Abstract
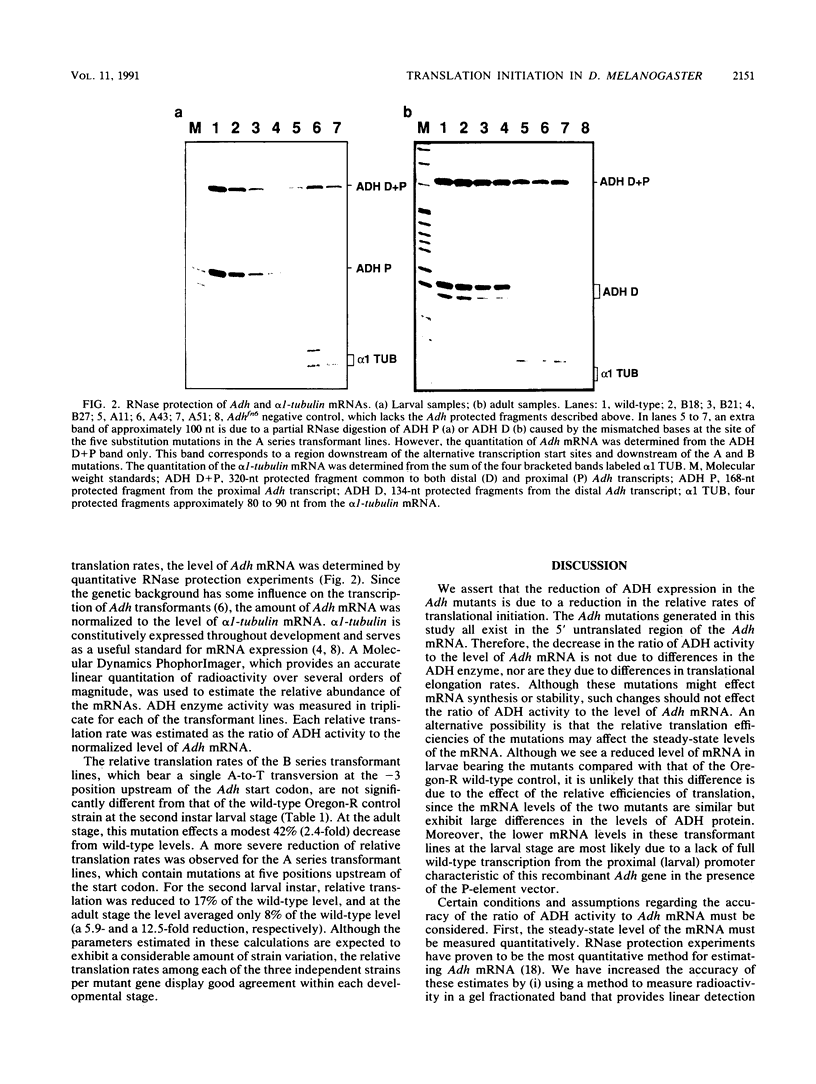
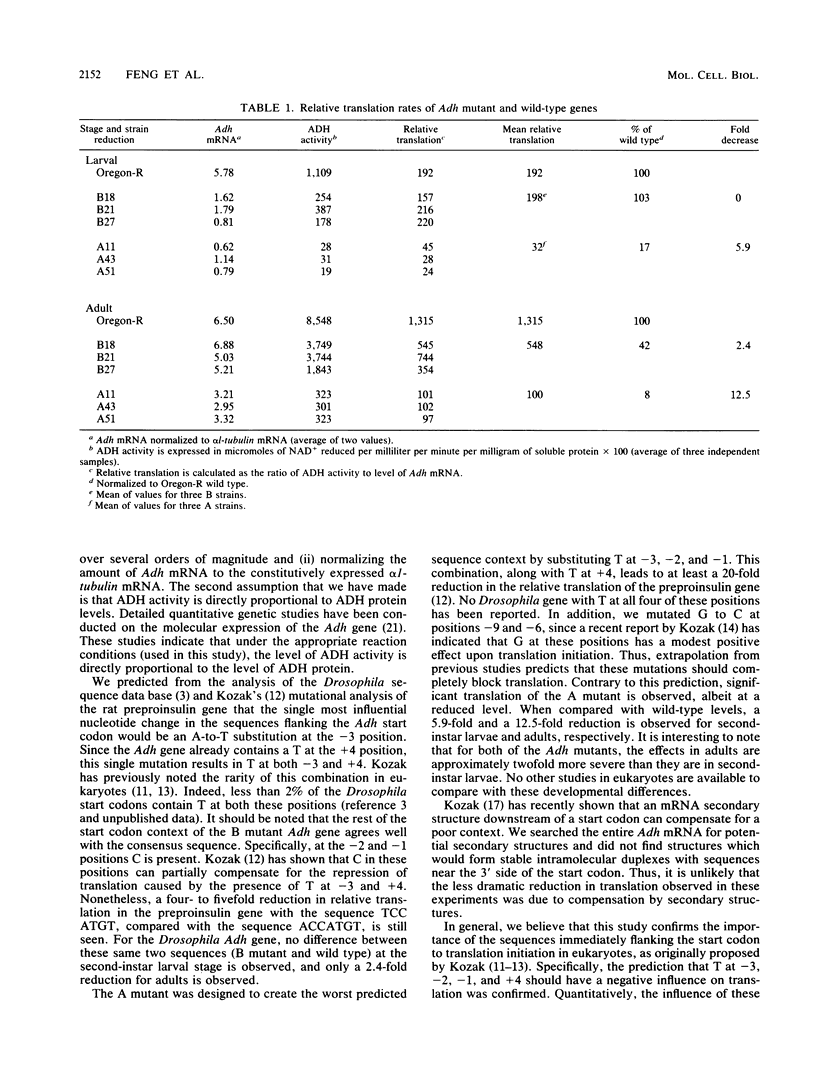
The importance to in vivo translation of sequences immediately upstream of the Drosophila alcohol dehydrogenase (Adh) start codon was examined at two developmental stages. Mutations were introduced into the Adh gene in vitro, and the mutant gene was inserted into the genome via germ line transformation. An A-to-T substitution at the -3 position did not affect relative translation rates of the ADH protein at the second-instar larval stage but resulted in a 2.4-fold drop in translation of ADH at the adult stage. A second mutant gene, containing five mutations in the region -1 to -9, was designed to completely block translation initiation. However, transformant lines bearing these mutations still exhibit detectable ADH, albeit at substantially reduced levels. The average fold reduction at the second-instar larval stage was 5.9, while at the adult stage a 12.5-fold reduction was observed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baim S. B., Sherman F. mRNA structures influencing translation in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1988 Apr;8(4):1591–1601. doi: 10.1128/mcb.8.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin V., Maniatis T. Role of transcriptional interference in the Drosophila melanogaster Adh promoter switch. Nature. 1989 Jan 19;337(6204):279–282. doi: 10.1038/337279a0. [DOI] [PubMed] [Google Scholar]
- Dasso M. C., Milburn S. C., Hershey J. W., Jackson R. J. Selection of the 5'-proximal translation initiation site is influenced by mRNA and eIF-2 concentrations. Eur J Biochem. 1990 Jan 26;187(2):361–371. doi: 10.1111/j.1432-1033.1990.tb15313.x. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R., Watanabe C. K., de Boer H. A. Compilation and comparison of the sequence context around the AUG startcodons in Saccharomyces cerevisiae mRNAs. Nucleic Acids Res. 1987 Apr 24;15(8):3581–3593. doi: 10.1093/nar/15.8.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. Developmental regulation of Drosophila alpha-tubulin genes. Cell. 1982 May;29(1):91–98. doi: 10.1016/0092-8674(82)90093-9. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. The phosphorylation state of eucaryotic initiation factor 2 alters translational efficiency of specific mRNAs. Mol Cell Biol. 1989 Mar;9(3):946–958. doi: 10.1128/mcb.9.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Weber U., Gehring W. J. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 1987 May 26;15(10):3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol. 1987 Aug 20;196(4):947–950. doi: 10.1016/0022-2836(87)90418-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Context effects and inefficient initiation at non-AUG codons in eucaryotic cell-free translation systems. Mol Cell Biol. 1989 Nov;9(11):5073–5080. doi: 10.1128/mcb.9.11.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8301–8305. doi: 10.1073/pnas.87.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Effects of intercistronic length on the efficiency of reinitiation by eucaryotic ribosomes. Mol Cell Biol. 1987 Oct;7(10):3438–3445. doi: 10.1128/mcb.7.10.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Laurie C. C., Stam L. F. Quantitative analysis of RNA produced by slow and fast alleles of Adh in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5161–5165. doi: 10.1073/pnas.85.14.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroni G., Laurie-Ahlberg C. C., Adams D. A., Wilton A. N. Genetic variation in the expression of ADH in Drosophila melanogaster. Genetics. 1982 Jul-Aug;101(3-4):431–446. doi: 10.1093/genetics/101.3-4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P. P., Hinnebusch A. G. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986 Apr 25;45(2):201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Murtha M. T., Cavener D. R. Ecdysteroid regulation of glucose dehydrogenase and alcohol dehydrogenase gene expression in Drosophila melanogaster. Dev Biol. 1989 Sep;135(1):66–73. doi: 10.1016/0012-1606(89)90158-9. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar H. K., Viitanen P. V., Padan E., Trumble W. R., Poonian M. S., McComas W., Kaback H. R. Oligonucleotide-directed site-specific mutagenesis of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:214–230. doi: 10.1016/s0076-6879(86)25019-3. [DOI] [PubMed] [Google Scholar]
- Stroeher V. L., Jorgensen E. M., Garber R. L. Multiple transcripts from the Antennapedia gene of Drosophila melanogaster. Mol Cell Biol. 1986 Dec;6(12):4667–4675. doi: 10.1128/mcb.6.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]



