Abstract
Urocortins (Ucns) injected peripherally decrease food intake and gastric emptying through peripheral CRF2 receptors in rodents. However, whether Ucns influence circulating levels of the orexigenic and prokinetic hormone, ghrelin has been little investigated. We examined plasma levels of ghrelin and blood glucose after intravenous (iv) injection of Ucn 1, the CRF receptor subtype involved and underlying mechanisms in ad libitum fed rats equipped with a chronic iv cannula. Ucn 1 (10 µg/kg, iv) induced a rapid onset and long lasting increase in ghrelin levels reaching 68% and 219% at 0.5 and 3 h post injection respectively and a 5-h hyperglycemic response. The selective CRF2 agonist, Ucn 2 (3 µg/kg, iv) increased fasting acyl (3 h: 49%) and des-acyl ghrelin levels (3 h: 30%) compared to vehicle while the preferential CRF1 agonist, CRF (3 µg/kg, iv) had no effect. Ucn 1’s stimulatory actions were blocked by the selective CRF2 antagonist, astressin2-B (100 µg/kg, iv). Hexamethonium (10 mg/kg, sc) prevented Ucn 1-induced rise in total ghrelin levels while not altering the hyperglycemic response. These data indicate that systemic injection of Ucns induces a CRF2-mediated increase in circulating ghrelin levels likely via indirect actions on gastric ghrelin cells that involves a nicotinic pathway independently from the hyperglycemic response.
Keywords: acyl ghrelin, astressin2-B, CRF, des-acyl ghrelin, radioimmunoassay, rat, urocortin
1. Introduction
The CRF signaling systems in mammals encompass CRF and three related peptides, urocortin (Ucn) 1, Ucn 2, and Ucn 3, as well as two main receptor subtypes, CRF1 and CRF2 [15]. CRF ligands interact with CRF receptors with distinct affinity, with CRF showing a 40-fold higher affinity to CRF1 than CRF2 receptors [15]. Ucn 1 displays a high affinity to both CRF1 and CRF2 receptors while Ucn 2 and Ucn 3 bind selectively to CRF2 [15]. While the CRF1 signaling system in the brain is well established to orchestrate the endocrine, behavioral, and visceral responses to stress [27,43,48], growing anatomical and physiological evidence also supports an important role of the peripheral CRF2 signaling system within the viscera, namely the gut and heart [12,28]. In particular, in the rat and human stomach CRF2 receptors are prominently expressed at the gene and protein levels although the cellular identification of CRF2 positive is still to be further investigated [4,5,55]. Convergent functional studies also showed that injection of Ucn 1 or 2 either intraperitoneally (ip) or intravenously (iv) inhibits gastric emptying, antral motility, and food intake in rodents [13,20,33,52]. We also reported that iv Ucn 1 induces a sustained hyperglycemia in fasted rats [50]. The demonstration that the selective antagonist, astressin2-B [37] injected peripherally prevents the ip or iv injected Ucn 1-induced inhibition of gastric emptying and food intake in mice and rats [13,31,37] while peripheral injection of CRF1 antagonists has no effect [29, 31] supports a mediation through CRF2 receptors.
Conversely, acyl ghrelin is a peptide hormone mainly released from gastric X/A-like cells, [8] stimulating food intake, gastric emptying and motility [6,18]. Acyl ghrelin release is increased by fasting or before a meal while being reduced postprandially in rodents and humans, indicative of a physiological role in meal initiation [7,44,46]. Previous reports indicate that visceral (abdominal surgery) or immunological (lipopolysaccharide, LPS, injected intraperitoneally at a low dose) stressors induced suppression of food intake and gastric emptying which was associated with the reduction of plasma ghrelin in rats [38,41,50]. In addition, LPS under these conditions up-regulated Ucns mRNA expression in the rat gastric corpus mucosa [55]. These findings indicate a potential influence of peripheral Ucns on circulating ghrelin which so far has been little investigated. One clinical study showed that Ucn 1 infused iv for one hour reduces the fasted ghrelin plasma levels starting at 2-h post infusion in healthy subjects [10] while an iv bolus of Ucn 1 in fasted rats did not change the plasma levels of total ghrelin and induced a hyperglycemia over the 5-h experimental period [50].
In the present study, we investigated the influence of Ucn 1, injected iv at a dose known to inhibit gastric emptying in rats [31,33], on plasma levels of ghrelin and blood glucose in ad libitum fed rats, linked with lower circulating levels of ghrelin compared with fasted conditions [42]. Next, we characterized the CRF receptor mediating the iv Ucn 1 action, using the selective CRF2 antagonist, astressin2-B [37]. We also examined whether the selective CRF2 agonist Ucn 2 injected iv would influence the fasting ghrelin levels including the acylated and the most abundant form, non-acylated (des-acyl) ghrelin that does not bind to the ghrelin receptor [22,23]. Lastly, in light of previous evidence that iv Ucn 1 activates brain nuclei regulating sympathetic outflow to the viscera as shown by Fos expression [51] and that ghrelin release is regulated by the autonomic nervous system [17], we also investigated the influence of ganglionic blockade by hexamethonium on ghrelin and glucose alterations induced by the iv injection of Ucn 1.
2. Materials and Methods
2.1. Animals
Adult male Sprague-Dawley rats (Harlan, San Diego, CA, USA, 280–320 g) were housed 4 animals/cage under conditions of controlled illumination (12:12 h light/dark cycle, lights on/off: 6.00 h/18.00 h) and temperature (22±2 °C) unless otherwise stated. Animals were fed a standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO, USA) and tap water ad libitum. Animal care and experimental procedures followed institutional ethic guidelines and conformed to the requirements of the federal authority for animal research conduct. All procedures were approved by the Animal Research Committee at the Veterans Affairs Greater Los Angeles Healthcare System (animal protocol # 05058-02).
2.2. Compounds
Rat CRF, rat Ucn 1, human Ucn 2 and astressin2-B were synthesized as described before [37] at the Clayton Foundation Laboratories (Peptide Biology Laboratories, Salk Institute, La Jolla, CA). Peptides, stored in powder form at −80 °C and hexamethonium (Sigma-Aldrich, San Louis, MO) stored at room temperature, were dissolved in vehicle immediately before use.
2.3. Blood collection and assays
2.3.1. Intravenous catheterization
Intravenous catheterization was performed as described in our previous studies [50]. Briefly, rats were anesthetized with a mixture of ketamine (75 mg/kg ip; Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (5 mg/kg ip; Mobay, Shawnee, KS) and a sterile PE-50 catheter was inserted into the right external jugular vein. The catheter, filled with saline-heparin (200 units/ml) to maintain lumen patency, was exteriorized between the scapulae via subcutaneous tunneling, then secured to the skin and closed using a wire. Rats were singly housed after surgery and allowed to recover for three days during which they were accustomed to the experimental procedures including light hand restraint for blood withdrawal. Body weight was monitored before the iv catheterization and three days after the surgery.
2.3.2. Blood withdrawal and processing
Blood (0.5 ml) was withdrawn into a syringe while rats were lightly hand restrained. The first blood sampling time started between 9.00 h and 10.00 h for each batch of rats. Blood samples were processed according to the RAPID method for the measurement of acyl and total ghrelin as previously described [42]. Briefly, immediately after withdrawal, blood was diluted 1:10 in an ice-cold buffer (pH 3.6) containing 0.1M ammonium acetate, 0.5 M NaCl, and enzyme inhibitors (diprotin A, E-64-d, antipain, leupeptin and chymostatin, 1 µg/ml; Peptides International, Louisville, KY), and immediately centrifuged at 3000 rpm for 10 min at 4 °C. Sep-Pak C18 cartridges (360 mg, 55–105 µm, product no. WAT051910, Waters Corporation, Milford, MA) were charged with 5 ml 100% acetonitrile and equilibrated with 10 ml 0.1% trifluoroacetate (TFA). The equilibrated cartridges were loaded with sample, rinsed with 3 ml 0.1% TFA and eluted with 2 ml 70% acetonitrile in 0.1% TFA. The eluted samples were dried by vacuum centrifugation and stored at −80°C until further processing.
For measurement of total ghrelin alone, blood was collected in ice-cooled tubes containing EDTA (7.5%, 10 µl/0.5 ml blood; Sigma-Aldrich) and aprotinin (0.6 trypsin Inhibitory Unit per 0.5 ml blood; ICN Pharmaceuticals, Costa Mesa, CA) as previously described [50]. Samples were kept on ice until centrifugation at 3000 rpm for 10 min at 4°C. Plasma was collected and stored at −80°C.
2.3.3. Determination of acyl ghrelin, des-acyl ghrelin and total ghrelin plasma levels
Radioimmunoassay was performed using a commercial RIA kit for rat/mouse total ghrelin (Phoenix Pharmaceuticals, Belmont, CA). The limit of the assay sensitivity was 54 pg/ml and the intra- and inter-assay variations were less than 5% and 14% respectively.
For acyl and des-acyl ghrelin determinations, samples were re-suspended in double distilled H2O according to the original volume of plasma and thereafter, acyl and total ghrelin were measured using specific radioimmunoassay kits according to the manufacturer’s instructions (# GHRT-89HK and GHRA-88HK, respectively, Millipore, Billerica, MA). Des-acyl ghrelin was calculated as the difference of total minus acyl ghrelin for each individual sample. Intra-assay variability was < 5% and all samples were processed in one batch.
2.3.4. Blood glucose levels
Blood glucose levels were determined using a glucometer (One-Touch Ultra; LifeScan, Milpitas, CA).
2.4. Experimental protocols
All experiments were performed between 9.00 h and 14.00 h. Rats had similar body weight before and three days after the surgery for iv catheter (277.3 ± 11.1 vs 277.3 ± 12.2 g; n=39).
2.4.1. Influence of iv Ucn 1 on plasma total ghrelin and blood glucose levels: Time course study
Freely fed rats implanted with a chronic intra-jugular catheter were injected iv (0.2 ml) with vehicle (pyrogen-free water) or Ucn 1 (10 µg/kg dissolved in vehicle) and returned to their home cages with access to water but not food to avoid potential confounding factors linked with differential influence of treatment on food intake. Blood (0.5 ml) was withdrawn before and at 0.5, 1, 3 and 5 h post injection and processed for plasma levels of total ghrelin and blood glucose measurements were performed as described above. The iv dose of Ucn 1 was selected based on our previous dose response studies in rats showing maximal suppression of gastric emptying in rats [33].
2.4.2. Influence of CRF2 receptor antagonist on total ghrelin and glucose responses to iv Ucn 1
Freely fed rats implanted with a chronic jugular catheter were injected iv (0.1 ml) with vehicle (pyrogen-free water) or astressin2-B (100 µg/kg dissolved in vehicle) and 15 min later with vehicle (pyrogen-free water) or Ucn 1 (10 µg/kg in vehicle). The regimen of astressin2-B administration was based on previous studies showing the blockade of gastric transit induced by iv Ucn 1 in rats [31]. Blood (0.5 ml) was withdrawn before iv injection of astressin2-B and 1 h post injection of iv Ucn 1 for determination of total ghrelin plasma and blood glucose levels.
2.4.3. Influence of Ucn 2 or CRF on fasted plasma acyl and des-acyl ghrelin levels
Rats implanted with a chronic intra-jugular catheter were deprived of food but not water overnight and injected iv (0.2 ml) with vehicle (saline or pyrogen-free water containing 0.1% bovine serum albumin, BSA, Sigma-Aldrich), Ucn 2 (3 µg/kg in pyrogen-free water containing 0.1% BSA), or CRF (3 µg/kg in sterile saline containing 0.1% BSA) and returned to their home cages without access to food but with water ad libitum. Blood (0.5 ml) was withdrawn at 3 and 5 h post injection of Ucn 2, CRF or vehicle and processed for total and acyl ghrelin measurements and the determination of des-acyl ghrelin from these individual values. Doses of peptides were selected based on their inhibitory effects on gastric emptying upon peripheral injection in rats [31,33].
2.4.4. Influence of hexamethonium on iv Ucn 1-induced rise in plasma total ghrelin and hyperglycemia
Rats fed ad libitum were injected subcutaneously (sc, 0.3 ml/rat) with vehicle (saline) or hexamethonium (10 mg/kg, dissolved in vehicle), 30 min before the iv injection (0.2 ml/rat) of vehicle (pyrogen-free water) or Ucn 1 (10 µg/kg, dissolved in pyrogen-free water). The dose of hexamethonium was modified from our previous studies showing that 15 mg/kg prevented the iv CRF induced decrease in intraluminal gastric pressure in anesthetized rats [36]. Blood (0.5 ml) was withdrawn at 1 h post injection and processed for total ghrelin and glucose determinations.
2.5. Statistical analysis
Data are expressed as mean ± SEM and analyzed by one-way or two-way analysis of variance (ANOVA) followed by Tukey post hoc test. Data obtained in the CRF2 antagonist and ganglionic blocker experiments were expressed as percentage of change calculated from basal level for each value in the same rat. Differences between groups were considered significant when p < 0.05.
3. Results
3.1. Intravenous Ucn 1 increases plasma total ghrelin and blood glucose levels: time course study
In freely fed rats, pre-injection levels of total ghrelin were similar in both groups (Fig. 1A). In the control group injected iv with vehicle, plasma total ghrelin levels increased during the 5-h observation period compared with pre-injection levels, reaching significance at 3 h post injection, which represented a 74% increase at this time point that was maintained at 5 h (0 h vs. 3 h: 0.77 ± 0.09 vs. 1.36 ± 0.19, p < 0.05 and vs. 5h: 1.39 ± 0.13 ng/ml; p < 0.05; n = 6; Fig. 1A). By contrast, blood glucose levels were stable throughout the 5-h experimental period in iv vehicle injected rats (0 h: 117.2 ± 2.2 vs. 5 h: 124.5 ± 3.5 mg/dl, n = 6; p > 0.05; Fig. 1B). Injection of Ucn 1 (10 µg/kg, iv) increased total plasma ghrelin levels compared to the vehicle-injected group at 0.5 h (1.41 ± 0.18 vs. 0.85 ± 0.11 ng/ml, n = 7; p < 0.05), 1 h (1.69 ± 0.18 vs. 1.19 ± 0.15 ng/ml; p < 0.05), and 3 h (2.61 ± 0.25 vs. 1.36 ± 0.19 ng/ml; p < 0.05) post injection, whereas at 5 h, no significant difference was observed compared to vehicle (1.81 ± 0.32 vs. 1.39 ± 0.13 ng/ml; p > 0.05; Fig. 1A). Likewise, blood glucose was significantly increased after iv Ucn 1 compared to vehicle at 0.5 h (155.7 ± 3.9 vs. 119.2 ± 2.7 mg/dl, p < 0.05) with a plateau increase at 1 h (164.4 ± 6.0 vs. 124.0 ± 4.7 mg/dl, p < 0.05) and 3 h (163.4 ±3.1 vs. 126.3 ± 1.7 mg/dl, p < 0.05), and a slow decrease at 5 h post injection (148.7 ± 3.2 vs. 124.5 ± 3.5 mg/dl, p < 0.05; Fig. 1B). Two way ANOVA showed that total ghrelin levels were significantly influenced by iv Ucn 1 treatment (F(1,55) = 22.6, p < 0.001) and time (F(4,55) = 11.4, p < 0.001), but not by treatment × time (F(4,55) = 2.5, p = 0.05). The increase in blood glucose levels was also significantly influenced by treatment (F(1,55) = 127.3, p < 0.001) and time (F(4,55) = 21.6, p < 0.001), as well as treatment × time (F(4,55) = 12.6, p < 0.001).
Fig. 1.
Time course of rise in plasma total ghrelin (A) and blood glucose (B) levels induced by iv urocortin 1 in freely moving rats. Freely fed rats implanted with a chronic iv catheter were injected iv with Ucn 1 or vehicle and returned to their home cages with access to water but not food. Blood was withdrawn at various time intervals. Blood glucose and plasma total ghrelin were measured. Data are expressed as mean ± SEM, n = 6–7/group, *: p < 0.05 vs. time 0 and #: vs. vehicle at the same time point.
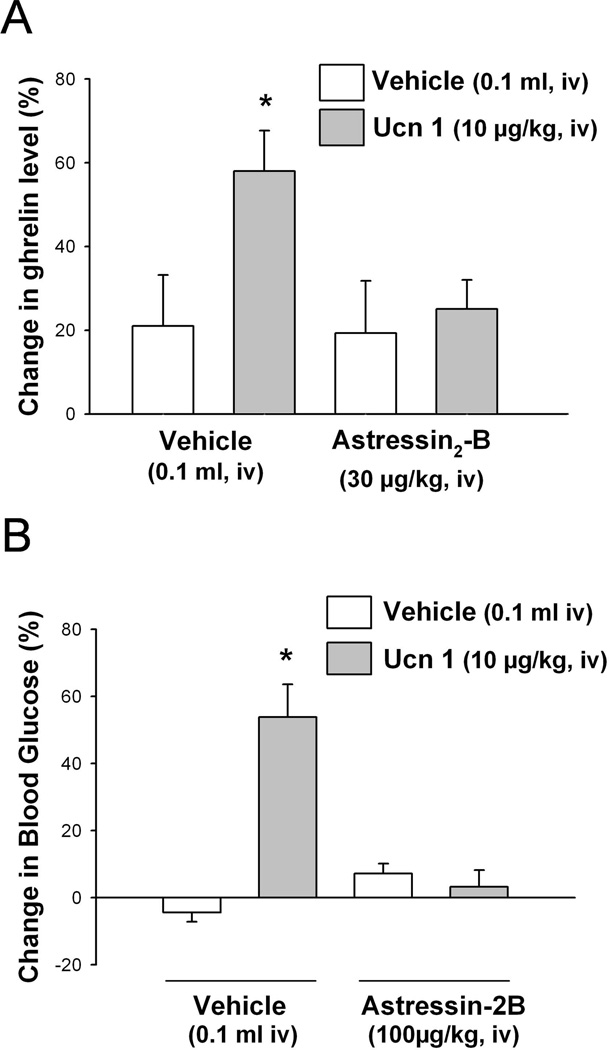
3.2. Intravenous Ucn 1-induced elevations of plasma ghrelin and blood glucose levels are CRF2 receptor mediated
In freely fed rats pretreated with iv vehicle, iv Ucn 1 (10 µg/kg) induced a significant 58.0 ± 9.7% increase in plasma ghrelin (Fig. 2A) and 53.8 ± 9.8% blood glucose levels compared with iv vehicle + iv vehicle as measured at 1 h after peptide injection (Fig. 2B). The selective peptide CRF2 antagonist astressin2-B (100 µg/kg, iv) completely blocked the iv Ucn 1-induced rise in ghrelin and glucose circulating levels (Fig. 2). When injected alone the antagonist had no effect on either total ghrelin or glucose levels compared to vehicle (p > 0.05; Fig. 2).
Fig. 2.
The CRF2 receptor antagonist, astressin2-B blocks the iv urocortin 1-induced increase in plasma total ghrelin (A) and blood glucose (B) levels in freely moving rats. Freely fed rats implanted with a chronic iv catheter were injected iv with astressin2-B or vehicle 15 min before iv injection of Ucn 1 or vehicle, and returned to their home cages with access to water but not food. Blood was withdrawn before injection of astressin2-B and at 1 h post injection of Ucn 1. Blood glucose and plasma total ghrelin were measured. Data are expressed as mean of percent changes ± SEM, n= 5–7/group. * p < 0.05 vs. vehicle/vehicle.
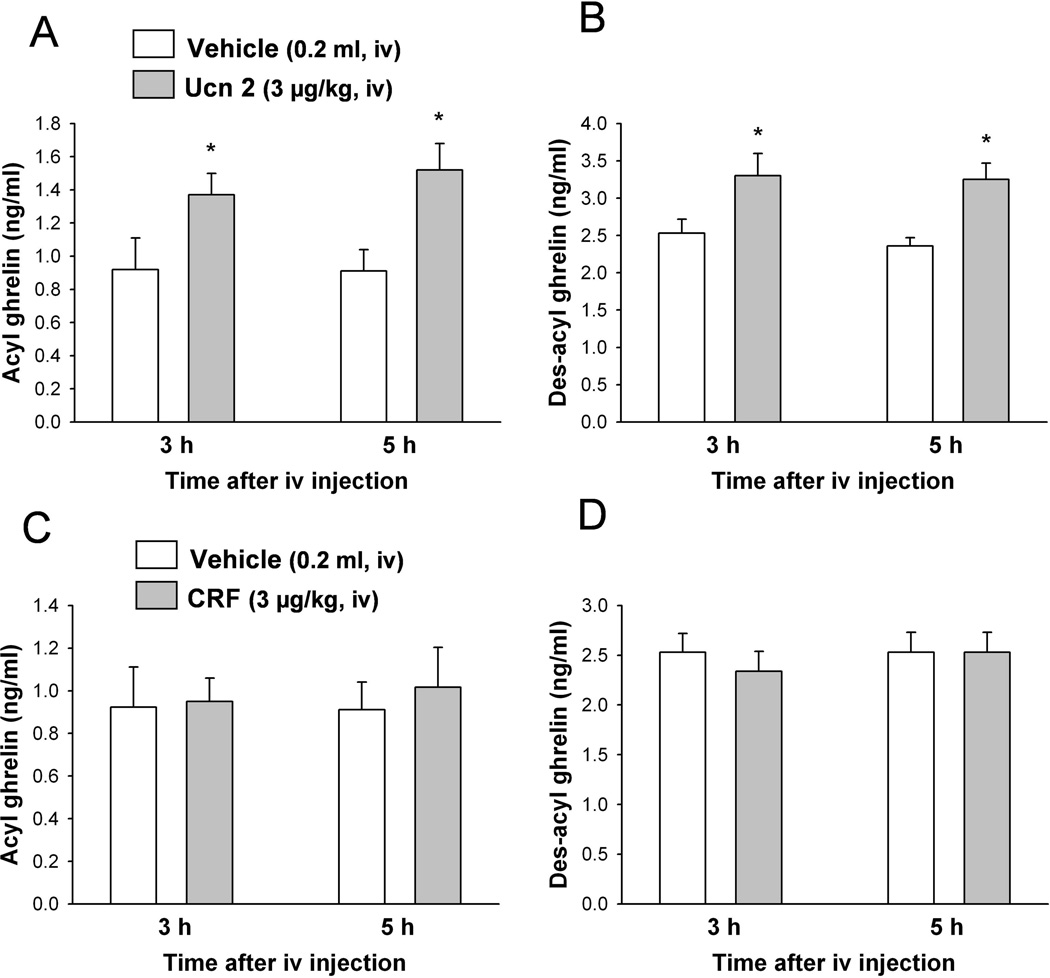
3.3. Intravenous Ucn 2, but not CRF, increases plasma acyl and des-acyl ghrelin
In overnight fasted rats, Ucn 2 (3 µg/kg, iv) significantly increased plasma acyl ghrelin levels compared with the iv vehicle group by 48.8% at 3 h (1.37 ± 0.13 vs. 0.92 ± 0.19 ng/ml, p < 0.05) and 66.8 % at 5 h post injection (1.52 ± 0.16 vs. 0.91 ± 0.13 ng/ml, p < 0.05; Fig. 3A). Two-way ANOVA showed a significant influence of treatment (F(1,16) = 11.9, p < 0.01) but not time (F(1,16) = 0.2, p > 0.05). Likewise, plasma des-acyl ghrelin levels were significantly elevated after iv Ucn 2 compared to vehicle injected animals by 30.4% at 3 h (3.30 ± 1.15 vs. 2.53 ± 0.19 ng/ml) and 37.7% at 5 h (3.25 ± 0.23 vs. 2.36 ± 0.30 ng/ml; Fig. 3B). Two-way ANOVA indicated a significant influence of treatment (F(1,16) = 14.5, p < 0.01) but not time (F(1,16) = 0.3, p > 0.05). By contrast, plasma levels of acyl and des-acyl ghrelin did not change significantly over the experimental period following the iv injection of the preferential CRF1 agonist, CRF (3 µg/kg, iv) compared to iv vehicle as monitored at 0.5, 1, 3 and 5 h post injection in fasted rats (p > 0.05; Fig. 3C, D).
Fig. 3.
Urocortin 2, unlike CRF, increases fasted levels of acyl and des-acyl ghrelin in freely moving rats. Overnight fasted rats implanted with chronic iv catheter were injected iv with Ucn 2, CRF or vehicle and returned in their home cages with access to water but not food. Blood was withdrawn at 3 and 5 h post injection of Ucn 2 (A, B) and CRF (C, D) and processed for acyl and total ghrelin measurements. Des-acyl ghrelin was determined as the difference between total and acyl ghrelin for each measurement. Data are expressed as mean of percent changes ± SEM, of n=5/group. * p < 0.05 and vs. vehicle.
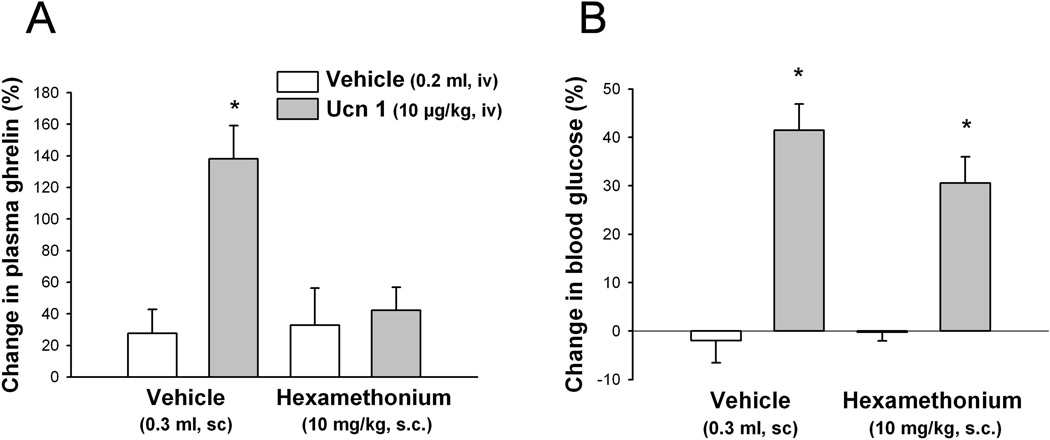
3.4. Intravenous Ucn 1-induced CRF2 receptor mediated increase in plasma total ghrelin levels is blocked by hexamethonium, unlike the hyperglycemia
Hexamethonium (10 mg/kg) injected sc 30 min before the iv injection of Ucn 1 (10 µg/kg, iv) completely blocked the increase of total ghrelin as measured at 1 h post iv injection in freely fed rats (hexamethonium/Ucn 1 vs. vehicle/Ucn 1: 42.3 ± 14.6% vs. 138.1 ± 21.0%, n = 7–8; p < 0.05; Fig. 4A). By contrast, the concomitant elevation of blood glucose was blunted without reaching statistical significance (hexamethonium/Ucn 1 vs. vehicle/Ucn 1, 30.1 ± 5.5% vs. 41.5 ± 5.5%, p > 0.05; Fig. 4B). Hexamethonium alone under these conditions did not influence levels of plasma ghrelin or blood glucose (Fig. 4).
Fig. 4.
Hexamethonium blocks the iv urocortin 1-induced increase in plasma total ghrelin but not blood glucose levels. Rats fed ad libitum implanted with a chronic iv catheter were injected sc with hexamethonium or vehicle and 30 min later with Ucn 1 or vehicle iv and blood was withdrawn at 1 h post injection and processed for total ghrelin (A) and glucose (B) measurements. Data are expressed as mean ± SEM, n= 5–6/group. * p < 0.05 vs. all other groups.
4. Discussion
The present data established that Ucn 1 injected intravenously increases ghrelin plasma levels compared with iv vehicle in freely fed conscious rats. Time course studies showed that the Ucn 1 response was rapid in onset and long lasting as shown by a 68% elevation at 30 min post iv Ucn 1 injection that reached 216% at 3 h compared to pre-injection levels with a return to vehicle levels at 5 h. The sustained response may be linked with pharmacokinetic properties of Ucn 1 that has a long half life in the circulation [34]. However, the underlying mechanisms contributing the peak response observed at 3 h post injection are not clear at the present time. It may be speculated that there is synergistic interaction between Ucn 1’s ghrelin-releasing action and additional mechanisms recruited at 3 h. Indeed, in the vehicle-injected group, we observed a gradual increase in ghrelin levels reaching significance at 3 h (76%) and maintained at 5 h (84%) post injection compared with pre-injection values which may be linked with known diurnal changes in circulating ghrelin with a peak occurring at 5 h after onset of the light phase in ad libitum fed rats [3]. Consistent with such a possibility is the drop to vehicle values at 5 h post urocortin 1 injection when the peptide (which has a half life of 2.9 h) is cleared [34].
We also showed that the response is not specific to Ucn 1, since the related peptide, Ucn 2 [15] injected iv also induced a sustained ghrelin elevation reaching 49% and 57% at 3 and 5 h post injection respectively compared with vehicle. As the Ucn 2 experiment was performed in fasted rats, a metabolic state well established to increase circulating levels of ghrelin compared to the fed state [42,46], these data provide the first evidence in rats that a systemic treatment with Ucns induces a consistent elevation of circulating ghrelin levels irrespective of the feeding status, overnight fasted or freely fed associated with low or already elevated endogenous ghrelin levels. However, the ghrelin response to iv Ucn 1 is still equivocal as in our previous studies in fasted rats, iv Ucn 1 at a similar dose did not influence plasma levels of total ghrelin [50], indicative that fasting may influence the effect of Ucn 1. In one clinical study, Ucn 1 (50 µg/healthy subject/h) or vehicle was infused iv for 1 h starting at 1 h after breakfast. There was a time-related elevation of ghrelin levels with a peak response at 3 h in vehicle group and Ucn 1 reduced the elevated plasma total ghrelin levels at 3 and 5 h post initiation of the infusion while not influencing the rise for the first 2 h [10]. Whether these differential patterns reflect species or dose differences remains to be investigated.
Total ghrelin encompasses two major circulating forms, acyl ghrelin, the only form binding to the ghrelin receptor, and des-acyl ghrelin, the predominant form in the blood [9,32]. Recent evidence indicates that the ratio of acyl and des-acyl ghrelin can be altered in experimental animals and humans under stress conditions [45]. We previously determined that the ratio of plasma acyl:des-acyl ghrelin was 1:3 (acyl:total ghrelin 1:5) in rats using the RAPID method based on blood processing to improve the recovery of acyl ghrelin compared with previous methods that yield a lower acyl:des-acyl ratio [16,38,42]. Likewise, in the present study, the acyl:des-acyl ghrelin ratio at 3 and 5 h after iv vehicle was 1:3.1 and 1:2.7, respectively (p > 0.05) in fasted rats. Ucn 2 injected iv did not significantly modify this ratio (1:2.5 and 1:2.2 at 3 and 5 h post injection, respectively p > 0.05 vs. vehicle), although there was a trend to enhance acyl ghrelin vs. des-acyl ghrelin. Ghrelin-O acyltransferase (GOAT) which is expressed in rat gastric corpus ghrelin cells [40], is the unique enzyme highly conserved across vertebrates able to catalyze the octanoylation of ghrelin, leading to the active form of the peptide [54]. The present data indicate that the rise in ghrelin after iv Ucn 2 may not reflect changes in GOAT expression or activity.
Next, we used a pharmacological approach to assess the receptor subtype(s) involved in the action of the CRF1/CRF2 agonist, Ucn 1 [15,47]. We demonstrated that the selective CRF2 antagonist, astressin2-B injected iv before Ucn 1 completely blocked the rise in ghrelin elicited by Ucn 1. Moreover, the rise in plasma ghrelin can be reproduced by the iv injection of the selective CRF2 agonist, Ucn 2 [15] while the preferential CRF1 agonist, CRF [15] had no effect throughout the 5-h experimental period including the 0.5 h sampling period as well as in the first hour and every two hours thereafter. Consistent with the lack of CRF action, a previous study showed that CRF microinfused through a probe inserted into the submucosal layer of the rat gastric corpus did not significantly influence the release of ghrelin monitored in the microdialysis perfusate while noradrenaline had a stimulatory effect [11]. Collectively, these data provide convergent pharmacological evidence that systemic injection of Ucns leads to a CRF2 receptor-mediated rapid onset and prolonged elevation of circulating acyl and des-acyl ghrelin under basal or fasting-stimulated conditions in rats. This contrasts with the intracerebroventricular injection of Ucn 1 in rats which was recently reported to decrease plasma ghrelin levels during the first 2 h post injection with a longer lasting inhibitory effect on des-acyl ghrelin than acyl ghrelin [53]. The opposite effects of Ucn 1 injected into the lateral brain ventricle vs. systemic circulation is indicative of distinct centrally vs. peripherally initiated Ucn 1 sites of action to influence circulating levels of ghrelin. In addition, the low basal entry of circulating Ucn 1 across the blood brain barrier compared with other peripheral peptides [19] does not support a direct action of iv Ucn 1 into the brain except in areas outside of the blood-brain barrier.
Then, we investigated the pathways involved in the CRF2-mediated Ucn 1 action. The majority of circulating forms of ghrelin is produced by ghrelin positive X/A-like cells that are located in both gastric oxyntic and, to a lesser extent, antral mucosa of rats [2]. Previously, we obtained pharmacologic and anatomic evidence indicative that the iv somatostatin (sst)-induced sst2 receptor mediated inhibition of circulating ghrelin may involve a direct action on sst2 receptors expressed on gastric corpus ghrelin cells [41]. However, there is no report that CRF2 immunoreactivity is present in endocrine cells of the rat gastric mucosa [5,35]. In addition, CRF2 mRNA is not expressed in a purified preparation of mouse gastric ghrelin cells carrying a reporter, M-cherry, analyzed by microarray compared to whole gastric mucosal scrape (Dr. N. Lambrecht, private communication). Studies available to date are indicative that the CRF2 mediated action of iv Ucn 1 is unlikely to be exerted directly on ghrelin producing cells in the gastric corpus. However, this contention will need to be ascertained using preparations of pure and not-immortalized rat ghrelin cells when available.
An indirect action is further supported by the complete blockade of the hyperghrelinemic response to iv Ucn 1 by the ganglionic blocker, hexamethonium. Of note, as reported previously [50], iv Ucn 1 induced a sustained rise in blood glucose measured concomitantly with ghrelin. However, while the hyperglycemia was also blocked by the CRF2 antagonist, hexamethonium had no effect. Previous reports established that the iv Ucn 3-induced hyperglycemia involves a CRF2 mediated action exerted directly on α-cells in rat pancreatic islets [26]. These data are consistent with the present demonstration that the CRF2 receptor mediated hyperglycemic responses to iv Ucn 1 are initiated by distinct independent mechanisms. It is well established that the activation of the vagal and sympathetic nervous system increases circulating ghrelin levels [17]. It may be speculated that CRF2 receptors expressed at peripheral sites such as the nodose ganglia or in brain areas outside the blood-brain barrier, namely the area postrema [25,30,49], can initiate reflex control over efferent autonomic mechanisms [14,21]. In line with this contention, we previously reported that the iv injection of Ucn 1 at a similar dose induced the expression of Fos, a marker of neuronal activation, in the nucleus tractus solitarius, area postrema and other hypothalamic brain nuclei involved in autonomic regulation [51]. In particular, iv Ucn 1 activates C1/A1 catecholaminergic neurons [51] unlike the dorsal motor nucleus of the vagus, favoring an involvement of the sympathetic nervous system. The implication of these neural mechanisms needs to be substantiated in future investigations.
In conclusion, systemic injection of Ucns induces a rapid onset and sustained CRF2 mediated increase in circulating ghrelin levels unlikely to be related to a direct action on gastric ghrelin cells but requiring the integrity of ganglionic nicotinic transmission in conscious rats. Circulating ghrelin is known to play a physiological orexigenic and gastric prokinetic role to regulate food intake and gastric motility [1,39]. The hyperghrelinemia in response to iv Ucn 1 and Ucn 2, rules out the role of ghrelin in mechanisms underlying peripheral Ucns-CRF2 mediated inhibition of food intake, gastric emptying and motility that is well established under similar conditions of peripheral administration [13,20,33,52]. Whether such a rise in ghrelin plays a role to dampen the Ucn 1 inhibitory action needs to be assessed using ghrelin antagonists. In addition, the CRF2 mediated hexamethonium-independent concomitant hyperglycemia induced by iv Ucn 1 strengthens the emerging role of Ucns-CRF2 signaling as novel peripheral modulators of glucose homeostasis and metabolic functions [24,26].
Highlights.
-
! !
Intravenous urocortin 1 increases plasma total ghrelin and blood glucose in fed rats
-
! !
Intravenous CRF2 receptor agonist, urocortin 2 increases fasted plasma acyl ghrelin levels.
-
! !
The increase in plasma ghrelin and glucose by urocortin 1 is CRF2 receptor mediated.
-
! !
Ghrelin but not glucose rise by urocortin 1 requires the integrity of autonomic transmission
Acknowledgements
This work was supported by the Veterans Administration Research Career Scientist Award, VA Merit Award, Center Grant NIH DK-41301 (Animal Core) and R01 NIH DK 33061 (Y.T.), German Research Foundation STE 1765/3-1 (A.S.) and Charité University Funding UFF 89-441-176 (A.S.). We thank Dr. Jean Rivier (Clayton Foundation Laboratories, Salk Institute, La Jolla, CA) for the generous supply of peptides. We are grateful to Mrs. Honghui Liang for the excellent technical support and Ms. Eugenia Hu for her review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have nothing to disclose. No conflicts of interest exist.
References
- 1.Ariga H, Tsukamoto K, Chen C, Mantyh C, Pappas TN, Takahashi T. Endogenous acyl ghrelin is involved in mediating spontaneous phase III-like contractions of the rat stomach. Neurogastroenterol. Motil. 2007;19:675–680. doi: 10.1111/j.1365-2982.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 2.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 3.Bodosi B, Gardi J, Hajdu I, Szentirmai E, Obal F, Jr, Krueger JM. Rhythms of ghrelin, leptin, and sleep in rats: effects of the normal diurnal cycle, restricted feeding, and sleep deprivation. Am. J. Physiol Regul. Integr. Comp Physiol. 2004;287:R1071–R1079. doi: 10.1152/ajpregu.00294.2004. [DOI] [PubMed] [Google Scholar]
- 4.Chatzaki E, Lambropoulou M, Constantinidis TC, Papadopoulos N, Tache Y, Minopoulos G, Grigoriadis DE. Corticotropin-releasing factor (CRF) receptor type 2 in the human stomach: protective biological role by inhibition of apoptosis. J Cell Physiol. 2006;209:905–911. doi: 10.1002/jcp.20792. [DOI] [PubMed] [Google Scholar]
- 5.Chatzaki E, Murphy BJ, Wang L, Million M, Ohning GV, Crowe PD, Petroski R, Tache Y, Grigoriadis DE. Differential profile of CRF receptor distribution in the rat stomach and duodenum assessed by newly developed CRF receptor antibodies. J. Neurochem. 2004;88:1–11. doi: 10.1046/j.1471-4159.2003.02078.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen CY, Asakawa A, Fujimiya M, Lee SD, Inui A. Ghrelin gene products and the regulation of food intake and gut motility. Pharmacol. Rev. 2009;61:430–481. doi: 10.1124/pr.109.001958. [DOI] [PubMed] [Google Scholar]
- 7.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 8.Date Y, Kojima M, Hosoda H, Sawaguchi A, Mondal MS, Suganuma T, Matsukura S, Kangawa K, Nakazato M. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 9.Davenport AP, Bonner TI, Foord SM, Harmar AJ, Neubig RR, Pin JP, Spedding M, Kojima M, Kangawa K. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol. Rev. 2005;57:541–546. doi: 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- 10.Davis ME, Pemberton CJ, Yandle TG, Lainchbury JG, Rademaker MT, Nicholls MG, Frampton CM, Richards AM. Urocortin-1 infusion in normal humans. J. Clin. Endocrinol. Metab. 2004;89:1402–1409. doi: 10.1210/jc.2003-031231. [DOI] [PubMed] [Google Scholar]
- 11.de la Cour CD, Norlen P, Hakanson R. Secretion of ghrelin from rat stomach ghrelin cells in response to local microinfusion of candidate messenger compounds: a microdialysis study. Regul. Pept. 2007;143:118–126. doi: 10.1016/j.regpep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Emeto TI, Moxon JV, Rush C, Woodward L, Golledge J. Relevance of urocortins to cardiovascular disease. J Mol. Cell Cardiol. 2011;51:299–307. doi: 10.1016/j.yjmcc.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Gourcerol G, Wang L, Wang YH, Million M, Tache Y. Urocortins and cholecystokinin-8 act synergistically to increase satiation in lean but not obese mice: involvement of corticotropin-releasing factor receptor-2 pathway. Endocrinology. 2007;148:6115–6123. doi: 10.1210/en.2007-0678. [DOI] [PubMed] [Google Scholar]
- 14.Hasser EM, Cunningham JT, Sullivan MJ, Curtis KS, Blaine EH, Hay M. Area postrema and sympathetic nervous system effects of vasopressin and angiotensin II. Clin. Exp. Pharmacol. Physiol. 2000;27:432–436. doi: 10.1046/j.1440-1681.2000.03261.x. [DOI] [PubMed] [Google Scholar]
- 15.Hauger RL, Grigoriadis DE, Dallman MF, Plotsky PM, Vale WW, Dautzenberg FM. International Union of Pharmacology. XXXVI. Current Status of the Nomenclature for Receptors for Corticotropin-Releasing Factor and Their Ligands. Pharmacol. Rev. 2003;55:21–26. doi: 10.1124/pr.55.1.3. [DOI] [PubMed] [Google Scholar]
- 16.Hosoda H, Doi K, Nagaya N, Okumura H, Nakagawa E, Enomoto M, Ono F, Kangawa K. Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin. Chem. 2004;50:1077–1080. doi: 10.1373/clinchem.2003.025841. [DOI] [PubMed] [Google Scholar]
- 17.Hosoda H, Kangawa K. The autonomic nervous system regulates gastric ghrelin secretion in rats. Regul. Pept. 2008;146(1–3):12–28. doi: 10.1016/j.regpep.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Hosoda H, Kojima M, Kangawa K. Ghrelin and the regulation of food intake and energy balance. Mol. Interv. 2002;2:494–503. doi: 10.1124/mi.2.8.494. [DOI] [PubMed] [Google Scholar]
- 19.Kastin AJ, Akerstrom V, Pan W. Activation of urocortin transport into brain by leptin. Peptides. 2000;21:1811–1817. doi: 10.1016/s0196-9781(00)00349-1. [DOI] [PubMed] [Google Scholar]
- 20.Kihara N, Fujimura M, Yamamoto I, Itoh E, Inui A, Fujimiya M. Effects of central and peripheral urocortin on fed and fasted gastroduodenal motor activity in conscious rats. Am. J Physiol Gastrointest. Liver Physiol. 2001;280:G406–G419. doi: 10.1152/ajpgi.2001.280.3.G406. [DOI] [PubMed] [Google Scholar]
- 21.Kitamura A, Torii K, Uneyama H, Niijima A. Role played by afferent signals from olfactory, gustatory and gastrointestinal sensors in regulation of autonomic nerve activity. Biol. Pharm Bull. 2010;33:1778–1782. doi: 10.1248/bpb.33.1778. [DOI] [PubMed] [Google Scholar]
- 22.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 23.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 24.Kuperman Y, Chen A. Urocortins: emerging metabolic and energy homeostasis perspectives. Trends Endocrinol. Metab. 2008;19:122–129. doi: 10.1016/j.tem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Lawrence AJ, Krstew EV, Dautzenberg FM, Ruhmann A. The highly selective CRF(2) receptor antagonist K41498 binds to presynaptic CRF(2) receptors in rat brain. Br. J. Pharmacol. 2002;136:896–904. doi: 10.1038/sj.bjp.0704783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, Rivier J, Smith MS, Vale W. Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology. 2003;144:3216–3224. doi: 10.1210/en.2002-0087. [DOI] [PubMed] [Google Scholar]
- 27.Lloyd RB, Nemeroff CB. The role of corticotropin-releasing hormone in the pathophysiology of depression: therapeutic implications. Curr. Top. Med. Chem. 2011;11:609–617. doi: 10.2174/1568026611109060609. [DOI] [PubMed] [Google Scholar]
- 28.Martinez V, Wang L, Million M, Rivier J, Tache Y. Urocortins and the regulation of gastrointestinal motor function and visceral pain. Peptides. 2004;25:1733–1744. doi: 10.1016/j.peptides.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 29.Martinez V, Wang L, Rivier JE, Vale W, Tache Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J. Pharmacol. Exp. Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 30.Mercer JG, Lawrence CB, Copeland PA. Corticotropin-releasing factor binding sites undergo axonal transport in the rat vagus nerve. J Neuroendocrinol. 1992;4:281–286. doi: 10.1111/j.1365-2826.1992.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 31.Million M, Maillot C, Saunders P, Rivier J, Vale W, Tache Y. Human urocortin II, a new CRF-related peptide, displays selective CRF(2)-mediated action on gastric transit in rats. Am. J. Physiol Gastrointest. Liver Physiol. 2002;282:G34–G40. doi: 10.1152/ajpgi.00283.2001. [DOI] [PubMed] [Google Scholar]
- 32.Nishi Y, Yoh J, Hiejima H, Kojima M. Structures and molecular forms of the ghrelin-family peptides. Peptides. 2011;32:2175–2182. doi: 10.1016/j.peptides.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 33.Nozu T, Martinez V, Rivier J, Tache Y. Peripheral urocortin delays gastric emptying: role of CRF receptor 2. Am. J. Physiol. 1999;276:G867–G874. doi: 10.1152/ajpgi.1999.276.4.G867. [DOI] [PubMed] [Google Scholar]
- 34.Patel K, Rademaker M, Kirkpatrick C, Charles C, Fisher S, Yandle T, Richards A. Comparative pharmacokinetics and pharmacodynamics of urocortins 1, 2 and 3 in healthy sheep. Br. J Pharmacol. 2012;166:1916–1925. doi: 10.1111/j.1476-5381.2012.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porcher C, Peinnequin A, Pellissier S, Meregnani J, Sinniger V, Canini F, Bonaz B. Endogenous expression and in vitro study of CRF-related peptides and CRF receptors in the rat gastric antrum. Peptides. 2006;27:1464–1475. doi: 10.1016/j.peptides.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Raybould HE, Koelbel CB, Mayer EA, Tache Y. Inhibition of gastric motor function by circulating corticotropin-releasing factor in anesthetized rats. Neurogastroenterology & Motility. 1999;2:265–272. [Google Scholar]
- 37.Rivier J, Gulyas J, Kirby D, Low W, Perrin MH, Kunitake K, DiGruccio M, Vaughan J, Reubi JC, Waser B, Koerber SC, Martinez V, Wang L, Tache Y, Vale W. Potent and long-acting corticotropin releasing factor (CRF) receptor 2 selective peptide competitive antagonists. J Med. Chem. 2002;45:4737–4747. doi: 10.1021/jm0202122. [DOI] [PubMed] [Google Scholar]
- 38.Stengel A, Goebel M, Wang L, Reeve JR, Jr, Tache Y, Lambrecht NW. Lipopolysaccharide differentially decreases plasma acyl and desacyl ghrelin levels in rats: potential role of the circulating ghrelin-acylating enzyme GOAT. Peptides. 2010;31:1689–1696. doi: 10.1016/j.peptides.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stengel A, Goebel M, Wang L, Tache Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010;31:357–369. doi: 10.1016/j.peptides.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stengel A, Goebel M, Wang L, Tache Y, Sachs G, Lambrecht NW. Differential distribution of ghrelin-O-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem. Biophys. Res. Commun. 2010;392:67–71. doi: 10.1016/j.bbrc.2009.12.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stengel A, Goebel-Stengel M, Wang L, Shaikh A, Lambrecht NW, Rivier J, Tache Y. Abdominal surgery inhibits circulating acyl ghrelin and ghrelin-O-acyltransferase levels in rats: role of the somatostatin receptor subtype 2. Am. J Physiol Gastrointest. Liver Physiol. 2011;301:G239–G248. doi: 10.1152/ajpgi.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, Tache Y, Reeve JR., Jr The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology. 2009;150:5113–5118. doi: 10.1210/en.2009-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stengel A, Tache Y. Corticotropin-releasing factor signaling and visceral response to stress. Exp. Biol. Med. (Maywood. ) 2010;235:1168–1178. doi: 10.1258/ebm.2010.009347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stengel A, Tache Y. Ghrelin - a pleiotropic hormone secreted from endocrine x/a-like cells of the stomach. Front Neurosci. 2012;6:24. doi: 10.3389/fnins.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stengel A, Wang L, Tache Y. Stress-related alterations of acyl and desacyl ghrelin circulating levels: mechanisms and functional implications. Peptides. 2011;32:2208–2217. doi: 10.1016/j.peptides.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tschop M, Wawarta R, Riepl RL, Friedrich S, Bidlingmaier M, Landgraf R, Folwaczny C. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Invest. 2001;24:RC19–RC21. doi: 10.1007/BF03351037. [DOI] [PubMed] [Google Scholar]
- 47.Tu H, Kastin AJ, Pan W. Corticotropin-releasing hormone receptor (CRHR)1 and CRHR2 are both trafficking and signaling receptors for urocortin. Mol. Endocrinol. 2007;21:700–711. doi: 10.1210/me.2005-0503. [DOI] [PubMed] [Google Scholar]
- 48.Turnbull AV, Rivier C. Corticotropin-releasing factor (CRF) and endocrine responses to stress: CRF receptors, binding protein, and related peptides. Proc. Soc. Exp. Biol. Med. 1997;215:1–10. doi: 10.3181/00379727-215-44108. [DOI] [PubMed] [Google Scholar]
- 49.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, Basa NR, Shaikh A, Luckey A, Heber D, St Pierre DH, Tache Y. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am. J. Physiol Gastrointest. Liver Physiol. 2006;291:G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Martinez V, Vale W, Tache Y. Fos induction in selective hypothalamic neuroendocrine and medullary nuclei by intravenous injection of urocortin and corticotropin-releasing factor in rats. Brain Res. 2000;855:47–57. doi: 10.1016/s0006-8993(99)02200-3. [DOI] [PubMed] [Google Scholar]
- 52.Wang L, Stengel A, Goebel M, Martinez V, Gourcerol G, Rivier J, Tache Y. Peripheral activation of corticotropin-releasing factor receptor 2 inhibits food intake and alters meal structures in mice. Peptides. 2011;32:51–59. doi: 10.1016/j.peptides.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yakabi K, Noguchi M, Ohno S, Ro S, Onouchi T, Ochiai M, Takabayashi H, Takayama K, Harada Y, Sadakane C, Hattori T. Urocortin 1 reduces food intake and ghrelin secretion via CRF(2) receptors. Am. J Physiol Endocrinol. Metab. 2011;301:E72–E82. doi: 10.1152/ajpendo.00695.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 55.Yuan PQ, Wu SV, Tache Y. Urocortins and CRF type 2 receptor isoforms expression in the rat stomach are regulated by endotoxin: role in the modulation of delayed gastric emptying. Am. J Physiol Gastrointest. Liver Physiol. 2012;303:G20–G31. doi: 10.1152/ajpgi.00547.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]