Abstract
A near-infrared (NIR)-responsive Aurod@pNIPAAm-PEGMA nanogel was synthesized in two steps, growing a PEGMA monolayer on the surface of gold nanorods (AuNRs), followed by in situ polymerization and cross-linking of N-iso-propylacrylamide (NIPAAm) and poly-(ethylene glycol)-methacrylate (PEGMA). The AuNRs and Aurod@pNIPAAm-PEGMA nanogel were characterized by UV–vis spectroscopy, Raman spectroscopy, Fourier transform infrared spectroscopy, and transmission electron microscopy, respectively. The lower critical solution temperature of the Aurod@pNIPAAm-PEGMA nanogel could be tuned by changing the molar ratio of NIPAAm/PEGMA. The NIR-mediated drug release behavior of the Aurod@pNIPAAm-PEGMA nanogel was studied with zinc phthalocyanines (ZnPc4) as a drug model. It was also demonstrated that the loaded ZnPc4 could keep the capability of generating singlet oxygen, and the in vitro study showed a great photodynamic therapy (PDT) effect on Hela cells. It thus indicated the potential of this Aurod@pNIPAAm-PEGMA nanogel for application as a drug carrier in PDT, which might make contributions to oncotherapy.
Keywords: NIR-responsive, Aurod@pNIPAAm-PEGMA nanogel, LCST, singlet oxygen, PDT
Background
Gold nanoparticles including nanoshells, nanocages, and nanorods have drawn increasing attention in photodynamic therapy (PDT), drug delivery, and diagnostic imaging field in recent years [1-5]. Among them, gold nanorods (AuNRs) are of particular interest due to their unique optical properties. With the different aspect ratios and the resulting longitudinal surface plasmon resonance (SPR), AuNRs exhibit an absorption band in the near-infrared (NIR) region [6], which conduces to higher photothermal conversion and also shows significant biomedical application in view of the penetration of NIR light into biological tissues [7,8].
Poly(N-isopropylacrylamide) (pNIPAAm) gel, as one of the most widely studied temperature-responsive polymers [9-11], undergoes phase transition in water when the temperature increases or decreases beyond its lower critical solution temperature (LCST; approximately 32°C) [12,13]. Besides, its LCST can be tuned by the addition of a comonomer during polymerization [14,15]. Combining this temperature-responsive gel and gold nanoparticles together, the temperature-responsive gel can be induced to collapse by the photothermal conversion of gold nanoparticles, which gives rise to much possibility for the application of the temperature-responsive gel in drug delivery. The composites of Au@pNIPAAm have been synthesized and studied in many works [16-18]. However, the combination mostly through the physical embedding effect or electrostatic interaction between gold nanoparticles and pNIPAAm may make the composites lack long-term stability, especially in the biological environment. Our previous work has reported the synthesis of a core-shell structured multifunctional hybrid Au@IPN-pNIPAAm nanogel in which the hydrogel could be chemically grafted onto a single gold nanoparticle [19].
Herein, we developed a new way to immobilize pNIPAAm combined with poly-(ethylene glycol)-methacrylate (PEGMA) on the surface of AuNRs through chemical grafting to obtain NIR-responsive Aurod@pNIPAAm-PEGMA nanogel. ZnPc4, a photosensitizer, was used as drug model to investigate the drug loading and release properties of the Aurod@pNIPAAm-PEGMA nanogel. The capacity of generating singlet oxygen of ZnPc4 after being loaded in the Aurod@pNIPAAm-PEGMA nanogel was measured, and the in vitro PDT was also studied. Our current results suggested the potential of Aurod@pNIPAAm-PEGMA nanogel as a carrier in PDT.
Methods
Synthesis of PEGMA-SH compound
Concentrations of 1.0 mmol 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) and 2.0 mmol dicyclohexylcarbodiimide (DCC) were dissolved in 50 mL of dichlormethane, followed by the addition of 2.2 mmol 4-dimethylaminopyridine (DMAP) and 2.0 mmol PEGMA. The mixture was degassed with nitrogen and then stirred for 48 h at room temperature. After filtration, the filtrate was washed sequentially with water, 5% acetic acid, and water. Then, the organic phase was dried over magnesium sulfate, filtered, and evaporated to dryness. The product was dissolved in 100 mL of water/ethanol (V/V, 4/1) with the addition of 2 mL of 1 M sodium borohydride (NaBH4) and stirred for 2 h, and was used without further purification.
Synthesis of Aurod@pNIPAAm-PEGMA nanogel
AuNRs with a length of 50 nm were synthesized using the seed-mediated growth method as reported previously [20]. Subsequently, 0.1 mmol PEGMA-SH was added to 25 mL of the as-prepared AuNRs suspension (1.6 × 10−6 μmol) and continuously stirred for 5 h at room temperature. Aurod@PEGMA was collected by centrifugation at 9,500 rpm for 12 min and then re-dispersed in 15 mL of the deionized water, followed by the addition of 1.8 mmol NIPAAm, 0.2 mmol PEGMA, 86.69 μmol sodium dodecyl sulfate (SDS), and 12.97 μmol N,N-methylenebisacrylamide (BIS). The mixture was heated to 75°C with stirring and maintained in vacuum. After equilibration for 1 h, the polymerization was initiated by adding 109.6 μmol ammonium persulfate (APS). The reaction was allowed to proceed for 4 h at 75°C and terminated by opening the system to air. The resulting Aurod@pNIPAAm-PEGMA nanogels were purified by repeated centrifugation (9,000 rpm for 12 min) and subsequently lyophilized for further use.
Characterization
The optical properties of AuNRs and Aurod@pNIPAAm-PEGMA nanogels were characterized by an UV–vis spectrophotometer (DUTM800, Beckman Coulter, Brea, CA, USA) with a scanning speed of 1,200 nm/min from 400 to 1,000 nm. The transmission electron microscopy (TEM) images were obtained from a JEM 2100 microscope (JEOL Ltd., Tokyo, Japan) operating at an acceleration voltage of 200 kV. Raman spectra were performed on an UV-1000x instrument (Renishaw, Wotton-under-Edge, UK) (path length = 200 nm) using a red light-emitting diode laser (λ = 785 nm, 0.5 mW). A Fourier transform interferometer (AVATAR360, Nicolet Instrument Corporation, Madison, WI, USA) was used to record the absorption spectra of AuNRs and Aurod@pNIPAAm-PEGMA nanogels between 400 and 4,000 cm−1 at a spectral resolution of 4 cm−1.
LCST measurement of Aurod@pNIPAAm-PEGMA nanogel
In order to investigate the thermal property of the Aurod@pNIPAAm-PEGMA nanogel, nanogels with different molar ratios of NIPAAm/PEGMA (1:0, 18:1, 12:1, 9:1, 6:1, 4.5:1) were synthesized. LCSTs of nanogels were measured through turbidimetric measurement. The concentration for each Aurod@pNIPAAm-PEGMA nanogel in the deionized water was maintained at 1 mg/mL. The light transmittances at 600 nm were then measured by an UV–vis spectrophotometer (TU-1901, Beijing Purkinje General Instrument Co. Ltd, Beijing, China) equipped with a temperature-controlled sample holder, and the heating rate was set at 0.1°C/min. The LCST was defined as the initial break point in the resulting transmittance versus temperature curves.
ZnPc4 loading and NIR-mediated ZnPc4 release
Two milligrams of Aurod@pNIPAAm-PEGMA nanogels and 2 mg of ZnPc4 were dispersed in 10 mL of N,N-dimethyl formamide (DMF) and stirred for 24 h at room temperature. The ZnPc4-loaded Aurod@pNIPAAm-PEGMA nanogels were then collected by centrifugation (9,000 rpm for 12 min). To determine the amount of unloaded ZnPc4, the supernatant was analyzed by an UV–vis spectrophotometer (DUTM800, Beckman Coulter) at 680 nm where ZnPc4 has a maximum absorption. The loading efficiency was calculated according to the following formula:
where Wt represents the total amount of ZnPc4 and W0 represents the unloaded amount of ZnPc4.
For the NIR-mediated ZnPc4 release, 5 mL of the ZnPc4-loaded Aurod@pNIPAAm-PEGMA nanogel suspension (1 mg/mL) was placed into dialysis bags (molecular weight cutoff, 8 to 14 kDa) and irradiated by an 808-nm laser (0 to 400 mW/cm2) for different times (0 to 60 min). To determine the amount of ZnPc4 released, the dialysate was removed and subsequently analyzed by an UV–vis spectrophotometer (DUTM800, Beckman Coulter). The release efficiency was calculated as follows:
where Wr represents the released amount of ZnPc4 and Wl represents the loaded amount of ZnPc4.
Singlet oxygen detection
The generation of singlet oxygen (1O2) from ZnPc4 loaded in the Aurod@pNIPAAm-PEGMA nanogel was determined by the transformation of 9,10-dimethylanthracene (DMA) which exhibits a maximum absorption at 377 nm [21]. The DMA can react irreversibly with 1O2 to yield an endoperoxide. The reaction could be monitored by recording the decrease in the absorption at 377 nm. In a typical experiment, 0.105 mg of the Aurod@pNIPAAm-PEGMA nanogel loaded with 0.0135 μmol ZnPc4 was dispersed in 3 mL of DMF, and then, 0.45 μmol DMA was added. Pure ZnPc4 (0.0135 μmol) was used as a control. The solutions were then irradiated with a LED lamp (680 nm, 10 mW/cm2) or a NIR laser (808 nm, 400 mW/cm2). The absorption measurements followed by irradiation were carried out every 5 min.
Light-induced in vitro PDT effect
Hela cells were seeded into 24-well cell culture plates (1 × 105 cells/well) and incubated for 24 h. After being treated with ZnPc4-loaded Aurod@pNIPAAm-PEGMA nanogels (300 μg/mL) in serum-free medium at 37°C for 22 h, chloroquine (10 mg/mL) was added into every well for another 2 h to promote endosomal escape [22]. Then, Hela cells were washed with PBS and incubated in a nanogel-free medium and treated with an 808-nm laser at 400 mW/cm2 for 15 min and a 680-nm LED lamp at 10 mW/cm2 for 40 min. For cell survival test, the irradiated plates were returned to the incubator, and cell viability was colorimetrically measured 48 h later with MTT assay [23].
Results and discussion
Synthesis of Aurod@pNIPAAm-PEGMA nanogel
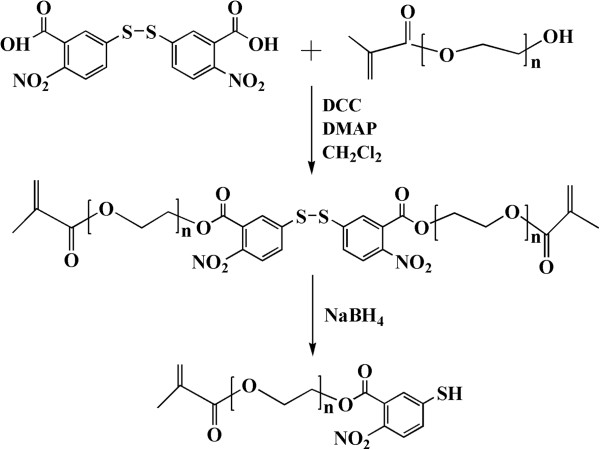
The synthesis of PEGMA-SH was shown in Figure 1. PEGMA-DTNB compound was firstly gained by the esterification reaction between the terminal hydroxyl group on the PEGMA and the carboxyl group on the DTNB with the DCC as medium and DMAP as catalyst [24,25]. Subsequently, the disulfide bond of PEGMA-DTNB was reduced by NaBH4 to yield the desired PEGMA-SH compound.
Figure 1.
Schematic description of the synthesis of PEGMA-SH.
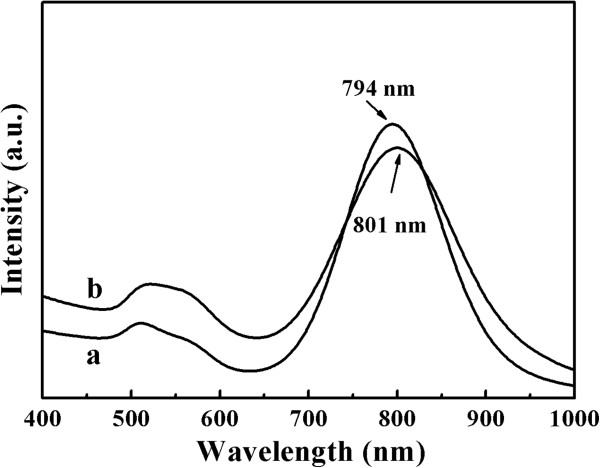
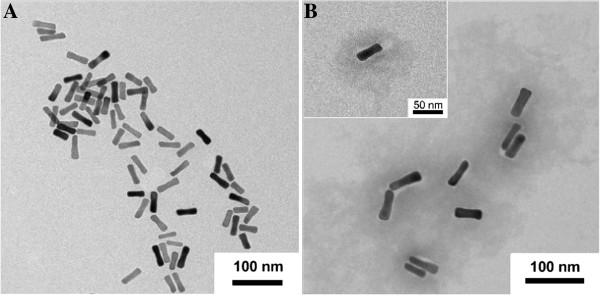
The strategy to prepare the Aurod@pNIPAAm-PEGMA nanogel involves two steps, growing a PEGMA monolayer on the surface of a AuNR, followed by in situ polymerization and cross-linking of NIPAAm and PEGMA, as depicted in Figure 2. In the first step, the AuNR surface was modified with a PEGMA self-assembled monolayer through a sulfhydryl-gold interaction. In the second step, PEGMA-modified AuNRs could be used as a template for in situ formation of hydrogel by polymerization and cross-linking of NIPAM and PEGMA with BIS as crosslinker, APS as initiator, and SDS as emulsifier. The coating of pNIPAAm-PEGMA on AuNRs can be reflected in the corresponding UV–vis spectra (Figure 3). AuNRs used in this work had a length of about 50 nm with an aspect ratio of approximately 3.2 (Figure 4A) which exhibited the maximum of the plasmon peak of 794 nm (Figure 3a). After the AuNRs were modified with pNIPAAm-PEGMA, a red shift from 794 to 801 nm occurred (Figure 3b). This red shift of SPR and the peak shape widening might be due to a change for AuNRs in the local refractive index produced by the pNIPAAm-PEGMA shell (Figure 4B) [26].
Figure 2.
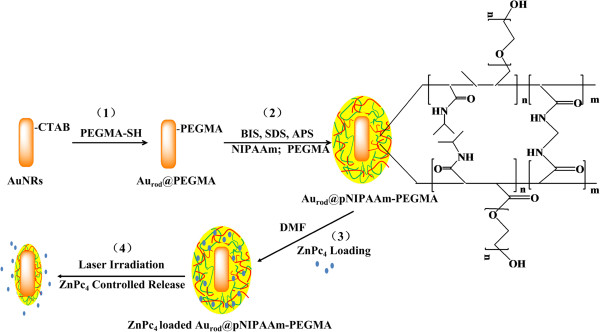
Preparation of the Aurod@pNIPAAm-PEGMA nanogel. (1, 2) Schematic of the sequence of steps in the synthesis of the hybrid Aurod@pNIPAAm-PEGMA nanogels, (3) ZnPc4 loading process, and (4) NIR-mediated ZnPc4 release.
Figure 3.
The UV–vis spectra of (a) AuNRs and (b) Aurod@pNIPAAm-PEGMA nanogel.
Figure 4.
The typical TEM images of AuNRs (A) before and (B) after modification with pNIPAAM-PEGMA, respectively.
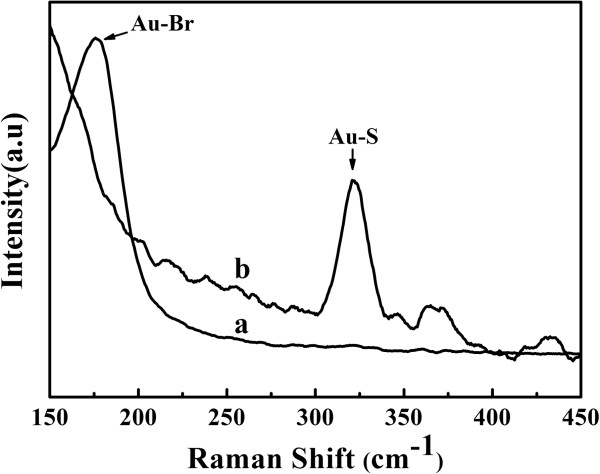
Raman spectra were also used to identify the synthesis of the Aurod@pNIPAAm-PEGMA nanogel. The Raman spectrum of the as-prepared AuNRs (Figure 5a) exhibited a band at 190 nm which was ascribable to the Au-Br bond on the surface of AuNRs [27]. This is because the as-prepared AuNRs were stabilized by the cationic detergent cetyltrimethylammonium bromide (CTAB) in the aqueous solution. After being modified with pNIPAAm-PEGMA (Figure 5b), the Au-Br band disappeared, and a band at 320 nm was observed, which was assigned to the Au-S bond [28]. It is thus suggested that PEGMA-SH might replace CTAB to form PEGMA-modified AuNRs through the Au-S bond, and then, PEGMA-SH on the surface of AuNRs might serve as the template for the following polymerization and cross-linking of NIPAAm and PEGMA.
Figure 5.
The Raman spectra of (a) AuNRs and (b) Aurod@pNIPAAm-PEGMA nanogel.
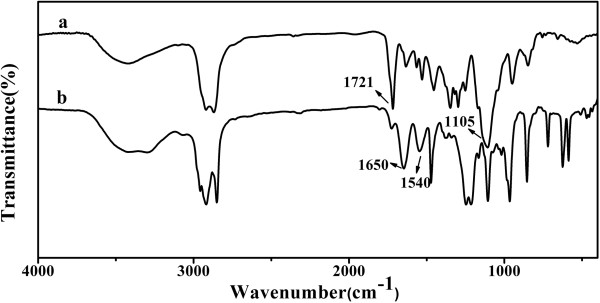
FTIR spectra (Figure 6) were recorded to confirm the structure of the polymer shell. In the FTIR spectrum of PEGMA-modified AuNRs (Figure 6a), the absorption peaks of PEGMA, including ν(C=O) (1,721 cm−1) and ν(C-O-C) (1,105 cm−1), were observed. The spectrum of Aurod@pNIPAAm-PEGMA nanogels (Figure 6b) exhibited the characteristic peaks of polymerized NIPAAm at 1,650 cm−1 (ν(C=O), amide I) and 1,550 cm−1 (δ(N-H), amide II). Hence, the FTIR results could provide evidence for the surface modification and polymerization on AuNRs.
Figure 6.
FTIR spectra of (a) Au@PEGMA and (b) Aurod@pNIPAAm-PEGMA nanogel.
Thermosensitive property of Aurod@pNIPAAm-PEGMA nanogel
Figure 7 and Table 1 showed the effect of the molar ratios of NIPAAm/PEGMA on the LCSTs of the Aurod@pNIPAAm-PEGMA nanogel. The Aurod@pNIPAAm (the molar ratio of NIPAAm/PEGMA, 1:0) exhibited an LCST of approximately 32°C, which was consistent with pure pNIPAAm [13]. It is clearly shown in Table 1 that the LCSTs of the Aurod@pNIPAAm-PEGMA nanogel could be tuned by changing the molar ratio of NIPAAm/PEGMA. Namely, as the molar ratio of NIPAAm/PEGMA decreased, the LCST of the nanogel increased. For example, when the molar ratio of NIPAAm/PEGMA was set at 18:1, the LCST of Aurod@pNIPAAm-PEGMA nanogels could be up to 36°C. The addition of hydrophilic PEGMA increased the hydrophilicity of pNIPAAm due to the strong interactions between water and hydrophilic groups on the polymer, which led to an increased LCST [29]. It is thus expected that this attractive property of tunable LCST might make Aurod@pNIPAAm-PEGMA nanogels more promising in drug delivery application.
Figure 7.
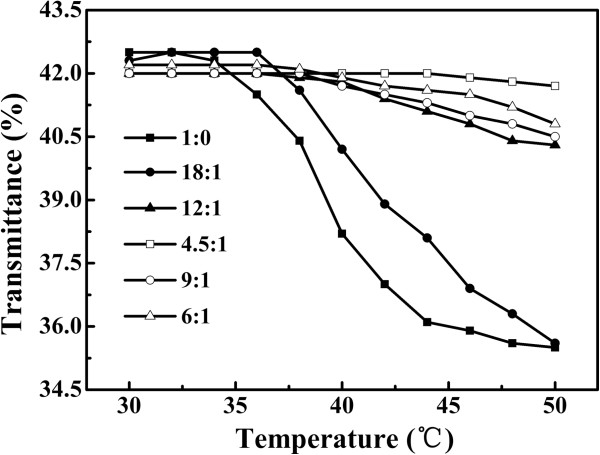
The transmittance versus temperature curves of Aurod@pNIPAAm-PEGMA nanogels. With different molar ratios of NIPAAm/PEGMA (1:0, 18:1, 12:1, 9:1, 6:1, 4.5:1, respectively).
Table 1.
The LCSTs of Aurod@pNIPAAm-PEGMA nanogels with different molar ratios of NIPAAm/PEGMA
| NIPAAm (mmol) | PEGMA (mmol) | NIPAAm/PEGMA (mmol/mmol) | LCST (°C) |
|---|---|---|---|
| 1.8 |
0 |
1:0 |
32 |
| 1.8 |
0.1 |
18:1 |
36 |
| 1.8 |
0.15 |
12:1 |
38 |
| 1.8 |
0.2 |
9:1 |
40 |
| 1.8 |
0.3 |
6:1 |
42 |
| 1.8 | 0.4 | 4.5:1 | 44 |
NIR-mediated ZnPc4 release
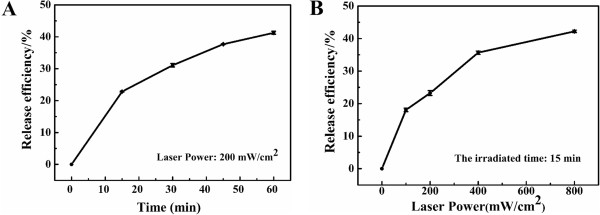
NIR-mediated release of ZnPc4 loaded in Aurod@pNIPAAm-PEGMA nanogels was investigated with the irradiation of a NIR laser (808 nm). When the sample was irradiated at 200 mW/cm2, the release efficiency was about 23.5% in the initial 20 min. As the irradiated time was prolonged, the cumulative release efficiency was up to 37.4% within 1 h (Figure 8A). This can be explained by the AuNRs of Aurod@pNIPAAm-PEGMA nanogels absorbing a certain SPR wavelength light and converting it into heat [30]. The heat diffused into the polymer shell and caused the shrinkage of the pNIPAAm-PEGMA nanogels and the release of ZnPc4.
Figure 8.
NIR-mediated release of ZnPc4. (A) Time- and (B) power-dependent of release of ZnPc4 from Aurod@pNIPAAm-PEGMA nanogels, respectively.
The effect of laser power density on drug release was studied (Figure 8B). Exposure of Aurod@pNIPAAm-PEGMA nanogels to an 808-nm laser with the power of 100 mW/ cm2 for 15 min caused 20% of the loaded ZnPc4 released. More loaded ZnPc4 (43.7%) in Aurod@pNIPAAm-PEGMA nanogels could be released upon the irradiation power of 800 mW/ cm2. This is because when irradiated with a low-power NIR laser, small shrinkage of nanogels occurred, whereas a laser at high power might make nanogels shrink considerably and rapidly [31], consequently more ZnPc4 could be released. It is thus speculated that the NIR-responsive Aurod@pNIPAAm-PEGMA nanogel, acting as drug delivery carriers, could offer specific drug delivery to the targeted site, such as a tumor zone.
Singlet oxygen detection
In PDT, the photosensitizing drugs should preferentially accumulate in target tissues and subsequently be activated by light with a matching wavelength to generate reactive singlet oxygen [32]. The singlet oxygen will cause the destruction of target cells by a complex cascade of chemical, biological, and physiological reactions [33]. The Aurod@pNIPAAm-PEGMA nanogels served as ZnPc4 carrier in PDT; besides the excellent properties of drug loading and release, its effect on the capability of loaded ZnPc4 to generate singlet oxygen was also investigated.
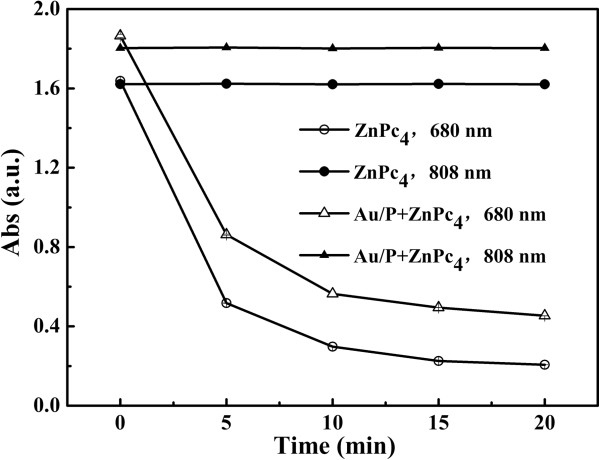
Photo-induced 1O2 of ZnPc4 was examined by a chemical method by using DMA, which could react with 1O2 to form an endoperoxide. The decrease in amount of DMA can be recorded by measuring the absorption at 377 nm. As shown in Figure 9, when ZnPc4-loaded Aurod@pNIPAAm-PEGMA nanogels or pure ZnPc4 was irradiated by an 808-nm laser, the absorption of DMA remained unchanged with the increase of exposure time to light, whereas the absorption of DMA continuously decreased as the ZnPc4-loaded Aurod@pNIPAAm-PEGMA nanogels or pure ZnPc4 was irradiated by a 680-nm light. This decrease indicated the production of 1O2, which can irreversibly react with DMA. Moreover, the generation curve of ZnPc4-loaded Aurod@pNIPAAm-PEGMA nanogels was similar with that of pure ZnPc4, demonstrating that the capacity of generating 1O2 of ZnPc4 was hardly affected after being loaded in Aurod@pNIPAAm-PEGMA nanogels. It is thus suggested that the Aurod@pNIPAAm-PEGMA nanogel might be a promising drug carrier for photodynamic therapy in the future.
Figure 9.
The generation profiles of singlet oxygen from ZnPc4-loaded Aurod@pNIPAAm-PEGMA nanogels (Au/P). The nanogels were irradiated by an 808-nm laser and a 680-nm LED lamp, respectively.
In vitro PDT study on Hela cells
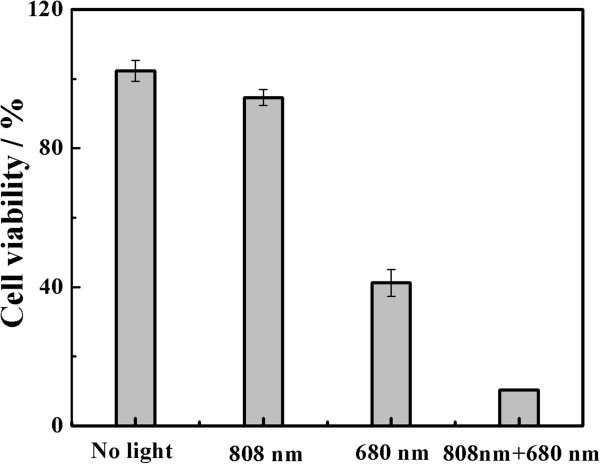
The in vitro PDT study, represented in Figure 10, showed the percentage of cell viability after treatment of Hela cells with the ZnPc4-loaded Aurod@pNIPAAm-PEGMA nanogel (300 μg/mL) at different irradiated conditions. Compared with the cells’ group with no light treatment, no significant difference of the cell viability was found in the 808-nm laser-treated group. However, for the 680-nm light-treated group, the cell viability decreased to 40%. It is interesting to note that when irradiated by the two lights, the cell viability decreased to 10%. This is because the 808-nm laser treatment might result in the release of ZnPc4 from nanogels, which could improve the efficiency of the generation of singlet oxygen after the 680-nm irradiation and thus enhance the PDT effect on Hela cells.
Figure 10.
The photodynamic therapy effect of ZnPc4-loaded Aurod@pNIPAAm-PEGMA nanogels on Hela cells at different irradiated conditions.
Conclusions
A facile approach to prepare near-infrared-responsive Aurod@pNIPAAm-PEGMA nanogels was described. The LCSTs of these Aurod@pNIPAAm-PEGMA nanogels could be tuned by changing the molar ratio of NIPAAm/PEGMA. The release of ZnPc4 loaded in Aurod@pNIPAAm-PEGMA nanogels increased with the extension of irradiated time and the increase of the power of the NIR laser. The loaded ZnPc4 in Aurod@pNIPAAm-PEGMA nanogels could generate singlet oxygen efficiently. The in vitro study showed obvious PDT effect on Hela cells. On these bases, the Aurod@pNIPAAm-PEGMA nanogels might serve as a promising drug carrier in PDT.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
RL conceived the study, participated in the experimental design, and helped draft the manuscript. TXH participated in the design of the study and performed the statistical analysis. ST and WCD carried out the preparation experiments and drafted the manuscript. LDH, KXB, YAQ, and CM participated in the characterization experiments. All authors read and approved the final manuscript.
Authors’ information
RL, TXH, and LDH are Ph.Ds. and professors. ST, WCD, KXB, YAQ, and CM are M.D. students in the Department of Biomaterials, College of Materials, Xiamen University.
Contributor Information
Ting Shang, Email: shangting2010@sina.com.
Cai-ding Wang, Email: licaiting109@gmail.com.
Lei Ren, Email: renlei@xmu.edu.cn.
Xin-hua Tian, Email: txhmd@163.com.
Dong-hui Li, Email: lidh@xmu.edu.cn.
Xue-bin Ke, Email: kcc2691@126.com.
Min Chen, Email: loveyuechunforever@163.coml.
An-qi Yang, Email: y003045@yahoo.com.cn.
Acknowledgments
This work was financially supported by the National Basic Research Program of China (2010CB732402, 2013CB933703) and the National Natural Science Foundation of China (30970733, 81171448).
References
- Han G, Ghosh P, Rotello VM. Functionalized gold nanoparticles for drug delivery. Nanomedicine. 2007;2:113–123. doi: 10.2217/17435889.2.1.113. [DOI] [PubMed] [Google Scholar]
- Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 2008;41:1842–1851. doi: 10.1021/ar800150g. [DOI] [PubMed] [Google Scholar]
- Tong L, Wei QS, Wei A, Cheng JX. Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects. Photochem Photobiol. 2009;85:21–32. doi: 10.1111/j.1751-1097.2008.00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrabalak SE, Chen J, Au L, Lu X, Li X, Xia Y. Gold nanocages for biomedical applications. Adv Mater. 2007;19:3177–3184. doi: 10.1002/adma.200701972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nann T. Nanoparticles in photodynamic therapy. Nano Biomed Eng. 2011;3:137–143. [Google Scholar]
- Jana NR, Gearheart L, Murphy CJ. Wet chemical synthesis of high aspect ratio cylindrical gold nanorod. J Phys Chem B. 2001;105:4065–4067. [Google Scholar]
- Liao HW, Hafner JH. Gold nanorod bioconjugates. Chem Mater. 2005;17:4636–4641. doi: 10.1021/cm050935k. [DOI] [Google Scholar]
- Cheng JS, Liang QQ, Chang HX, Zhu WJ. Redox approaches derived Tin (IV) oxide nanoparticles/graphene nanocomposites as the near-infrared absorber for selective human prostate cancer cells destruction. Nano Biomed Eng. 2012;4:76–82. [Google Scholar]
- Rahimi M, Wadajkar A, Subramanian K, Yousef M, Cui W, Hsieh JT, Nguyen KT. In vitro evaluation of novel polymer-coated magnetic nanoparticles for controlled drug delivery. Nanomedicine. 2010;6:672–680. doi: 10.1016/j.nano.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Chen JY, Wang P, Wang PN, Guo J, Yang WL, Wang CC, Peng Q. Poly(N-isopropylacrylamide)-coated thermo-responsive nanoparticles for controlled delivery of sulfonated Zn-phthalocyanine in Chinese hamster ovary cells in vitro and zebra fish in vivo. Nanotechnology. 2007;18:5. [Google Scholar]
- Fujimoto KL, Ma ZW, Nelson DM, Hashizume R, Guan JJ, Tobita K, Wagner WR. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao P, Niu QS, Wang ZB, Cao DP. Synthesis of thermosensitive micelles based on poly(N-isopropylacrylamide) and poly(l-alanine) for controlled release of adriamycin. Chem Eng J. 2010;159:257–263. doi: 10.1016/j.cej.2010.02.035. [DOI] [Google Scholar]
- Inoue T, Chen GH, Nakamae K, Hoffman AS. Temperature sensitivity of a hydrogel network containing different LCST oligomers grafted to the hydrogel backbone. Polymer Gels and Networks. 1997;5:561–575. [Google Scholar]
- Wang ZC, Xu XD, Chen CS, Wang GR, Cheng SX, Zhang XZ, Zhu RX. In situ formation of thermosensitive P(NIPAAm-co-GMA)/PEI hydrogels. React Funct Polym. 2009;69:14–19. doi: 10.1016/j.reactfunctpolym.2008.10.004. [DOI] [Google Scholar]
- Karg M, Hellweg T. New “smart” poly(NIPAM) microgels and nanoparticle microgel hybrids: properties and advances in characterisation. Curr Opin Colloid In. 2009;14:438–450. doi: 10.1016/j.cocis.2009.08.002. [DOI] [Google Scholar]
- Contreras-Cáceres R, Pacifico J, Pastoriza-Santos I, Perez-Juste J, Fernández-Barbero A, Liz-Marzán LM. Au@pNIPAM thermosensitive nanostructures: control over shell cross-linking, overall dimensions, and core growth. Adv Funct Mater. 2009;19:3070–3076. doi: 10.1002/adfm.200900481. [DOI] [Google Scholar]
- Chen T, Chang DP, Zhang JM, Jordan R, Zauscher S. Manipulating the motion of gold aggregates using stimulus-responsive patterned polymer brushes as a motor. Adv Funct Mater. 2012;22:429–434. doi: 10.1002/adfm.201101795. [DOI] [Google Scholar]
- Li WY, Cai X, Kim CH, Sun GR, Zhang Y, Deng R, Yang MX, Chen JY, Achilefu S, Wang LV, Xia YN. Gold nanocages covered with thermally-responsive polymers for controlled release by high-intensity focused ultrasound. Nanoscale. 2011;3:1724–1730. doi: 10.1039/c0nr00932f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XQ, Wang TX, Liu W, Wang CD, Wang D, Shang T, Shen LH, Ren L. Multifunctional Au@IPN-pNIPAAm nanogels for cancer cell imaging and combined chemo-photothermal treatment. J Mater Chem. 2011;21:7240–7247. doi: 10.1039/c1jm10277j. [DOI] [Google Scholar]
- Sau TK, MurPhy CJ. Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc. 2004;126:8648–8649. doi: 10.1021/ja047846d. [DOI] [PubMed] [Google Scholar]
- Tempesti TC, Alvarez MG, Durantini EN. Synthesis and photodynamic properties of amphiphilic A3B-phthalocyanine derivatives bearing N-heterocycles as potential cationic phototherapeutic agents. Dyes Pigments. 2011;91:6–12. doi: 10.1016/j.dyepig.2011.02.004. [DOI] [Google Scholar]
- Douglas KL, Piccirillo CA, Tabrizian M. Cell line-dependent internalization pathways and intracellular trafficking determine transfection efficiency of nanoparticle vectors. Eur J Pharm Biopharm. 2008;68:676–687. doi: 10.1016/j.ejpb.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Tu J, Wang TX, Shi W, Wu GS, Tian XH, Wang YH, Ge DT, Ren L. Multifunctional ZnPc-loaded mesoporous silica nanoparticles for enhancement of photodynamic therapy efficacy by endolysosomal escape. Biomaterials. 2012;33:7903–7914. doi: 10.1016/j.biomaterials.2012.07.025. [DOI] [PubMed] [Google Scholar]
- Siskou IC, Rekka EA, Kourounakis AP, Chrysselis MC, Tsiakitzis K, Kourounakis PN. Design and study of some novel ibuprofen derivatives with potential nootropic and neuroprotective properties. Bioorg Med Chem Lett. 2007;15:951–961. doi: 10.1016/j.bmc.2006.10.056. [DOI] [PubMed] [Google Scholar]
- Pomroy NC, Deber CM. Solubilization of hydrophobic peptides by reversible cysteine PEGylation. Biochem Bioph Res Co. 1998;245:618–621. doi: 10.1006/bbrc.1998.8493. [DOI] [PubMed] [Google Scholar]
- Singh N, Lyon LA. Au nanoparticle templated synthesis of pNIPAm nanogels. Chem Mater. 2007;19:719–726. doi: 10.1021/cm061878d. [DOI] [Google Scholar]
- Leedham TJ, Powell DB, Scott JGV. Infrared and Raman spectra of 1,5-cyclooctadiene complexes of copper (І), silver (І), gold (І), and gold (III), and the nature of the gold compounds. Spectrochimi Acta A. 1973;29:559–565. doi: 10.1016/0584-8539(73)80037-6. [DOI] [Google Scholar]
- Levin CS, Janesko BJ, Bardhan R, Scuseria GE, Hartgerink JD, Halas NJ. Chain-length-dependent vibrational resonances in alkanethiol self-assembled monolayers observed on plasmonic nanoparticle substrates. Nano Lett. 2006;6:2617–2621. doi: 10.1021/nl062283k. [DOI] [PubMed] [Google Scholar]
- Feil H, Bae YH, Jan FJ, Kim SW. Effect of comonomer hydrophilicity and ionization on the lower critical solution temperature of N-isopropylacrylamide copolymers. Macromolecules. 1993;26:2496–2500. doi: 10.1021/ma00062a016. [DOI] [Google Scholar]
- Kawano T, Niidome Y, Mori T, Katayama Y, Niidome T. PNIPAM gel-coated gold nanorods for targeted delivery responding to a near-infrared laser. Bioconjugate Chem. 2009;20:209–212. doi: 10.1021/bc800480k. [DOI] [PubMed] [Google Scholar]
- Palewska K, Sujka M, Urasińska-Wόjcik B, Sworakowski J, Lipiński J, Nešpůrek S, Rakušan J, Karásková M. Light-induced effects in sulfonated aluminum phthalocyanines - potential photosensitizers in the photodynamic therapy spectroscopic and kinetic study. J Photoch Photobio A. 2008;197:1–12. doi: 10.1016/j.jphotochem.2007.11.025. [DOI] [Google Scholar]
- Lopez RF, Lange N, Guy R, Bentley MV. Photodynamic therapy of skin cancer: controlled drug delivery of 5-ALA and its esters. Adv Drug Deliv Rev. 2004;56:77–94. doi: 10.1016/j.addr.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Cerńy JR, Karásková M, San JR, Nešpůrek S. Reactive oxygen species produced by irradiation of some phthalocyanine derivatives. J Photochem Photobiol A. 2010;210:82–88. doi: 10.1016/j.jphotochem.2009.11.016. [DOI] [Google Scholar]