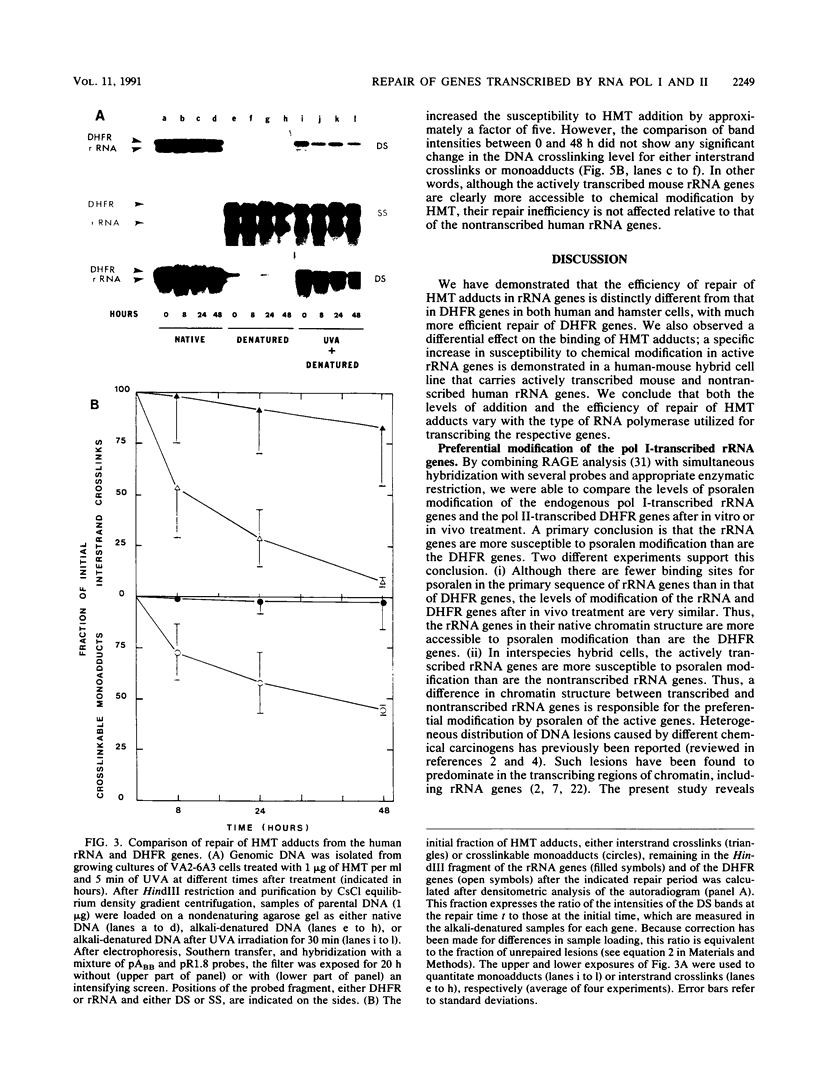
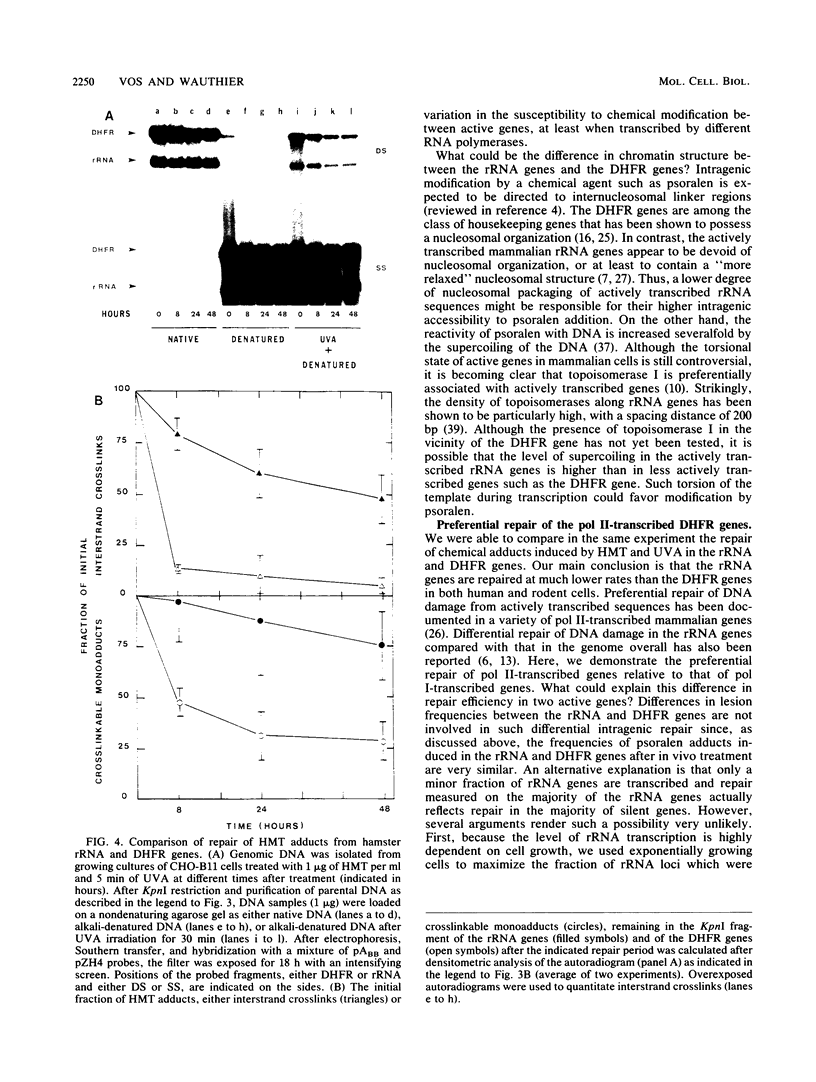
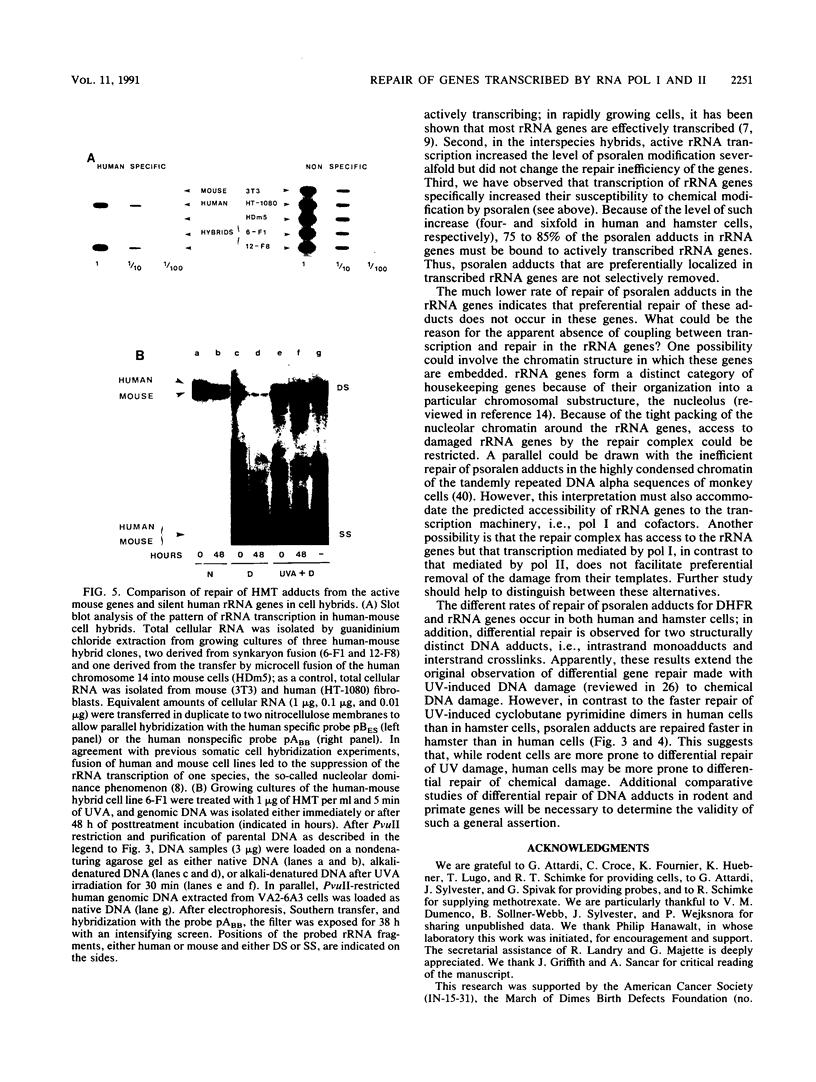
Abstract
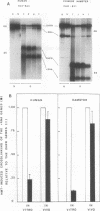
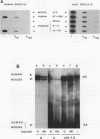
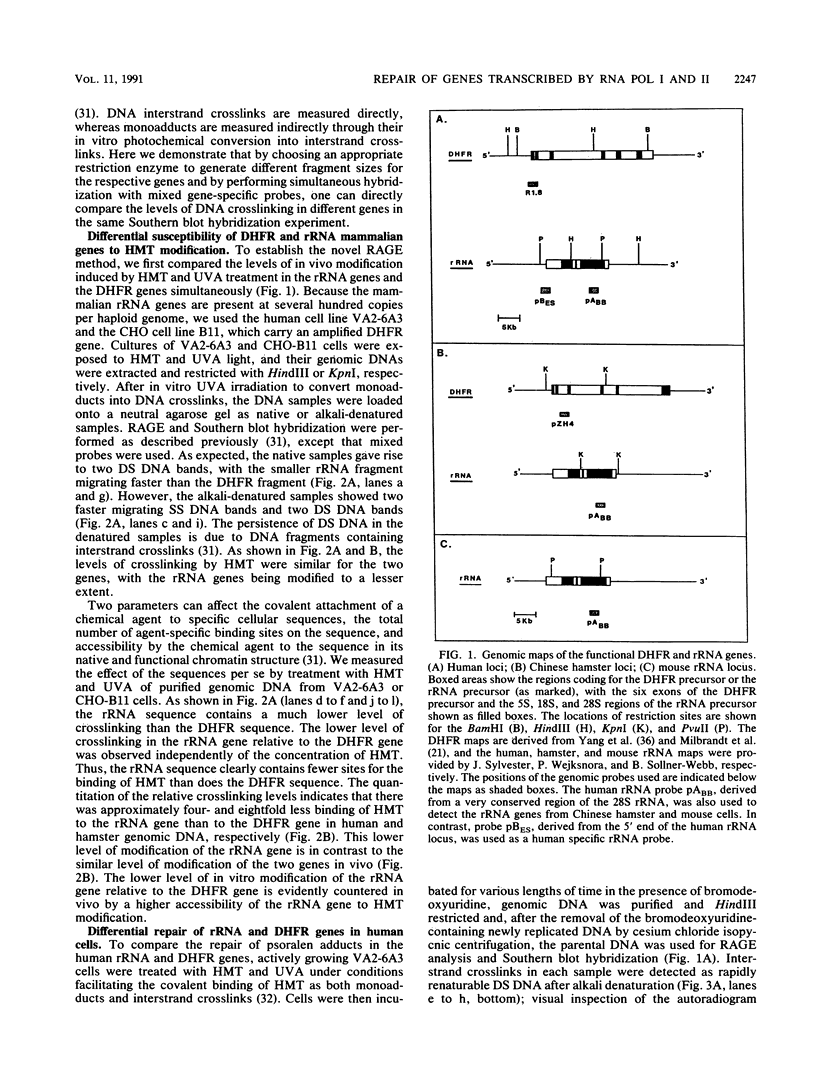
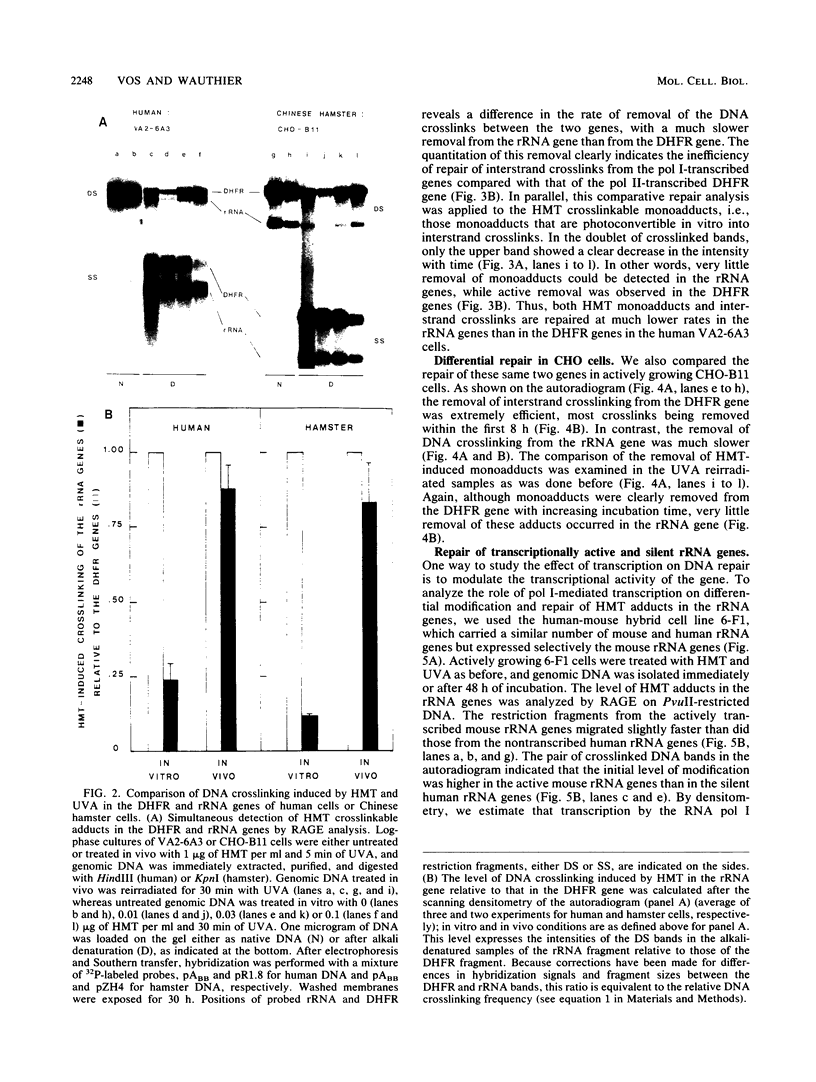
We have developed a general quantitative method for comparing the levels of drug-induced DNA crosslinking in specific mammalian genes. We observed a dramatic difference between the efficiency of the removal of both psoralen monoadducts and interstrand crosslinks from the rRNA genes and the efficiency of their removal from the dihydrofolate reductase (DHFR) gene in cultured human and hamster cells. While 90% of the interstand crosslinks were removed from the human DHFR gene in 48 h, less than 25% repair occurred in the rRNA genes. Similarly, in Chinese hamster ovary cells, 85% repair of interstrand crosslinks occurred within 8 h in the DHFR gene versus only 20% repair in the rRNA genes. The preferential repair of the DHFR gene relative to that of the rRNA genes was also observed for psoralen monoadducts in cells from both mammalian species. In human-mouse hybrid cells, the active mouse rRNA genes were five times more susceptible to psoralen modification than are the silent rRNA human genes, but adduct removal was similarly inefficient for both classes. We conclude that the repair of chemical damage such as psoralen photoadducts in an expressed mammalian gene may depend upon the class of transcription to which it belongs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Xeroderma pigmentosum: a human disease in which an initial stage of DNA repair is defective. Proc Natl Acad Sci U S A. 1969 Jun;63(2):428–435. doi: 10.1073/pnas.63.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn S. M., Lieberman M. W. The use of antibodies to 5-bromo-2'-deoxyuridine for the isolation of DNA sequences containing excision-repair sites. J Biol Chem. 1984 Oct 25;259(20):12456–12462. [PubMed] [Google Scholar]
- Conconi A., Widmer R. M., Koller T., Sogo J. M. Two different chromatin structures coexist in ribosomal RNA genes throughout the cell cycle. Cell. 1989 Jun 2;57(5):753–761. doi: 10.1016/0092-8674(89)90790-3. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Loss of mouse chromosomes in somatic cell hybrids between HT-1080 human fibrosarcoma cells and mouse peritioneal macrophages. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3248–3252. doi: 10.1073/pnas.73.9.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. H., Reudelhuber T. L., Garrard W. T. Varigated chromatin structures of mouse ribosomal RNA genes. J Mol Biol. 1983 Jun 15;167(1):133–155. doi: 10.1016/s0022-2836(83)80038-2. [DOI] [PubMed] [Google Scholar]
- Futcher B. Supercoiling and transcription, or vice versa? Trends Genet. 1988 Oct;4(10):271–272. doi: 10.1016/0168-9525(88)90166-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez I. L., Gorski J. L., Campen T. J., Dorney D. J., Erickson J. M., Sylvester J. E., Schmickel R. D. Variation among human 28S ribosomal RNA genes. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7666–7670. doi: 10.1073/pnas.82.22.7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Retinoblastoma and the progression of tumor genetics. Trends Genet. 1988 May;4(5):125–128. doi: 10.1016/0168-9525(88)90134-5. [DOI] [PubMed] [Google Scholar]
- Irvin T. R., Wogan G. N. Quantitative and qualitative characterization of aflatoxin B1 adducts formed in vivo within the ribosomal RNA genes of rat liver DNA. Cancer Res. 1985 Aug;45(8):3497–3502. [PubMed] [Google Scholar]
- Jordan G. At the heart of the nucleolus. Nature. 1987 Oct 8;329(6139):489–490. doi: 10.1038/329489a0. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Lee M. M., Scotto J. DNA repair protects against cutaneous and internal neoplasia: evidence from xeroderma pigmentosum. Carcinogenesis. 1984 Apr;5(4):511–514. doi: 10.1093/carcin/5.4.511. [DOI] [PubMed] [Google Scholar]
- Leys E. J., Crouse G. F., Kellems R. E. Dihydrofolate reductase gene expression in cultured mouse cells is regulated by transcript stabilization in the nucleus. J Cell Biol. 1984 Jul;99(1 Pt 1):180–187. doi: 10.1083/jcb.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo T. G., Handelin B., Killary A. M., Housman D. E., Fournier R. E. Isolation of microcell hybrid clones containing retroviral vector insertions into specific human chromosomes. Mol Cell Biol. 1987 Aug;7(8):2814–2820. doi: 10.1128/mcb.7.8.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher V. M., Dorney D. J., Mendrala A. L., Konze-Thomas B., McCormick J. J. DNA excision-repair processes in human cells can eliminate the cytotoxic and mutagenic consequences of ultraviolet irradiation. Mutat Res. 1979 Sep;62(2):311–323. doi: 10.1016/0027-5107(79)90087-3. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. D., Azizkhan J. C., Greisen K. S., Hamlin J. L. Organization of a Chinese hamster ovary dihydrofolate reductase gene identified by phenotypic rescue. Mol Cell Biol. 1983 Jul;3(7):1266–1273. doi: 10.1128/mcb.3.7.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross P. M., Yu H. S. Interstrand crosslinks due to 4,5',8-trimethylpsoralen and near ultraviolet light in specific sequences of animal DNA. Effect of constitutive chromatin structure and of induced transcription. J Mol Biol. 1988 May 20;201(2):339–351. doi: 10.1016/0022-2836(88)90142-8. [DOI] [PubMed] [Google Scholar]
- Sauerbier W., Hercules K. Gene and transcription unit mapping by radiation effects. Annu Rev Genet. 1978;12:329–363. doi: 10.1146/annurev.ge.12.120178.001553. [DOI] [PubMed] [Google Scholar]
- Sentenac A. Eukaryotic RNA polymerases. CRC Crit Rev Biochem. 1985;18(1):31–90. doi: 10.3109/10409238509082539. [DOI] [PubMed] [Google Scholar]
- Shimada T., Nienhuis A. W. Only the promoter region of the constitutively expressed normal and amplified human dihydrofolate reductase gene is DNase I hypersensitive and undermethylated. J Biol Chem. 1985 Feb 25;260(4):2468–2474. [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]
- Thacker J. The molecular nature of mutations in cultured mammalian cells: a review. Mutat Res. 1985 Jun-Jul;150(1-2):431–442. doi: 10.1016/0027-5107(85)90140-x. [DOI] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987 Aug 28;50(5):789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- Wauthier E. L., Hanawalt P. C., Vos J. M. Differential repair and replication of damaged DNA in ribosomal RNA genes in different CHO cell lines. J Cell Biochem. 1990 Jun;43(2):173–183. doi: 10.1002/jcb.240430208. [DOI] [PubMed] [Google Scholar]
- Weber C. A., Salazar E. P., Stewart S. A., Thompson L. H. Molecular cloning and biological characterization of a human gene, ERCC2, that corrects the nucleotide excision repair defect in CHO UV5 cells. Mol Cell Biol. 1988 Mar;8(3):1137–1146. doi: 10.1128/mcb.8.3.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Yang J. K., Masters J. N., Attardi G. Human dihydrofolate reductase gene organization. Extensive conservation of the G + C-rich 5' non-coding sequence and strong intron size divergence from homologous mammalian genes. J Mol Biol. 1984 Jun 25;176(2):169–187. doi: 10.1016/0022-2836(84)90419-4. [DOI] [PubMed] [Google Scholar]
- Yoakum G. H., Cole R. S. Cross-linking and relaxation of supercoiled DNA by psoralen and light. Biochim Biophys Acta. 1978 Dec 21;521(2):529–546. doi: 10.1016/0005-2787(78)90295-2. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Soreng A. L., Bowe A. E. Fragile sites are targets of diverse mutagens and carcinogens. Oncogene. 1987 Mar;1(1):59–69. [PubMed] [Google Scholar]
- Zhang H., Wang J. C., Liu L. F. Involvement of DNA topoisomerase I in transcription of human ribosomal RNA genes. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1060–1064. doi: 10.1073/pnas.85.4.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolan M. E., Cortopassi G. A., Smith C. A., Hanawalt P. C. Deficient repair of chemical adducts in alpha DNA of monkey cells. Cell. 1982 Mar;28(3):613–619. doi: 10.1016/0092-8674(82)90216-1. [DOI] [PubMed] [Google Scholar]
- van Duin M., de Wit J., Odijk H., Westerveld A., Yasui A., Koken M. H., Hoeijmakers J. H., Bootsma D. Molecular characterization of the human excision repair gene ERCC-1: cDNA cloning and amino acid homology with the yeast DNA repair gene RAD10. Cell. 1986 Mar 28;44(6):913–923. doi: 10.1016/0092-8674(86)90014-0. [DOI] [PubMed] [Google Scholar]