drug claim for at least one (for first measure) or at least two HRMs (for second measure).
Independent Variables
Descriptive variables defined at the individual level included age (65–69, 70–74, 75–79, 80–85, ≥ 85), sex, race (white, black, other), low personal income (defined by receipt of Medicare State Buy-in assistance22), U.S. census division of residence (refer to eTable 2 in online appendix for list of states in each division), and socioeconomic status (SES) index score (by quintile). The SES index score is a composite measure of area-level socioeconomic status derived from zip code-level employment, housing, income, education and crowding data. Composition and creation of the score is described in detail by the Agency for Healthcare Research and Quality.23 Plan-level independent variables included model type (group/staff, independent practice association, mixed/network), plan age (determined by the date the plan began its operation: before 1980, 1980–1999, and after 2000), beneficiary enrollment per plan (0–24,999, 25,000–99,999, ≥ 100,000), and plan tax status (for-profit, not-for-profit). All variables were tested for non-collinearity.
Statistical Analysis
We determined the percentage of eligible enrollees who met the numerator criteria for the two HEDIS indicator measures in each sociodemographic and health plan-level subgroup and used χ2-square or t-tests to compare the characteristics of enrollees receiving a HRM to those who did not. To identify predictors of HRM use, we fit generalized linear models to predict receipt of a HRM using the independent variables described above. We modeled outcomes on the risk-difference scale and accounted for clustering of enrollees within health plans using generalized estimating equations. Three models were fit: model 1 controlled for the individual-level characteristics of age, sex, race, and low personal income; model 2 included all variables from model 1, as well as community-level characteristics of zip code-based SES index score and census division of enrollee residence; and model 3 included all variables from models 1 and 2, as well as health plan characteristics (model type, profit status, enrollment size, and plan age).
To determine the extent of geographic variation in HRM use, we calculated the proportion of total eligible enrollees in each HRR that met the criteria for each of the HEDIS indicators. National maps were created to display the geographic variation in rates of HRM use for each measure.
Statistical tests were two-sided, with only P < 0.005 considered statistically significant, due to the large sample size. All analyses were performed using SAS statistical software (version 9.3; Cary, North Carolina) and maps were created using ArcGIS software (version 9.3; Redlands, California). The study was approved by Brown University’s Human Research Protections Office.
RESULTS
In the final sample, the rates of receipt of at least one HRM and of at least two HRMs were 21.5 % and 4.8 %, respectively. The mean age of enrollees was 74.5 years; 58 % were women and 83 % were white. The characteristics of persons receiving and not receiving HRMs are presented in Table 1.
Table 1.
Sociodemographic, Geographic, and Health Plan Characteristics of Medicare Advantage Enrollees Receiving and Not Receiving at Least One High Risk Medication (HRM), Based on HEDIS’ Drugs to Avoid in the Elderly Measure*
| Characteristics | Received ≥ 1 HRM (n = 1,333,307) | Did not receive ≥ 1 HRM (n = 4,871,517) |
|---|---|---|
| Women (%) | 71 | 55 |
| Age group, years (%) | ||
| 65–69 | 31 | 30 |
| 70–74 | 25 | 25 |
| 75–79 | 20 | 20 |
| 80–84 | 14 | 14 |
| ≥ 85 | 10 | 10 |
| Race (%) | ||
| White | 84 | 83 |
| Black | 10 | 10 |
| Other | 7 | 7 |
| Low Personal Income (%) | 16 | 11 |
| SES indicator(zip code), mean (SD) | 50.7 (4) | 51.2 (4) |
| Geographic division (%) | ||
| New England | 3 | 5 |
| Middle Atlantic | 15 | 21 |
| East North Central | 9 | 10 |
| West North Central | 6 | 7 |
| South Atlantic | 21 | 16 |
| East South Central | 6 | 4 |
| West South Central | 11 | 7 |
| Mountain | 9 | 10 |
| Pacific | 20 | 21 |
| Plan size (%) | ||
| 0–24,999 | 29 | 28 |
| 25,000–99,999 | 47 | 46 |
| ≥100,000 | 25 | 26 |
| Model Type (%) | ||
| Group/Staff | 7 | 10 |
| Independent Practice | 10 | 11 |
| Association | ||
| Network/Mixed | 83 | 79 |
| Plan Start Year (%) | ||
| Before 1980 | < 1 | < 1 |
| 1980–1999 | 60 | 64 |
| After 2000 | 39 | 35 |
| Profit Status (%) | ||
| For Profit | 74 | 70 |
Abbreviations: HEDIS Healthcare Effectiveness Data and Information Set; SES socioeconomic status
Note: The SES index score is derived from zip code-level US census variables based on the Agency for Healthcare Research and Quality’s specifications; higher SES values imply higher SES (U.S. mean, 50).23
All numbers refer to percentages, with the exception of SES index score; values rounded to nearest whole number
*The χ2 or t test was performed to compare the characteristics of individuals who received high risk medications with those who did not; all tests resulted in P values of less than 0.001
In fully adjusted analyses (Table 2), female gender, low personal income, and white race were significant predictors of receipt of a HRM at the individual level. In comparison to the reference age group, which included those 65–69 years of age, the oldest age category appeared to have a protective effect, with significantly lower rates of receipt of HRMs among those enrollees. At the community level, SES index quintile showed a graded response, with lower SES scores associated with higher rates of receipt of HRMs. Although most of the census divisions had significantly higher rates of receipt of HRMs when compared to the reference New England division (with the exception of Middle Atlantic and the West North Central divisions), the extent of this difference was highest (> 10 %) among divisions in the Southern U.S. region, including the South Atlantic, East South Central, and West South Central divisions. Plan-level characteristics did not predict use of HRM medications.
Table 2.
Observed and Adjusted Rates of HEDIS Drugs to Avoid in the Elderly Measure, Receipt of at Least One High Risk Medication*
| Characteristics | Unadjusted Difference, % points | Model 1 | Model 2 | Model 3 |
|---|---|---|---|---|
| Sex | ||||
| Women | 11.2 | 10.8 (10.3, 11.3)† | 10.6 (10.1, 11.2) | 10.6 (10.0, 11.2) |
| Age group, years | ||||
| 65–69 | Reference | Reference | Reference | Reference |
| 70–74 | −0.4 | −0.4 (−0.7, 0.0) | −0.2 (−0.5, 0.1) | −0.2 (−0.4, 0.1) |
| 75–79 | −0.3 | −0.5 (−1.0, 0.0) | −0.1 (−0.5, 0.2) | −0.1 (−0.4, 0.3) |
| 80–84 | −0.3 | −0.8 (−1.4, −0.3) | −0.3 (−0.7, 0.1) | −0.3 (−0.7, 0.1) |
| ≥ 85 | −0.8 | −2.1 (−2.7, −1.4) | −1.3 (−1.8, -0.8) | −1.2 (−1.7, −0.8) |
| Race | ||||
| White | Reference | Reference | Reference | Reference |
| Black | −1.2 | −2.6 (−3.8, –1.5) | −4.2 (−4.8, –3.5) | −4.0 (−4.6, –3.3) |
| Other | −0.8 | −1.7 (−3.5, 0.1) | −2.1 (−3.2, –0.9) | −2.0 (−3.1, –1.0) |
| Personal income | ||||
| Low | 8.6 | 7.8 (6.4, 9.2) | 6.6 (5.6, 7.5) | 6.5 (5.5, 7.5) |
| Zip-code based SES index Score | ||||
| Quintile 1 (low) | 6.2 | – | 2.8 (2.0, 3.7) | 2.7 (1.9, 3.4) |
| Quintile 2 | 3.4 | – | 1.9 (1.4, 2.4) | 1.8 (1.3, 2.3) |
| Quintile 3 | 2.4 | – | 1.4 (0.9, 1.9) | 1.3 (0.8, 1.8) |
| Quintile 4 | 1.4 | – | 1.1 (0.8, 1.5) | 1.1 (0.7, 1.4) |
| Quintile 5 (high) | Reference | – | Reference | Reference |
| Geographic division | ||||
| New England | Reference | – | Reference | Reference |
| Middle Atlantic | 1.2 | – | 1.3 (0.1, 2.6) | 0.4 (−1.4, 2.3) |
| East North Central | 4.6 | – | 4.6 (3.3, 5.9) | 3.4 (1.5, 5.3) |
| West North Central | 3.0 | – | 2.8 (1.4, 4.2) | 1.9 (−0.0, 3.9)‡ |
| South Atlantic | 11.9 | – | 11.3 (9.0, 13.5) | 10.3 (7.7, 12.9) |
| East South Central | 14.0 | – | 12.4 (10.7, 14.0) | 11.2 (9.0, 13.2) |
| West South Central | 14.2 | – | 13.1 (9.7, 16.5) | 12.0 (8.6, 15.4) |
| Mountain | 6.0 | – | 5.3 (3.1, 7.6) | 4.4 (1.5, 7.4) |
| Pacific | 5.9 | – | 5.6 (3.5, 7.7) | 5.8 (3.6, 8.1) |
| Plan size | ||||
| 0–24,999 | 1.6 | – | – | 0.3 (−1.6, 2.2) |
| 25,000–99,999 | 1.3 | – | – | 1.0 (−0.8, 2.9) |
| ≥ 100,000 | Reference | – | – | Reference |
| Model Type | ||||
| Group/Staff | Reference | – | – | Reference |
| Independent practice association | 2.3 | – | – | 2.9 (−0.0, 5.8)‡ |
| Mixed/Network | 5.7 | – | – | 3.1 (0.8, 5.5)∥ |
| Year HMO began contract | ||||
| Before 1980 | Reference | – | – | Reference |
| 1980–1999 | −0.1 | – | – | −0.2 (−3.9, 3.5) |
| After 2000 | 2.8 | – | – | 0.6 (−2.9, 4.2) |
| Profit Status | ||||
| For Profit | 3.7 | – | – | 0.0 (−1.5, 1.5) |
Abbreviations: HEDIS Healthcare Effectiveness Data and Information Set; CI confidence interval; HMO health maintenance organization; SES socioeconomic status
*All estimates on the risk difference scale, reported as percentage point difference (95 % CI)
†Except where noted, confidence intervals that do not cross 0 are significant at P < 0.005
‡Confidence interval crosses 0, not significant at P < 0.005
∥P-value =0.008
HRRs vary substantially with respect to HEDIS measures of quality prescribing (Fig. 1). The HRR with the highest proportion of MA enrollees receiving at least one HRM was Albany, Georgia, where 38.2 % of enrollees received at least one HRM. The HRR with the highest proportion of enrollees receiving at least two HRMS was Alexandria, Louisiana, where 13.5 % received at least two different HRMs. The rate in Albany was approximately four times higher than the HRR with the lowest rate of receipt of at least one HRM—Mason City, Iowa. Of additional note, the rate of receipt of at least two HRMs in Alexandria was 20 times higher as compared to the HRR with the lowest rate—Worcester, Massachusetts (13 % vs. 0.7 %). The twenty lowest performing HRRs were all in the Southern region of the U.S. In contrast, only one of the twenty highest performing HRRs was in the South; the remainder were either in the Midwest or Northeast census regions. The results for the use of two or more HRMs were similar and are therefore not shown here.
Figure 1.
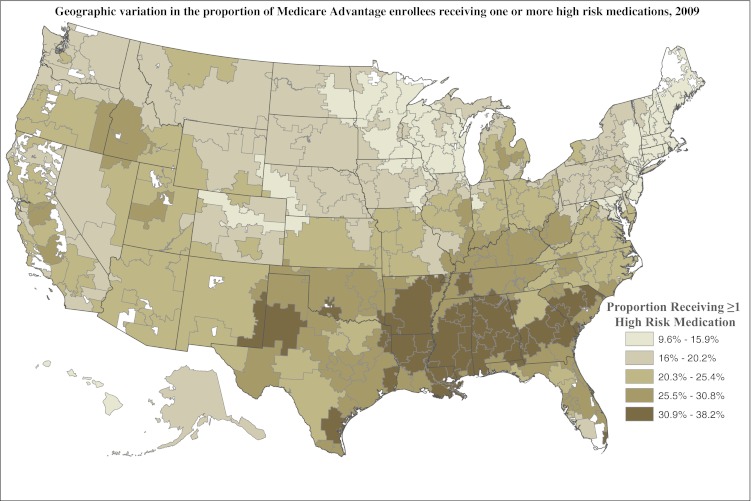
Quintiles of performance on HEDIS measure of quality of drug prescribing, according to hospital-referral region. Note: lower rates indicate better quality.
COMMENTS
In 2009, of the 6,204,824 eligible Medicare Advantage enrollees included in this population-based, cross-sectional analysis, 21.5 % received at least one HRM and 4.8 % received at least two, as defined by the HEDIS quality measures. In fully adjusted analyses, females had a 10.6 higher percentage point rate of receipt of at least one HRM (95 % CI: 10–11.2) compared to males. Persons residing in the West South Central, East South Central and South Atlantic census divisions had a percentage point rate of 10 or higher of receiving HRMs compared with those residing in New England. We observed increased rates of use of HRMs among white enrollees, individuals with low personal income, and those residing in areas of lower socioeconomic status.
The direction of the gender difference was not surprising, as some of the medications categorized as high risk by HEDIS are prescribed only to women (e.g. oral estrogens), or are treatments for conditions that are more prevalent in women. Examples include psychopharmacologic agents such as long-acting benzodiazepines,24 analgesics,25 and anticholinergics.26,27 Previous work examining use of HRMs in the community-dwelling elderly has also consistently identified males as having decreased risk of receipt of HRM medications.9
The magnitude of the gender difference is of particular note. One study that examined the persistent gender differences in utilization of HRMs reported that even after controlling for sociodemographic characteristics, number of medications, and comorbidities, women remained at significantly higher risk of receipt of HRMs.28 That racial minority status is associated with lower use of mental health services may also explain the slightly lower risk of HRM use in blacks and other non-white groups.29–31 More research is needed to better elucidate the process by which these gender and racial differences arise.
In addition to gender, region of residence appears to be one of the strongest predictors, with rate differences greater than 10 percentage points for the three Southern census divisions, as compared to the reference New England division. This finding is reflected in Figure 1, which suggests that geographic variation in HRM use is consistent with patterns described in the Medicare fee-for-service population,20 where the HRR with the highest rate of inappropriate medication use was also Alexandria, Louisiana. The prevailing maxim for the elderly Medicare population that “geography is destiny” seems applicable to the use of HRMs.
The existence of substantial geographic variation in healthcare quality, costs, and delivery has been well established in the literature.32 However, the interpretation of such extensive variation remains controversial. It is uncertain who or what principal actors or agents in the process of prescribing may underlie the geographic disparity of the magnitude seen with these HRM measures. One possible explanation is increased poly-pharmacy among those populations in the Southern region with the highest rates of HRM use. This population may also have a greater prevalence of chronic conditions, including for example obesity,33 which may precipitate use of such medications.34 Previous research has pointed to both poor health and poly-pharmacy9,35 as predictors for receipt of HRMs. However, a recent study of the Medicare fee-for-service population did not find an association between increased HRM use and overall expenditures on prescription drugs.20 Another possible explanation could be prescribing differences mediated organizationally and geographically vis-à-vis clinician training and education. Further, clinician prescribing behavior may reflect differences in patient preferences or formulary structure in health plan prescription drug coverage.36,37 Additional qualitative and quantitative research to better understand the causes and potential adverse consequences of this variation is warranted.
The primary strength of this study lies in its inclusion of a broad set of predictors of HRM use in a recent, nationally representative sample of all elderly Medicare managed care enrollees. Prior research studies of HRM use in the elderly have used older data,9,38 or focused on specific healthcare settings17,39–42 such as nursing homes or home health. In addition, no existing studies have included socioeconomic status and health plan-level variables that may also help explain propensity to receive a HRM. We found that, after accounting for geographic region, the organizational characteristics of health plans are not associated with the likelihood of receiving HRMs.
In 2006, the NCQA reported the use of HRMs among elderly MA enrollees to be 23.1 %.43 In the Medicare fee-for-service population, the rate was estimated at 25.8 % in 2010.20 The relative stability of the estimates over time and across different elderly populations underscores the challenges of changing prescribing behavior, as well as the need to further emphasize medication management of geriatric patients in clinical training.
Our study has some limitations. HEDIS data lacks information on the number of prescriptions and diagnoses or clinical comorbidities of enrollees; both have been found to be potential predictors of HRM use.9 As a result, we were not able to determine whether particular medications account for the majority of the HRM use in the MA population. The most recent research utilizing national survey data to assess HRM use in the community-dwelling elderly determined that the most prevalent HRMs of use include propoxyphene, amitriptyline, antihistamines, diazepam, muscle relaxants, gastrointestinal antispasmodics and indomethacin.35
We were unable to assess whether and to what extent differences in the prevalence of comorbid conditions may account for the geographic variation in HRM use. If, in fact, increased clinical comorbidities may explain the high rates of use of HRMs in the Southern region of the U.S., further efforts to target those populations for more thorough medication management and review should be supported. That there are in many cases alternative medications to those found on the HEDIS list underscores this point.44,45 These patients may also be at increased risk for other potentially inappropriate prescribing, including drug–disease interactions and/or drug–drug interactions.
The utility of quality measures of HRM use have been criticized for ignoring contextual factors associated with prescribing by using a “hit list” approach for identifying HRMs.46 Some clinicians argue that medications on the HEDIS list are in fact appropriate and safe for a subset of their patients, and therefore, the prevalence of HRM use may overestimate true rates of inappropriate medication use. Empirical evidence definitively linking use of HRMs to adverse health outcomes has also been limited, further mitigating efforts to reduce prescribing of such agents in routine clinical practice.
Despite these challenges, the HEDIS measures allow clinicians, health plans, and policymakers to assess HRM use over time and characterize elderly groups that are more likely to receive medications that may predispose them to increased morbidity and mortality. Moreover, prior studies found significant associations between use of specific HRMs and falls, fractures, and self-reported adverse drug events.13–15 Most HRMs also have alternatives that are safer for use in the elderly population.45
CONCLUSION
Understanding predictors of HRM use at the population level can inform quality improvement efforts to reduce the use of these agents. This study examined the prevalence and predictors of HRMs among a national sample of elderly managed care enrollees. We observed wide geographic variation in the use of these medications that were not explained by sociodemographic characteristics, with the Southern region of the United States having markedly higher rates of use of HRMs. Female gender, white race, low SES index score, and low personal income also predicted receipt of HRMs. Efforts to reduce prescribing of these medications among the elderly should consider the role these factors play in predicting their use.
Electronic Supplementary Material
(PDF 288 kb)
Acknowledgements
The authors would like to thank Dr. Gabriela Schmajuk for her assistance with the SES index score analysis. Both Dr. Qato and Dr. Trivedi had full access to all the data in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Funders
This work was supported by the Agency for Healthcare Research and Quality (1T32HS019657) and National Institute on Aging (5RC1AG036158). The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation of the manuscript.
Prior Presentations
These findings were presented during an oral presentation at the AcademyHealth conference in June, 2012, in Orlando, Florida.
Conflict of Interest
The authors declare that they do not have any conflicts of interest.
REFERENCES
- 1.Institute of Medicine (U.S.). Aspden P, Wolcott JA, Bootman JL, Cronenwett LR, Eds. Preventing Medication Errors. Committee on Identifying and Preventing Medication Errors. . Washington: National Academies Press; 2007.
- 2.Hohl CM, Zed PJ, Brubacher JR, Abu-Laban RB, Loewen PS, Purssell RA. Do emergency physicians attribute drug-related emergency department visits to medication-related problems? Ann Emerg Med. 2010;55(6):493–502. doi: 10.1016/j.annemergmed.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Hohl CM, Dankoff J, Colacone A, Afilalo M. Polypharmacy, adverse drug-related events, and potential adverse drug interactions in elderly patients presenting to an emergency department. Ann Emerg Med. 2001;38(6):666–671. doi: 10.1067/mem.2001.119456. [DOI] [PubMed] [Google Scholar]
- 4.Bond CA, Raehl CL. 2006 national clinical pharmacy services survey: clinical pharmacy services, collaborative drug management, medication errors, and pharmacy technology. Pharmacotherapy. 2008;28(1):1–13. doi: 10.1592/phco.28.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000;109(2):87–94. doi: 10.1016/S0002-9343(00)00451-4. [DOI] [PubMed] [Google Scholar]
- 6.Cahir C, Fahey T, Teeling M, Teljeur C, Feely J, Bennett K. Potentially inappropriate prescribing and cost outcomes for older people: a national population study. Br J Clin Pharmacol. 2010;69(5):543–552. doi: 10.1111/j.1365-2125.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007;147(11):755–U726. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 8.Fick D, Semla T, Beizer J, et al. American Geriatrics Society Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2012;60(4):616–631. doi: 10.1111/j.1532-5415.2012.03922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan C, Sangl J, Bierman AS, et al. Potentially inappropriate medication use in the community-dwelling elderly: findings from the 1996 Medical Expenditure Panel Survey. JAMA. 2001;286(22):2823–2829. doi: 10.1001/jama.286.22.2823. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenberg FR, Sun SX. The impact of medicare part D on prescription drug use by the elderly. Health Aff. 2007;26(6):1735–1744. doi: 10.1377/hlthaff.26.6.1735. [DOI] [PubMed] [Google Scholar]
- 11.Fu AZ, Tang AS, Wang N, Du DT, Jiang JZ. Effect of Medicare Part D on potentially inappropriate medication use by older adults. J Am Geriatr Soc. 2010;58(5):944–949. doi: 10.1111/j.1532-5415.2010.02809.x. [DOI] [PubMed] [Google Scholar]
- 12.Riley G, Zarabozo C. Trends in the health status of medicare risk contract enrollees. Health Care Financ Rev. Winter. 2006;28(2):81–95. [PMC free article] [PubMed] [Google Scholar]
- 13.Stockl KM, Le L, Zhang S, Harada AS. Clinical and economic outcomes associated with potentially inappropriate prescribing in the elderly. Am J Manag Care. 2010;16(1):e1–10. [PubMed] [Google Scholar]
- 14.Chrischilles EA, VanGilder R, Wright K, Kelly M, Wallace RB. Inappropriate medication use as a risk factor for self-reported adverse drug effects in older adults. J Am Geriatr Soc. 2009;57(6):1000–1006. doi: 10.1111/j.1532-5415.2009.02269.x. [DOI] [PubMed] [Google Scholar]
- 15.Berdot S, Bertrand M, Dartigues JF, Fourrier A, Tavernier B, Ritchie K, et al. Inappropriate medication use and risk of falls—a prospective study in a large community-dwelling elderly cohort. BMC Geriatr. 2009;9(1):30. doi: 10.1186/1471-2318-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Use of high-risk medications in the elderly: Percentage of Medicare members 65 years of age and older who received at least one high-risk medication. Available at: http://www.qualitymeasures.ahrq.gov/content.aspx?id=34685. Accessed September 25, 2012.
- 17.Pugh MJV, Hanlon JT, Zeber JE, Bierman A, Cornell J, Berlowitz DR. Assessing potentially inappropriate prescribing in the elderly veterans affairs population using the HEDIS 2006 quality measure. J Manag Care Pharm. 2006;12(7):537–545. doi: 10.18553/jmcp.2006.12.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Dartmouth atlas of health care, 1999. Chicago Ill.: American Hospital Publishing; 1999.
- 19.Landon BE, Keating NL, Barnett ML, et al. Variation in patient-sharing networks of physicians across the United States. JAMA. 2012;308(3):265–273. doi: 10.1001/jama.2012.7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Baicker K, Newhouse JP. Geographic variation in the quality of prescribing. N Engl J Med. 2010;363(21):1985–1988. doi: 10.1056/NEJMp1010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baicker K, Chandra A, Skinner JS, Wennberg JE. Who you are and where you live: how race and geography affect the treatment of medicare beneficiaries. Health Aff. 2004;Suppl Variation:VAR33-44. [DOI] [PubMed]
- 22.Koroukian SM, Dahman B, Copeland G, Bradley CJ. The utility of the state buy-in variable in the Medicare denominator file to identify dually eligible Medicare-Medicaid beneficiaries: a validation study. Health Serv Res. 2010;45(1):265–282. doi: 10.1111/j.1475-6773.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agency for Healthcare Research and Quality. Creation of new race-ethnicity codes and socioeconomic status (SES) indicators for medicare beneficiaries. Available at: http://www.ahrq.gov/qual/medicareindicators. Accessed September 25, 2012.
- 24.Yang HWK, Simoni-Wastila L, Zuckerman IH, Stuart B. Benzodiazepine use and expenditures for Medicare beneficiaries and the implications of Medicare Part D exclusions. Psychiatr Serv. 2008;59(4):384–391. doi: 10.1176/appi.ps.59.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goulding MR. Inappropriate medication prescribing for elderly ambulatory care patients. Arch Intern Med. 2004;164(3):305–312. doi: 10.1001/archinte.164.3.305. [DOI] [PubMed] [Google Scholar]
- 26.Morabia A, Fabre J, Dunand JP. The influence of patient and physician gender on prescription of psychotropic drugs. J Clin Epidemiol. 1992;45(2):111–116. doi: 10.1016/0895-4356(92)90003-6. [DOI] [PubMed] [Google Scholar]
- 27.Simoni-Wastila L. Gender and psychotropic drug use. Med Care. 1998;36(1):88–94. doi: 10.1097/00005650-199801000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Bierman AS, Pugh MJ, Dhalla I, et al. Sex differences in inappropriate prescribing among elderly veterans. Am J Geriatr Pharmacother. 2007;5(2):147–161. doi: 10.1016/j.amjopharm.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Bao Y, Alexopoulos GS, Casalino LP, et al. Collaborative depression care management and disparities in depression treatment and outcomes. Arch Gen Psychiatr. 2011;68(6):627–636. doi: 10.1001/archgenpsychiatry.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alegria M, Chatterji P, Wells K, et al. Disparity in Depression Treatment Among Racial and Ethnic Minority Populations in the United States. Psychiatr Serv. 2008;59(11):1264–1272. doi: 10.1176/appi.ps.59.11.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keyes KM, Hatzenbuehler ML, Alberti P, Narrow WE, Grant BF, Hasin DS. Service utilization differences for Axis I psychiatric and substance use disorders between white and black adults. Psychiatr Serv. 2008;59(8):893–901. doi: 10.1176/appi.ps.59.8.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenthal T. Geographic variation in health care. Annu Rev Med. 2012;63:493–509. doi: 10.1146/annurev-med-050710-134438. [DOI] [PubMed] [Google Scholar]
- 33.Maps of Diagnosed Diabetes and Obesity in 1994, 2000, and 2010 2011. Available at: http://www.cdc.gov/diabetes/statistics/slides/maps_diabetesobesity94.pdf. Accessed September 25, 2012.
- 34.Ogden CL, Flegal KM. Prescription Medication Use Among Normal Weight, Overweight, and Obese Adults, United States, 2005–2008. Ann Epidemiol. 2012;22(2):8p. doi: 10.1016/j.annepidem.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Zhang YJ, Liu WW, Wang JB, Guo JJ. Potentially inappropriate medication use among older adults in the USA in 2007. Age Ageing. 2011;40(3):398–401. doi: 10.1093/ageing/afr012. [DOI] [PubMed] [Google Scholar]
- 36.Wang YR, Pauly MV. Spillover effects of restrictive drug formularies on physician prescribing behavior: Evidence from Medicaid. J Econ Manage Strat Fal. 2005;14(3):755–773. doi: 10.1111/j.1530-9134.2005.00081.x. [DOI] [Google Scholar]
- 37.Joyce GF, Escarce JJ, Solomon MD, Goldman DP. Employer drug benefit plans and spending on prescription drugs. JAMA. 2002;288(14):1733–1739. doi: 10.1001/jama.288.14.1733. [DOI] [PubMed] [Google Scholar]
- 38.Fu AZ, Liu GG, Christensen DB. Inappropriate medication use and health outcomes in the elderly. J Am Geriatr Soc. 2004;52(11):1934–1939. doi: 10.1111/j.1532-5415.2004.52522.x. [DOI] [PubMed] [Google Scholar]
- 39.Bao YH, Shao HB, Bishop TF, Schackman BR, Bruce ML. Inappropriate Medication in a National Sample of US Elderly Patients Receiving Home Health Care. J Gen Intern Med. 2012;27(3):304–310. doi: 10.1007/s11606-011-1905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Gollarte F, Baleriola-Julvez J, Ferrero-Lopez I, Cruz-Jentoft AJ. Inappropriate Drug Prescription at Nursing Home Admission. J Am Med Dir Assoc. Jan 2012;13(1). [DOI] [PubMed]
- 41.Parsons C, Johnston S, Mathie E, et al. Potentially Inappropriate Prescribing in Older People with Dementia in Care Homes A Retrospective Analysis. Drugs Aging. 2012;29(2):143–155. doi: 10.2165/11598560-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Ryan C, O’Mahony D, Kennedy J, Weedle P, Byrne S. Potentially inappropriate prescribing in an Irish elderly population in primary care. Br J Clin Pharmacol. 2009;68(6):936–947. doi: 10.1111/j.1365-2125.2009.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Committee for Quality Assurance. Continuous improvement and the expansion of quality improvement: The state of health care quality 2011. Available at: http://www.ncqa.org/LinkClick.aspx?fileticket=J8kEuhuPqxk%3d&tabid=836. Accessed September 25, 2012.
- 44.Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170(18):1648–1654. doi: 10.1001/archinternmed.2010.355. [DOI] [PubMed] [Google Scholar]
- 45.PL Detail-Document, Potentially Harmful Drugs in the Elderly: Beers List. Pharmacist’s Letter/Prescriber’s Letter. Document # 280610. Available at: http://pharmacistsletter.therapeuticresearch.com/pl/ArticleDD.aspx?nidchk=1&cs=&s=PL&pt=2&segment=4413&dd=280610. Accessed September 25, 2012.
- 46.Avorn J. Improving drug use in elderly patients: getting to the next level. JAMA. 2001;286(22):2866–2868. doi: 10.1001/jama.286.22.2866. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 288 kb)


