Abstract
Background
Reverse transcription quantitative PCR has become a powerful technique to monitor mRNA transcription in response to different environmental conditions in many bacterial species. However, correct evaluation of data requires accurate and reliable use of reference genes whose transcription does not change during the course of the experiment. In the present study exposure to different growth conditions was used to validate the transcription stability of eight reference gene candidates in three strains from two subspecies of Francisella noatunensis, a pathogen causing disease in both warm and cold water fish species.
Results
Relative transcription levels for genes encoding DNA gyrase (gyrA), RNA polymerase beta subunit (rpoB), DNA polymerase I (polA), cell division protein (ftsZ), outer membrane protein (fopA), riboflavin biosynthesis protein (ribC), 16S ribosomal RNA (16S rRNA) and DNA helicases (uvrD) were quantified under exponential, stationary and iron-restricted growth conditions. The suitability of selected reference genes for reliable interpretation of gene expression data was tested using the virulence-associated intracellular growth locus subunit C (iglC) gene.
Conclusion
Although the transcription stability of the reference genes was slightly different in the three strains studied, fopA, ftsZ and polA proved to be the most stable and suitable for normalization of gene transcription in Francisella noatunensis ssp.
Keywords: Gene expression, RT-qPCR, Francisella noatunensis, Fish, Francisellosis
Background
Systemic infection caused by the Gram-negative bacterium Francisella noatunensis remains a serious threat to Atlantic cod Gadhus morhua L. farming in Norway. Similar diseases associated with different strains of F. noatunensis have been reported in both fresh- and seawater farmed fish in Taiwan and Japan [1] Chile [2], America [3], and Great Britain [4]. Large knowledge gaps exist, however, in relation to pathogenesis and mechanisms of disease development. Recent genome analysis has revealed the presence of several virulence determinant loci in fish pathogenic Francisella spp., which share a high degree of sequence identity with the human pathogen F. tularensis[5]. Some of the most interesting genes are localized on the 33-kb Francisella Pathogenicity Island (FPI) [6,7], including genes of the intracellular growth locus operon iglABCD. The iglC gene has also been shown to be important for virulence in F. noatunensis ssp. orientalis[8,9].
One way of investigating disease pathogenesis at the molecular level is by reverse transcription quantitative PCR (RT-qPCR) analysis of gene transcription at different stages of disease development. However, use of RT-qPCR for gene transcription studies has its pitfalls. The method has an intrinsic requirement for normalization of target gene transcription levels against that of reference genes to ensure reliable data interpretation, as exemplified by Dedha et al. [10], and Guitierrez et al. [11]. The use of a minimum of three validated reference genes has been suggested [12]. Similarly, the importance of standardization of RNA extraction techniques, evaluation of RNA quality and the use of reference genes has been emphasized in several publications [13-15].
In this study, the transcriptional stability of eight reference genes in Francisella noatunensis ssp. was investigated under three different environmental conditions using the excel-based software geNorm [12]. To accurately quantify changes in transcription levels of specific mRNA targets, the iglC gene was chosen and normalized against the selected reference genes subjected to the same growth conditions using the established protocol.
Methods
Bacterial strains and growth conditions
The three isolates used in this study represent different fish pathogenic F. noatunensis strains. F. noatunensis spp. noatunensis (NCIMB14265T) was isolated from diseased Atlantic cod Gadus morhua L. in Norway [16]. F. noatunensis ssp. noatunensis PQ 1106 was isolated from diseased Atlantic salmon Salmo salar L. in Chile [2]. F. noatunensis ssp. orientalis DSM21254T was isolated from three-line grunt, Parapristipoma trilinineatum L. in Japan [1].
The strains were stored at -80°C in growth medium containing 20% glycerol. Prior to experimentation cultures were maintained on Eugon Chocolate Ferric Agar (ECFA) plates and incubated at 20 – 22°C. ECFA consists of 30.4 g/l BD Bacto™ Eugon Broth (Difco Laboratories) 15 g/l Microbiology Agar (Merck), 5% bovine blood (Håtunlab AB) and 2 mM FeCl3 (Sigma-Aldrich). BD Bacto™ Eugon Broth supplemented with 2 mM FeCl3 (EBF) was used for liquid cultures.
The experimental conditions tested included early exponential growth phase, stationary growth phase and an iron-depleted environment. For the growth phase studies, 10 ml EBF was inoculated with colony material from ECFA plates and incubated at 20 – 22°C with gentle shaking (150 rpm). Two parallel cultures were made for each strain on subsequent days. Optical density (OD) at 600 nm was measured with a Genesys 20 spectrophotometer (Thermo Scientific). For the iron depletion studies, the strains were inoculated into 10 ml EBF and incubated for 3 days at 22°C with shaking. The bacteria were subsequently pelleted by centrifugation (4000 g, 5 min), resuspended in fresh Eugon Broth without supplemented FeCl3 (EB) and grown for 24 hrs. Thereafter, the cultures were washed twice with PBS and once with EB supplemented with 2, 2′-dipyridyl (DP; Sigma-Aldrich) at a final concentration of 100 μM (EB/DP), to ensure removal of iron carried over from the previous medium. Washed bacteria were then used to inoculate cultures of EBF or EB/DP. The cultures were grown with shaking at 22°C for 24 h, after which the cells were immediately stabilized by adding 2 volumes of RNAProtect Bacteria reagent (QIAGEN).
Primer design, PCR efficiency
Potential reference genes were chosen based on a literature review of reference genes used in RT-qPCR for Francisella spp. (see Additional file 1), in addition to other commonly used bacterial reference genes. Primers were designed to target eight potential reference genes and the putative virulence gene iglC (see Table 1). The examined reference genes were uvrD (helicase, separates two annealed nucleic acid strands during DNA replication, transcription, translation, recombination, DNA repair and ribosome biogenesis) rpoB (β subunit of RNA polymerase), gyrA (DNA topoisomerase II), polA (DNA polymerase I), fopA (Francisella outer membrane protein A), ftsZ (encoding a prokaryotic cytoskeletal protein important for cell division), ribC (riboflavin synthetase) and 16S rRNA (small ribosomal RNA subunit). Primer efficiencies were determined using 10-fold dilution series of cDNA and genomic DNA as template for qPCR reactions.
Table 1.
Primers used for RT-qPCR in the present study
| |
|
|
|
Primer efficiency |
|
|
|
|---|---|---|---|---|---|---|---|
| Target gene | Forward primer (5′- 3′) | Reverse primer (5′- 3′) | Amplicon size | Fn. ssp. noatunensis | Fn. ssp. orientalis | Gene ID | Position |
|
uvrD |
ACTATTTGTCGCGGGTCCTT |
TCAAAGAAACGAAAACCTCCA |
82 bp |
2.050 |
1.959 |
12951493 |
596634-596715 |
|
rpoB |
GTGGTAAAGCGCAATTTGGT |
CAGCACCATATGCTTGTAACG |
72 bp |
1.986 |
1.988 |
12951517 |
620011-620082 |
|
gyrA |
CGAGCTTTACGAGCTGCTTC |
TCTTTTAGAGAACCCTAAAGAGGCT |
87 bp |
1.982 |
2.000 |
12952071 |
1187616-1187702 |
|
polA |
AGCTGGAACTGGTCGTAATCA |
ATCAGCATCTTCAGCAGCATA |
82 bp |
1.959 |
1.950 |
12951484 |
584274-584355 |
|
fopA |
TACTGGTGCATGGGATGTTG |
TCTTGGAGCCATTGTCTGAA |
100 bp |
1.902 |
1.938 |
12952182 |
1297252-1297351 |
|
ftsZ |
TACCATACTCAGCGGCTTTC |
GCGCCTGTAGTTGCTGAAGT |
112 bp |
1.986 |
1.997 |
12952792 |
136099-136210 |
|
ribC |
ATCTCAACTAGCCACGCTCC |
CGGTGGACACATGGTACAAG |
87 bp |
1.946 |
1.950 |
12952738 |
84136-84222 |
|
16S rRNA |
AACGACTGTTAATACCGCATAATATCTG [17] |
CCTTACCCTACCAACTAGCTAATCCA [17] |
101 bp |
1.954 |
1.954 |
12951375 |
463870-463970 |
| iglC | TAGGCGTATAACACTGGCTGC | TGCTATAGAAGGCGGAGAGG | 70 bp | 2.006 | 1.905 | 12951826 | 930145-930214 |
The complete genome of F. noatunensis ssp. orientalis strain Toba 04 (Accession number NC_017909), was used as a reference to assign the Gene ID.
RNA isolation and reverse transcription
Bacterial cells were stabilized with RNAProtect Bacteria Reagent (QIAGEN) and total RNA extracted from 500 μl early exponential growth phase cultures and 250 μl stationary growth phase cultures using RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions. A 15 minute on-column DNase digestion with RNase-Free DNase (QIAGEN) was performed to ensure removal of contaminating genomic DNA, as suggested by the manufacturer. For each strain, three RNA extractions were performed from exponential and stationary growth phases, while two extractions were performed for the iron-depletion experiment. RNA concentration and purity, determined by 260/280 and 260/230 ratios, were measured with a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies Inc.). RNA integrity was assessed by gel electrophoresis as described by BioRad Technical Note 5396 [18] using 500 ng of the extracted RNA and evaluation of the ratio between the bands corresponding to 16S and 23S ribosomal RNA. Reverse transcription of 1 μg extracted RNA in 20 μl reactions was performed with QuantiTect Reverse Transcription kit (QIAGEN) using random primers according to the manufacturer’s instructions. A control with omitted reverse transcriptase was performed for each extraction to check for the presence of contaminating genomic DNA. After reverse transcription, the samples were diluted 1:10 in DEPC treated H2O and used as templates for qPCR.
Reference gene validation
Quantitative PCR carried out with the Stratagene Mx3005 thermal cycler (Stratagene, La Jolla, San Diego, CA) was performed in a 25 μl reaction volume containing 2 μl of the appropriate cDNA or genomic DNA, 12.5 μl 2 × High power SYBR green PCR Master Mix (Applied Biosystems) and 300 nM concentration of the appropriate forward and reverse primers (Invitrogen). The thermal cycling conditions for the PCR were as follows: 1 cycle at 95°C for 10 min, 45 cycles of amplification at 95°C for 15 s and annealing at 60°C for 1 min. The data were collected during each elongation step. Melting-curve analysis consisting of 1 cycle at 95°C for 30 s, 55°C for 30 s and 95°C for 30 s was also performed after SYBR green I PCR to check the specificity of the amplification products. Negative (DEPC treated H2O) and no-reverse transcriptase controls were included in each run. All samples and controls were analyzed in triplicate.
The data was analyzed using the excel-based software geNorm [12], which compares the relative expression of reference genes in a pair-wise manner, and awards an M-value (expression stability) to each gene. The lowest M-value corresponds to the most stable reference gene. The worst scoring reference gene was then excluded from the analysis, and the process repeated in a step-wise manner until only the two best reference genes remained. The reference genes were ranked 1-8, with the most stable reference gene given the lowest value. The overall stability of each reference gene was determined by the sum of the ranking values from all four datasets combined. For calculation of the number of reference genes needed for reliable quantification of a target gene, the recommended cut-off for the pair-wise variation value V of 0.15 was used.
Normalization of iglC transcription
The relative transcription of the potential virulence gene iglC was investigated using the reference genes recommended by geNorm. For NCIMB14265T and DSM21254T, the five most stably transcribed reference genes were used, while for PQ 1106, the four most stably expressed reference genes were used. The relative transcription of iglC was determined in all samples by normalization against a normalization factor calculated by geNorm (based upon the geometric mean of the selected reference genes). The normalized values were divided by the arithmetic mean of the normalized log-phase expression values of iglC of each strain for presentation purposes. The statistical analysis of the normalized iglC transcription data was performed using JMP 8.0.2. (SAS Institute Inc.). The difference in iglC transcription levels between different growth phases, and the effect of an iron-depleted medium, were compared and determined as statistically significant if p-value <0.05 by Student’s t-test assuming unequal variance.
Results
Primer specificity and efficiency
Melting curve analysis revealed good specificity for the target genes with single peaks obtained for all primer sets. The primer efficiencies and amplicon size are shown in Table 1.
Transcription and stability of reference genes
To identify stably expressed reference genes in F. noatunensis ssp., eight candidate reference genes were initially analyzed. The bacterial cells were harvested in early exponential (OD600nm 0.3-0.5) growth phase and upon entry into stationary phase at 3 and 5 days post-inoculation for ssp. orientalis and ssp. noatunensis strains, respectively (see Figure 1). The purity of extracted total RNA used for reverse transcription was good, with 260/280 ratios above 2.1, and 260/230 ratios above 2.2 in all samples. No RNA degradation was observed as evaluated by gel electrophoresis. The data from the three strains combined suggested polA, ribC and ftsZ as the most stably transcribed reference genes across all strains, as indicated by the lowest M-values (Table 2). The pooled data, however, could not be used for analysis due to substantial pairwise variation (Figure 2). On individual analysis, we identified stably expressed reference genes for each strain. For F. noatunensis ssp. noatunensis NCIMB14265T, fopA, ftsZ and ribC were the three most stably transcribed genes, while ftsZ, polA and fopA; and ftsZ, gyrA and polA were the three most stably transcribed genes in F. noatunensis ssp. noatunensis PQ 1106 and F. noatunensis ssp. orientalis DSM21254T, respectively. Regardless of strain studied, 16S rRNA and rpoB consistently scored worst, and therefore constitute poor reference genes for gene transcription studies in Francisella noatunensis ssp. The optimal number of reference genes for each strain was determined based upon the average pairwise variation value V calculated by geNorm (Figure 2). For NCIMB14265T and DSM21254T, the optimal number of reference genes is five, while for PQ 1106 the optimal number is four.
Figure 1.
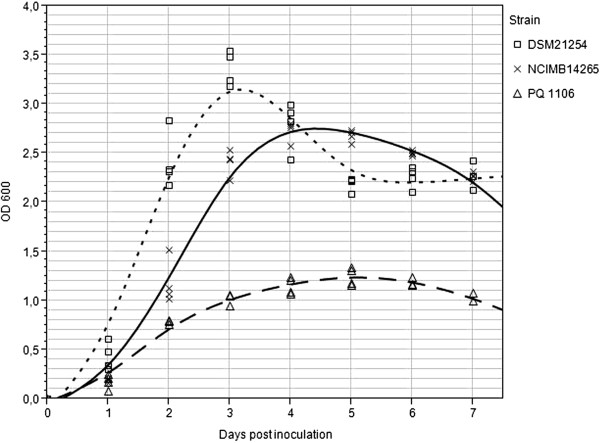
Growth curves for the strains used in the study. NCIMB14265 = F. noatunensis ssp. noatunensis strain NCIMB14265T. PQ 1106 = F. noatunensis ssp. noatunensis strain PQ 1106. DSM21254 = F. noatunensis ssp. orientalis strain DSM21254T. The growth curve for NCIMB14265T corresponds well to previously published data [19]. The two F. noatunensis ssp. noatunensis strains reach stationary growth phase at the same time point, after approximately 5 days, although PQ 1106 grows to lower OD600nm compared to NCIMB14265T. DSM21254T reaches a higher OD, and stationary growth phase after 3 days.
Table 2.
Expression stability index (M-value) and ranking of the candidate reference genes
| M-value | ||||||||
|---|---|---|---|---|---|---|---|---|
|
Strain |
ftsz |
polA |
fopA |
ribC |
gyrA |
uvrD |
16S rRNA |
rpoB |
|
NCIMB14265T |
0.531 |
0.818 |
0.531 |
0.751 |
0.949 |
1.050 |
2.004 |
2.563 |
|
DSM21254T |
0.555 |
0.692 |
0.716 |
1.009 |
0.555 |
0.892 |
1.496 |
2.343 |
|
PQ 1106 |
0.603 |
0.603 |
0.653 |
0.842 |
0.880 |
1.241 |
2.570 |
1.781 |
|
All strains combined |
0.979 |
0.790 |
1.535 |
0.790 |
1.498 |
1.675 |
2.411 |
2.126 |
| Ranking order | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
The lowest M-value corresponds to the most stable gene.
Figure 2.
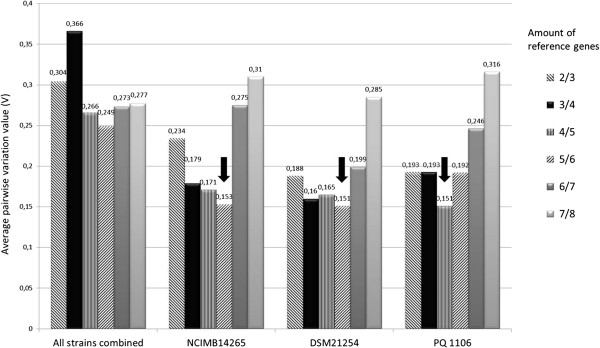
Average pairwise variation value V. NCIMB14265 = F. noatunensis ssp. noatunensis strain NCIMB14265T. PQ 1106 = F. noatunensis ssp. noatunensis strain PQ 1106. DSM21254 = F. noatunensis ssp. orientalis strain DSM21254T. Arrows indicate the number of reference genes needed for accurate normalization of a target gene for each strain.
Effect of growth phase and culture conditions on iglC transcription
The selected reference genes were used to assess the possible differential expression of iglC, which is known to be regulated by environmental cues [20,21]. All three strains displayed increased transcription of iglC in stationary compared to exponential growth phase, although for strain NCIMB14265T the increase was not statistically significant (see Figure 3). Transcription increases of 3.69 fold and 4.32 fold were recorded for iglCPQ 1106 and iglCDSM21254T, respectively. In an iron-depleted medium, PQ 1106 displayed a significant 6.72 fold increase in iglC transcription and a trend towards increased transcription was identified in NCIMB14265T (8.79 fold). No such increase was observed in DSM21254T.
Figure 3.
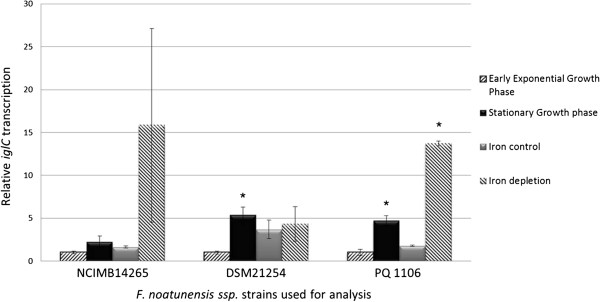
Statistical analysis of differential transcription of iglC under different growth conditions. Stars denote statistically significant differences with p-value <0.05. Bars indicate standard error of the mean. NCIMB14265 = F. noatunensis ssp. noatunensis strain NCIMB14265T. PQ 1106 = F. noatunensis ssp. noatunensis strain PQ 1106. DSM21254 = F. noatunensis ssp. orientalis strain DSM21254T.
Discussion
Identification of bacterial reference genes stably transcribed in the stationary growth phase can be challenging [22]. This is probably due to the physiological changes occurring in a bacterial culture on adaption to a nutrient-depleted environment, as reviewed by Navarro Llorens et al. [23]. We did, however, identify several reference genes which are stably transcribed during exponential growth, stationary growth and in an iron-depleted environment. While transcription stability of the various reference genes varied slightly in the three strains, ftsZ, polA and fopA provided relatively good scores for individual strains with geNorm expression stability measures below 1.0, which has been regarded as suitable for normalization in several studies [24-26]. Subspecies differences were apparent in our dataset. The four top scoring reference genes were identical in both strains of F. noatunensis ssp. noatunensis, although they ranked differently. The observed differences between ssp. noatunensis and orientalis may be explained by inter-strain evolutionary distances. Although several genes may appear useful for evaluation of gene transcription in the tested F. noatunensis strains, 16S rRNA and rpoB should be discouraged unless these genes are properly validated for each experimental condition.
On examination of iglC transcription in relation to bacterial growth phase, all strains show a trend toward higher iglC transcription in stationary compared to early exponential growth phase. The increase was, however, only statistically significant in two of the three studied strains (PQ 1106 and DSM21254T). The iglC gene has been shown to be important for intracellular growth in F. noatunensis ssp. orientalis[27]. The nutrient-depleted environment encountered in the stationary growth phase may mimic some aspects of the intra macrophage environment. Virulence associated factors, up-regulated in the stationary growth phase have also been shown in other intracellular pathogenic bacteria [28,29] to be up-regulated in infected macrophages. Our results indicate that transcription of iglC is increased in an environment mimicking intracellular conditions, thus suggesting a virulence-related intracellular survival role for this gene in F. noatunensis ssp.
The overall increase in iglC transcription in F. noatunensis ssp. noatunensis strains in an iron-depleted environment is consistent with the situation in F. tularensis ssp. holarctica Live Vaccine Strain [20]. Exposure to iron-limitation after reaching stationary growth phase might have prevented further induction of iglC transcription in DSM21254T. This is partially supported by data from the early logarithmic growth, where a 3.31 fold increase in iglC transcription was observed.
Generally, analysis of RT-qPCR gene expression data using a single reference gene is not acceptable, as inclusion of multiple reference genes results in much more accurate and reliable normalization. The geNorm calculated V values give the optimal number of reference genes to be used in an expression study [12]. Although the use of three reference genes is a valid normalization strategy in most cases, our results showed that four to five reference genes are required to achieve accurate normalization of gene transcription in all F. noatunensis ssp. Besides increasing the workload and cost, applying a large reference gene set could also pose constraints on limited sample availability. On normalization of iglC transcription with the three most stable genes across all three strains, we achieved basically the same results as normalizing with the optimal number of four or five reference genes, but with a slightly higher degree of variation (data not shown). Thus, it is always a trade-off between accuracy and practical considerations when it comes to the optimal number of reference genes to include in the analysis. For accurate quantification of small changes in gene transcription, it might be desirable to use the specified optimal number of reference genes. However, if only large differences in gene transcription are of interest, use of a smaller number of stably expressed reference genes might be justifiable.
In conclusion, the present study investigated the most reliable reference genes for normalization of gene expression data in F. noatunensis under different in vitro growth conditions. Although there were many potential suitable references genes, ftsZ, polA and fopA were the best, while 16S rRNA and rpoB proved to be the least stable under all tested conditions. These data emphasize the need for proper validation of candidate reference genes in any experimental expression study. Extrapolation of results from one strain to another must therefore be done with extreme caution. It also provides baseline data on selection of reference genes for many future studies investigating expression of virulence in pathogenic Francisella strains.
Availability of supporting data
The dataset supporting the results of this article is included within the article (and its additional file).
Competing interests
We declare no competing interests.
Authors’ contributions
BE performed the experiments, analyzed and interpreted the data and wrote the manuscript. WLHC and CDJ were involved in the design, interpretation of the data and writing of the manuscript. DS designed and performed some of the experiments, was involved in the interpretation of the data and writing of the manuscript. All authors read and approved the final manuscript.
Supplementary Material
List of reference genes used in publications for RT-qPCR gene expression analyses in Francisella spp. [30-43].
Contributor Information
Espen Brudal, Email: espen.brudal@nvh.no.
Hanne Cecilie Winther-Larsen, Email: hannewi@farmasi.uio.no.
Duncan John Colquhoun, Email: duncan.colquhoun@vetinst.no.
Samuel Duodu, Email: samuel.duodu@vetinst.no.
Acknowledgements
This work was supported financially by the Norwegian Research Council (Grant no. 185362) and the Norwegian School of Veterinary Science (NSVS), to whom we express our gratitude.
References
- Kamaishi T, Fukuda Y, Nishiyama M, Kawakami M, Matsuyama T, Yoshinaga T, Oseko N. Identification and pathogenicity of intracellular Francisella bacterium in three-line grunt Parapristipoma trilineatum. Fish Pathology. 2005;40:67–71. doi: 10.3147/jsfp.40.67. [DOI] [Google Scholar]
- Birkbeck TH, Bordevik M, Frøystad MK, Baklien A. Identification of Francisella sp. from Atlantic salmon, Salmo salar L., in Chile. J Fish Dis. 2007;30:505–507. doi: 10.1111/j.1365-2761.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- Mauel MJ, Soto E, Moralis JA, Hawke J. A piscirickettsiosis-like syndrome in cultured Nile tilapia in Latin America with Francisella spp. as the pathogenic agent. J Aquat Anim Health. 2007;19:27–34. doi: 10.1577/H06-025.1. [DOI] [PubMed] [Google Scholar]
- Jeffery KR, Stone D, Feist SW, Verner-Jeffreys DW. An outbreak of disease caused by Francisella sp. in Nile tilapia Oreochromis niloticus at a recirculation fish farm in the UK. Dis Aquat Organ. 2010;91:161–165. doi: 10.3354/dao02260. [DOI] [PubMed] [Google Scholar]
- Sjödin A, Svensson K, Öhrman C, Ahlinder J, Lindgren P, Duodu S, Hnath J, Burans JP, Johansson A, Colquhoun DJ. Genome characterisation of the genus Francisella reveals insight into similar evolutionary paths in pathogens of mammals and fish. BMC Genomics. 2012;13:268. doi: 10.1186/1471-2164-13-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano FE, Zhang N, Cowley SC, Klose KE, Cheung KK, Roberts MJ, Ludu JS, Letendre GW, Meierovics AI, Stephens G. A Francisella tularensis pathogenicity island required for intramacrophage growth. J Bacteriol. 2004;186:6430–6436. doi: 10.1128/JB.186.19.6430-6436.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson P, Oyston PC, Chain P, Chu MC, Duffield M, Fuxelius HH, Garcia E, Hälltorp G, Johansson D, Isherwood KE, Karp PD, Larsson E, Liu Y, Michell E, Prior J, Prior R, Malfatti S, Sjöstedt A, Svensson K, Thompson N, Vergez L, Wagg JK, Wren BW, Lindler LE, Andersson SG, Forsman M, Titball RW. The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat Genet. 2005;37:153–159. doi: 10.1038/ng1499. [DOI] [PubMed] [Google Scholar]
- Soto E, Fernandez D, Hawke JP. Attenuation of the fish pathogen Francisella sp. by mutation of the iglC* gene. J Aquat Anim Health. 2009;21:140–149. doi: 10.1577/H08-056.1. [DOI] [PubMed] [Google Scholar]
- Soto E, Wiles J, Elzer P, Macaluso K, Hawke JP. Attenuated Francisella asiatica iglC mutant induces protective immunity to francisellosis in tilapia. Vaccine. 2010;29(3):593–598. doi: 10.1016/j.vaccine.2010.06.040. [DOI] [PubMed] [Google Scholar]
- Dheda K, Huggett JF, Chang JS, Kim LU, Bustin SA, Johnson MA, Rook GA, Zumla A. The implications of using an inappropriate reference gene for real-time reverse transcription PCR data normalization. Anal Biochem. 2005;344:141–143. doi: 10.1016/j.ab.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Mauriat M, Guenin S, Pelloux J, Lefebvre JF, Louvet R, Rusterucci C, Moritz T, Guerineau F, Bellini C. The lack of a systematic validation of reference genes: a serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol J. 2008;6:609–618. doi: 10.1111/j.1467-7652.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De PK, Pattyn F, Poppe B, Van RN, De PA, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034.1-RESEARCH0034.11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Bustin SA. Why the need for qPCR publication guidelines?–The case for MIQE. Methods. 2010;50:217–226. doi: 10.1016/j.ymeth.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Olsen AB, Mikalsen J, Rode M, Alfjorden A, Hoel E, Straum-Lie K, Haldorsen R, Colquhoun DJ. A novel systemic granulomatous inflammatory disease in farmed Atlantic cod, Gadus morhua L., associated with a bacterium belonging to the genus Francisella. J Fish Dis. 2006;29:307–311. doi: 10.1111/j.1365-2761.2006.00714.x. [DOI] [PubMed] [Google Scholar]
- Ottem KF, Nylund A, Isaksen TE, Karlsbakk E, Bergh Ø. Occurrence of Francisella piscicida in farmed and wild Atlantic cod, Gadus morhua L., in Norway. J Fish Dis. 2008;31:525–534. doi: 10.1111/j.1365-2761.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- BioRad technical note 5396. http://www3.biorad.com/cmc_upload/Literature/195246/Bulletin_5396.pdf.
- Furevik A, Pettersen EF, Colquhoun D, Wergeland HI. The intracellular lifestyle of Francisella noatunensis in Atlantic cod (Gadus morhua L.) leucocytes. Fish Shellfish Immunol. 2011;30:488–494. doi: 10.1016/j.fsi.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Deng K, Blick RJ, Liu W, Hansen EJ. Identification of Francisella tularensis genes affected by iron limitation. Infect Immun. 2006;74:4224–4236. doi: 10.1128/IAI.01975-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett KRO, Cirillo KA. Environmental Adaptation of Francisella tularensis. Microbes Infect. 2009;11:828–834. doi: 10.1016/j.micinf.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derzelle S, Dilasser F, Duquenne M, Deperrois V. Differential temporal expression of the staphylococcal enterotoxins genes during cell growth. Food Microbiol. 2009;26:896–904. doi: 10.1016/j.fm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Navarro Llorens JM, Tormo A, Martinez-Garcia E. Stationary phase in gram-negative bacteria. FEMS Microbiol Rev. 2010;34:476–495. doi: 10.1111/j.1574-6976.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- Nielsen KK, Boye M. Real-time quantitative reverse transcription-PCR analysis of expression stability of Actinobacillus pleuropneumoniae housekeeping genes during in vitro growth under iron-depleted conditions. Appl Environ Microbiol. 2005;71(6):2949–2954. doi: 10.1128/AEM.71.6.2949-2954.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botteldoorn N, Van Coillie E, Grijspeerdt K, Werbrouck H, Haesebrouck F, Donné E, D'Haese E, Heyndrickx M, Pasmans F, Herman L. Real-time reverse transcription PCR for the quantification of the mntH expression of Salmonella enterica as a function of growth phase and phagosome-like conditions. J Microbiol Methods. 2006;66:125–135. doi: 10.1016/j.mimet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Tasara T, Stephan R. Evaluation of housekeeping genes in Listeria monocytogenes as potential internal control references for normalizing mRNA expression levels in stress adaptation models using real-time PCR. FEMS Microbiol Lett. 2007;269:265–272. doi: 10.1111/j.1574-6968.2007.00633.x. [DOI] [PubMed] [Google Scholar]
- Soto E, Fernandez D, Thune R, Hawke JP. Interaction of Francisella asiatica with tilapia (Oreochromis niloticus) innate immunity. Infect Immun. 2010;78:2070–2078. doi: 10.1128/IAI.01308-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B, Swanson MS. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Bose M, Brahmachari V. Analysis of expression profile of mammalian cell entry (mce) operons of Mycobacterium tuberculosis. Infect Immun. 2003;71:6083–6087. doi: 10.1128/IAI.71.10.6083-6087.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrly TD, Chong A, Virtaneva K, Sturdevant DE, Child R, Edwards JA, Brouwer D, Nair V, Fischer ER, Wicke L. Intracellular biology and virulence determinants of Francisella tularensis revealed by transcriptional profiling inside macrophages. Cell Microbiol. 2009;11:1128–1150. doi: 10.1111/j.1462-5822.2009.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan G, Meeker A, Dragulev B. fslE is necessary for siderophore-mediated iron acquisition in Francisella tularensis Schu S4. J Bacteriol. 2008;190:5353–5361. doi: 10.1128/JB.00181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson PE Jr, Carroll JA, O'Dee DM, Nau GJ. Modulation of virulence factors in Francisella tularensis determines human macrophage responses. Microb Pathog. 2007;42:204–214. doi: 10.1016/j.micpath.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzempa J, Carlson PE Jr, O'Dee DM, Shanks RM, Nau GJ. Global transcriptional response to mammalian temperature provides new insight into Francisella tularensis pathogenesis. BMC Microbiol. 2008;8:172. doi: 10.1186/1471-2180-8-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postic G, Frapy E, Dupuis M, Dubail I, Livny J, Charbit A, Meibom KL. Identification of small RNAs in Francisella tularensis. BMC Genomics. 2010;11:625. doi: 10.1186/1471-2164-11-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grall N, Livny J, Waldor M, Barel M, Charbit A, Meibom KL. Pivotal role of the Francisella tularensis heat-shock sigma factor RpoH. Microbiology. 2009;155:2560–2572. doi: 10.1099/mic.0.029058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni S, Ernst RK, Muszynski A, Mohapatra NP, Berry MP, Vinogradov E, Carlson RW, Gunn JS. Francisella tularensis blue–gray phase variation involves structural modifications of lipopolysaccharide O-antigen, core and lipid A and affects intramacrophage survival and vaccine efficacy. Front Microbiol. 2011;1:129. doi: 10.3389/fmicb.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JT, Jeffery EF, Shannon JD, Ramakrishnan G. Characterization of the siderophore of Francisella tularensis and role of fslA in siderophore production. J Bacteriol. 2006;188:3785–3795. doi: 10.1128/JB.00027-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charity JC, Costante-Hamm MM, Balon EL, Boyd DH, Rubin EJ, Dove SL. Twin RNA polymerase-associated proteins control virulence gene expression in Francisella tularensis. PLoS Pathog. 2007;3(6):e84. doi: 10.1371/journal.ppat.0030084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchan BW, McCaffrey RL, Lindemann SR, Allen LA, Jones BD. Identification of migR, a regulatory element of the Francisella tularensis live vaccine strain iglABCD virulence operon required for normal replication and trafficking in macrophages. Infect Immun. 2009;77:2517–2529. doi: 10.1128/IAI.00229-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell BL, Mohapatra NP, Gunn JS. Regulation of virulence gene transcripts by the Francisella novicida orphan response regulator PmrA: role of phosphorylation and evidence of MglA/SspA interaction. Infect Immun. 2010;78:2189–2198. doi: 10.1128/IAI.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapatra NP, Soni S, Bell BL, Warren R, Ernst RK, Muszynski A, Carlson RW, Gunn JS. Identification of an orphan response regulator required for the virulence of Francisella spp. and transcription of pathogenicity island genes. Infect Immun. 2007;75:3305–3314. doi: 10.1128/IAI.00351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotcke A, Weiss DS, Kim CC, Chain P, Malfatti S, Garcia E, Monack DM. Identification of MglA-regulated genes reveals novel virulence factors in Francisella tularensis. Infect Immun. 2006;74:6642–6655. doi: 10.1128/IAI.01250-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotcke A, Monack DM. Identification of fevR, a novel regulator of virulence gene expression in Francisella novicida. Infect Immun. 2008;76:3473–3480. doi: 10.1128/IAI.00430-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of reference genes used in publications for RT-qPCR gene expression analyses in Francisella spp. [30-43].


