Abstract
Calcium (Ca2+) is an essential signal transduction element involved in the regulation of several cellular activities and it is required at various key stages of the cell cycle. Intracellular Ca2+ is crucial for the orderly cell cycle progression and plays a vital role in the regulation of cell proliferation. Recently, it was demonstrated by in vitro and in vivo studies that nucleoplasmic Ca2+ regulates cell growth. Even though the mechanism by which nuclear Ca2+ regulates cell proliferation is not completely understood, there are reports demonstrating that activation of tyrosine kinase receptors (RTKs) leads to translocation of RTKs to the nucleus to generate localized nuclear Ca2+ signaling which are believed to modulate cell proliferation. Moreover, nuclear Ca2+ regulates the expression of genes involved in cell growth. This review will describe the nuclear Ca2+ signaling machinery and its role in cell proliferation. Additionally, the potential role of nuclear Ca2+ as a target in cancer therapy will be discussed.
Keywords: Nucleoplasmic reticulum, Nuclear calcium channels, Nuclear calcium, Cell proliferation
Introduction
Intracellular calcium (Ca2+) participates as a second messenger in several signaling pathways coordinating key events in a variety of cellular functions [1]. Ca2+ Signals are generally initiated by the binding of a hormone or growth factor to a transmembrane receptor, most commonly G protein coupled receptor (GPR) or tyrosine kinase receptor (RTK). The activation of such receptors recruits several second messengers, including phospholipase C (PLC) that, once activated, cleaves phosphatidylinositol 4,5-biphosphate (PIP2) producing diacylglycerol and inositol-1,4,5-trisphosphate (InsP3). InsP3 then binds to the InsP3 receptor (InsP3R), activating its channel to release Ca2+ from the endoplasmic reticulum. Once in the cytosol, Ca2+ can participate in several intracellular cascades and activate another class of Ca2+ channels, the ryanodine receptor (RyR), triggering a process denoted Ca2+-induced Ca2+ release [1]. The type II and III RyR are also sensitive to cyclic ADP-ribose (cADPR) [2,3], a process first demonstrated in sea urchin eggs [4], but now known to mobilize Ca2+ in a wide range of mammalian cell types [3,5-8]. Members of a third family of intracellular Ca2+ channels, the two pore channels (TPCs), are activated by nicotinic acid adenine dinucleotide phosphate (NAAD) which promotes release of Ca2+ from acidic organelles [9,10]. Like cADPR, NAADP was discovered in sea urchin eggs [11] and has now been found to induce Ca2+ signaling in mammalian cells as well [9,10]. Interactions among these different intracellular Ca2+ channels coordinate cellular responses mediated by Ca2+, both in health and disease. However little is known regarding the interaction of intracellular Ca2+ channels in the regulation of nuclear Ca2+ signaling.
One way by which intracellular Ca2+ regulates multiple cell functions is through spatial segregation of Ca2+ signaling. Indeed, subcellular increases in Ca2+ modulate not only physiological but also pathological events. For example, the physiological secretion of zymogen granules in pancreatic acinar cells is triggered by a localized sub-apical Ca2+ increase that does not spread throughout the entire cell [12]. In the other hand, the hypertrophic response in cardiomyocytes depends mostly on nuclear Ca2+ signals [13]. Additional examples of cellular processes modulated by subcellular Ca2+ signaling include the extension of growth cones in neuronal cells [14,15] and the establishment of specific gene transcription signatures [16], regulating development and differentiation [17-20], among others [21].
Moreover, the presence of another regulatory nuclear Ca2+ domain, denoted the nucleoplasmic reticulum [22,23] was reported in a wide variety of cells, from plants to animals (reviewed in [24]). The nucleoplasmic reticulum, of which 2 classes have been described, is a reticular membrane network of Ca2+ stores that is continuous with the endoplasmic reticulum and the nuclear envelope. The type I contains invaginations of the inner membrane of the nuclear envelope, and the type II contains both the inner and outer nuclear envelope membrane. These two classes of the nucleoplasmic reticulum can coexist within the same nucleus (reviewed in [24]), and their structure undergoes dynamic remodeling [25]. With the capacity to regulate Ca2+ signals in subnuclear regions, the presence of such machinery might provide a potential mechanism by which nucleoplasmic Ca2+ could simultaneously regulate many independent processes in the nucleus.
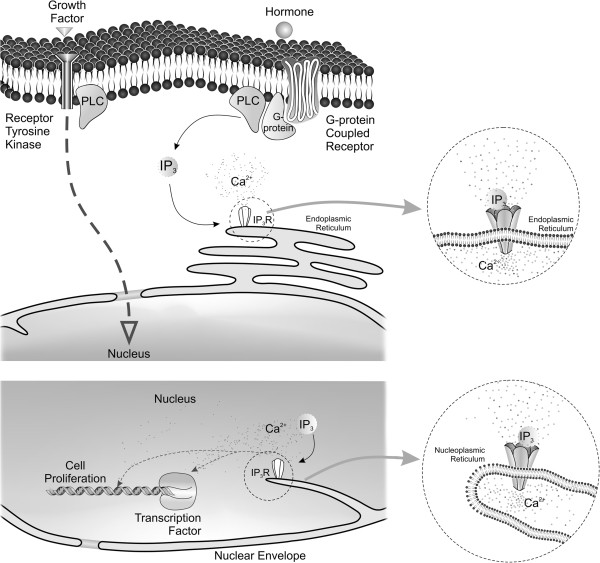
Although it is well known that nuclear Ca2+ has biological effects that differ from those mediated by increases in cytosolic Ca2+[21], the mechanisms by which Ca2+ is specifically increased in the nucleoplasm are a topic of debate. It was initially proposed that nuclear Ca2+ signaling would occur by passive diffusion of cytosolic Ca2+ across the nuclear envelope into the nucleoplasm. However, as it will be discussed in more detail, the nuclear interior has all the machinery required to produce localized Ca2+ signals, supporting the concept of the nuclear compartment as an independent apparatus to trigger Ca2+ signals. Moreover, the mechanisms and pathways by which localized Ca2+ signals in the nucleus regulate cell growth have only recently been investigated. The emerging model (Figure 1) shows that upon growth factor stimulation, RTKs translocate to the nucleus to induce hydrolysis of nuclear PIP2, generating InsP3 in the nucleoplasm, which leads to nuclear Ca2+ signals that can control cell growth [26-28] (Figure 1). This review highlights recent advances on nuclear Ca2+ signaling and its role in cell proliferation.
Figure 1.
Schematic representation of events involved in nuclear Ca2+ release and cellular proliferation. Ca2+ signals can be activated in the cells by the binding of a hormone or growth factor to a transmembrane receptor, most commonly a G protein coupled receptor or tyrosine kinase receptor. Through complex signaling cascades and networks, these effectors lead to the activation of several second messengers. One of the signaling pathways activated during cell proliferation is phospholipase C (PLC) that, once activated, cleaves phosphatidylinositol 4,5-biphosphate (PIP2) producing diacylglycerol and inositol-1,4,5-trisphosphate (InsP3). InsP3 then binds to the InsP3 receptor (InsP3R), activating its channel to release Ca2+. InsP3Rs can be found in the endoplasmic reticulum, in the nuclear envelope and in the nucleus along the nucleoplasmic reticulum. InsP3R-induced Ca2+ release specifically in the nucleus has been involved in the regulation of gene expression during different pathophysiological conditions, as well as during cellular proliferation. The cartoon shows the type I nucleoplasmic reticulum structure, with an invagination of the inner nuclear membrane alone. A Type II nucleoplasmic reticulum structure, with a double membrane walled invagination has also been reported in many cell types, although it is not represented here. In the nucleus, these cellular invaginations can provide focal release of Ca2+ that can bind directly to DNA structure or can modulate transcription factors involved in cell proliferation.
Ca2+ signaling in the nucleus
The nucleus is separated from the cytosol by the nuclear envelope, which is a specialized region of the endoplasmic reticulum, comprised of phospholipid bilayers [24]. However, the nuclear envelope contains pores that are permeable to molecules up to 60 kDa in size [29]. In the absence of a gating mechanism, a pore of this size would allow rapid equilibration of Ca2+ between the nucleus and cytosol. Indeed, under certain circumstances, free diffusion of Ca2+ through the nuclear pore occurs [30]. For example, stimulation of basophilic leukemia cells with antigen or photoreleased InsP3 triggered Ca2+ waves that spread from the cytosol into the nucleus [31]. Similar observations have been made in hepatocytes stimulated with vasopressin [32]. In contrast, several reports have demonstrate the existence of a nuclear-cytosolic Ca2+ gradient in a number of cell types [33,34], indicating that the permeability of nuclear pores to this ion can be regulated. However, the detection of such gradient can be incorrectly inferred depending on the technique used to measure intracellular Ca2+ in different compartments. Some of the commonly used organic Ca2+ indicators can display uneven distribution in the interior of the cells and can preferentially accumulate in membrane compartments such as the ER and the nucleoplasm. More importantly, the affinity of fluorescent probes for Ca2+ can vary depending on the cellular environment (reviewed [35]). Although each method for analyzing Ca2+ has certain drawbacks it is now appreciated that Ca2+ signaling is regulated at the subcellular level, and that this level of regulation is necessary for Ca2+ to act as a second messenger that regulates multiple cell functions simultaneously.
The nuclear envelope itself is a Ca2+ rich compartment, accumulating Ca2+ via a Ca2+-ATPase pump (SERCA) and a Na+/Ca2+-exchanger [36-38] and releasing it via channels that are sensitive to InsP3[37,39], cADPR [39,40], and NAADP [41]. The Ca2+-ATPase pump was shown to be present only in the outer membrane of the nuclear envelop, while the Na+/Ca2+-exchanger, is expressed in the inner membrane [38,42]. Regarding the intracellular Ca2+ channels, the RyRs appears to be present on both leaflets of the nuclear envelope [43]. Similarly, there are reports of InsP3Rs in the inner and outer membrane [28,44,45]. In addition, it was shown that ADP-ribosyl (CD38), an enzyme required for generation of cADPR, is located on the inner membrane of the nuclear envelope [40]. In Aplysia neurons, depolarization is the signal that triggers the translocation of CD38 to the nucleus [46]. Moreover, the nuclear envelope possess the tool kit necessary to produce InsP3, including PIP2, and PLC [47], and this machinery may be activated selectively through tyrosine kinase pathway [48].
However, the nuclear envelope is not the only nuclear site containing the Ca2+ signaling machinery. The nucleoplasmic reticulum represents another specialized cellular compartment involved in regulation in time and space of specific intracellular Ca2+ signaling events. For instance, both the InsP3R and the RyR are found in the nucleoplasmic reticulum [22,23]. Importantly, the InsP3-Kinase (IP3KB), the isoform that inactivates the InsP3 by phosphorylating it, was also reported to be located in the nucleoplasmic reticulum, where it may function to terminate the InsP3 mediated Ca2+ signal [49]. In addition, SERCA was also shown to be expressed along invaginations of the nucleoplasmic reticulum [50]. Therefore, there are several reports describing an active Ca2+ signaling regulatory domain deep in the nucleus, along the nucleoplasmic reticulum, providing further spatial control of Ca2+ within this cellular compartment [22,51,52].
Corroborating these findings, there is a growing body of data demonstrating that the nucleus has the capacity to independently generate Ca2+ signals. Several in vitro studies have shown that InsP3 releases Ca2+ directly from the nuclear envelop into the nucleus [33,39,44,53,54]. Accordingly, it has been demonstrated in a liver cell line that extracellular ATP can activate nuclear Ca2+ release, via an InsP3-dependent mechanism [55]. In cardiomyocytes, endothelin-1 has also been shown to elicit a local nuclear envelope Ca2+ release via InsP3R that activates nuclear CaMKII triggering HDAC5 phosphorylation and its nuclear export [56]. This signaling pathway has been implicated in the regulation of gene transcription in adult ventricular myocytes in response to neurohumoral signals during hypertrophy. Similar to InsP3, cADPR can also increase Ca2+ in isolated cell nuclei [33,39,40].
One of the proposed mechanism by which InsP3 generates nuclear Ca2+ signaling is via translocation of activated RTKs from the plasma membrane to the nuclear interior. For instance, it was shown that IGF-1 and integrins caused PIP2 breakdown in the nucleus but not at the plasma membrane [48]. Similarly, activation of the hepatocyte growth factor (HGF) receptor c-Met in a liver cell line and insulin receptor in primary hepatocytes caused PIP2 breakdown in the nucleus resulting in InsP3 formation that was followed by nuclear Ca2+ signals [26,27] (Figure 1). The triggering of this highly localized cascade was dependent on the rapid translocation of the activated HGF receptor to the nucleus, through a mechanism that depends on the adaptor protein Gab-1 and importin-B [26]. Moreover, it also has been hypothesized that relocation of MAP kinase to the nucleus activates nuclear phospholipase C to generate InsP3 there [43].
Once in the nucleus, Ca2+ signals directly regulate signaling pathways distinct from those mediated by cytosolic Ca2+, for instance they stimulate the intranuclear activity of PKC [22] and CaMK-IV [57]. Nuclear Ca2+ also plays a significant role in regulating the transcription factor CRE-binding protein and its coactivator, CREB-binding protein (CBP) [58]. Transcriptional activation of Elk-1 by EGF was also shown to depend on nuclear rather than cytosolic Ca2+[59]. On the other hand, nuclear Ca2+ can negatively regulate the activity of transcription factors, such as TEAD [60]. Moreover, nuclear Ca2+ has also been implicated in modulating cardiac hypertrophy [13,51] and within the nucleus Ca2+ was shown to bind to and directly regulate DNA structure [61]. Another evidence of the role of nuclear Ca2+ signaling pathway came from studies showing that in skeletal muscle cell, two-photon photorelease of caged Ca2+ near the nucleoplasmic reticulum was found to elicit a Ca2+-induced Ca2+ release event within the nucleus [23]. More recently, it was demonstrated that nuclear rather than cytosolic Ca2+ signals specifically control the progression through early prophase, showing that nucleoplasmic Ca2+ regulates cell proliferation [62].
Nuclear Ca2+ and cell proliferation
It has been long recognized that Ca2+ signals have an important role throughout the mammalian cell cycle and are especially important in early G1 and G1/S and G2/M transitions [63], with the first major Ca2+ transient occurring just prior to entry into mitosis, and the second one occurring during the metaphase-anaphase transition [63,64]. Indeed, Ca2+ is the most prominent messenger required through these cycle points [65,66] and downstream targets of Ca2+ have also been implicated in cell cycle progression as well [67,68].
Heterologous expression of the Ca2+ binding protein parvalbumin has been used to study the role of Ca2+ signals in the regulation of the cell cycle. This protein is normally expressed in skeletal muscle and neurons [69], and is known to buffer Ca2+[70]. The first report using parvalbumin as a molecular tool to buffer intracellular Ca2+ and study cellular growth, showed that reducing Ca2+ slowed progression through the cell cycle [68]. However, it is now known that the effects of Ca2+ on proliferation correlate with the subcellular compartment where Ca2+ is released. Using parvalbumin variants, selectively targeted to distinct intracellular Ca2+ rich compartments, it was found that buffering mitochondrial Ca2+ inhibits apoptosis and accelerates hepatocyte proliferation [71]. In contrast, buffering cytosolic Ca2+ was shown to retard liver regeneration and progression through the cell cycle after partial hepatectomy [72]. Since cytosolic Ca2+ can increase through a number of mechanisms, it is believed that, in this compartment, Ca2+ may have different effects on cell growth [71,73,74]. On the other hand, it was shown that nucleoplasmic rather than cytosolic Ca2+ is essential for liver cell line proliferation, and is necessary in particular for progression through early prophase [62]. It was also found that liver tumors implanted in nude mice grew much more slowly when expressing parvalbumin in their nuclei compared to the cytosol [62]. Moreover, HGF and insulin, two potent growth factors in liver, that induce cell proliferation during liver regeneration, were shown to selectively form InsP3 in the nucleus to initiate nuclear Ca2+ signals [22,27] (Figure 1). Since the nucleoplasmic reticulum is known to be abundant in many tumor cell types [24], one would expect that the existence of these nuclear invaginations could provide further specificity to cell proliferation by allowing the focal delivery of Ca2+ to particular sites within the nucleus.
Although, the proteins that link nuclear Ca2+ signals to cell proliferation have not been clearly identified, more recent findings in liver tumor cells indicated the endopeptidase legumain (LGMN) as a novel target of nuclear Ca2+[75]. Using Rapid Subtraction Hybridization (RaSH) to subtract genes in liver cells expressing the Ca2+ buffer protein parvalbumin targeted to the nucleus, from genes in cells expressing a mutated form of nuclear-targeted parvalbumin which has one of the two Ca2+-binding sites inactivated. The authors identified thirteen genes whose expression was affected by a small alteration in nuclear Ca2+ concentration. LGMN was one of such genes and upon further validation was demonstrated to be regulated by nuclear Ca2+ signals at the transcriptional level. LGMN was first recognized in plants [76] and later in humans and mice [77]. It is present in the tumor microenvironment where it is expressed by macrophages and contributes to metastatic behavior by promoting cell migration and tissue invasion. It is known that increased expression of LGMN is associated with poor tumor differentiation [78]. For instance, it was demonstrated that LGMN co-localizes with integrins at the invading front of tumors and expression of this enzyme was shown to be associated with increased invasiveness [78,79]. So, it was shown that when Ca2+ was buffered in the nucleus of the cells, LGMN expression decreased, impairing cell proliferation [75]. Additionally, this work also provided evidence that nuclear Ca2+ signals regulate cell proliferation at least in part through the modulation of gene expression (Figure 1). Other targets for nuclear Ca2+ that are involved in cell proliferation still remain to be described.
Altered nuclear morphology is a common feature of many cancers [24] and it has been proposed that information regarding the nucleoplasmic reticulum invaginations could be used in combination with other nuclear anomalies as markers of malignancy [80]. More recently, it was also proposed that nuclear Ca2+ buffering could be used in conjunction with radiotherapy as a therapeutic potential for the treatment of carcinoma. Ionizing radiation concomitant with nuclear Ca2+ buffering showed superior outcome, compared to irradiation alone [81]. Corroborating previous findings, the beneficial effect of nuclear Ca2+ buffering in the proposed antitumor therapy was shown to be due to changes caused in expression level of genes involved in the regulation of cell proliferation [59]. Moreover, it was also shown that buffering nuclear Ca2+ reduced the rate of tumor cell proliferation, without affecting cells from normal tissue [81], suggesting higher selectivity of nuclear Ca2+ towards controlling cancer cell growth. Further studies are required to determine the mechanistic basis for the differential sensitivity of normal versus cancer cell proliferation to changes in nuclear Ca2+. Nonetheless, these findings suggest that buffering nuclear Ca2+ could be one strategy employed to inhibit the growth of tumors without affecting normal tissue, either alone or in association therapy.
Conclusions
Ca2+ is important to several signaling pathways among virtually every cell type. The central mechanism by which Ca2+ regulates protein functions depends on how and where it is released into the cell. The role of nuclear Ca2+ in cell proliferation was demonstrated in vitro by showing that nuclear Ca2+ buffering reduced proliferation rate through blocking cell cycle in G2/M phase. It was also demonstrated that nuclear Ca2+ plays a role on tumor growth in vivo and it can alter the expression of genes involved in cell proliferation. Moreover, modulation of nuclear Ca2+ signaling was shown to be a potential target to treat cancer. However further studies are needed to better understand how nuclear Ca2+ can be generated and how it regulates cell proliferation and cell cycle progression. These findings would have strong potential as therapeutic targets in degenerative diseases or cancer.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors contributed in the conception and writing of the manuscript. All authors edited and approved the final version.
Contributor Information
Rodrigo R Resende, Email: rrresende@hotmail.com.
Lidia M Andrade, Email: lidia.pesquisa@gmail.com.
Andre G Oliveira, Email: agoufmg@gmail.com.
Erika S Guimarães, Email: erikasousaguimaraes@gmail.com.
Silvia Guatimosim, Email: silvia.guatimosim@gmail.com.
M Fatima Leite, Email: leitemd@dedalus.lcc.ufmg.br.
Acknowledgements
We thank Howard Hughes Medical Institute (HHMI), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), PRONEX and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
References
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- Sonnleitner A, Conti A, Bertocchini F, Schindler H, Sorrentino V. Functional properties of the ryanodine receptor type 3 (RyR3) Ca2+ release channel. EMBO J. 1998;17:2790–2798. doi: 10.1093/emboj/17.10.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meszaros LG, Bak J, Chu A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature. 1993;364:76–79. doi: 10.1038/364076a0. [DOI] [PubMed] [Google Scholar]
- Galione A, Lee HC, Busa WB. Ca(2+)-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- Cancela JM, Petersen OH. The cyclic ADP ribose antagonist 8-NH2-cADP-ribose blocks cholecystokinin-evoked cytosolic Ca2+ spiking in pancreatic acinar cells. Pflugers Arch. 1998;435:746–748. doi: 10.1007/s004240050578. [DOI] [PubMed] [Google Scholar]
- Kuemmerle JF, Makhlouf GM. Agonist-stimulated cyclic ADP ribose. Endogenous modulator of Ca(2+)-induced Ca2+ release in intestinal longitudinal muscle. J Biol Chem. 1995;270:25488–25494. doi: 10.1074/jbc.270.43.25488. [DOI] [PubMed] [Google Scholar]
- Clementi E, Riccio M, Sciorati C, Nistico G, Meldolesi J. The type 2 ryanodine receptor of neurosecretory PC12 cells is activated by cyclic ADP-ribose. Role of the nitric oxide/cGMP pathway. J Biol Chem. 1996;271:17739–17745. doi: 10.1074/jbc.271.30.17739. [DOI] [PubMed] [Google Scholar]
- Guse AH, da Silva CP, Berg I, Skapenko AL, Weber K, Heyer P. Regulation of calcium signalling in T lymphocytes by the second messenger cyclic ADP-ribose. Nature. 1999;398:70–73. doi: 10.1038/18024. [DOI] [PubMed] [Google Scholar]
- Macgregor A, Yamasaki M, Rakovic S, Sanders L, Parkesh R, Churchill GC. NAADP controls cross-talk between distinct Ca2+ stores in the heart. J Biol Chem. 2007;282:15302–15311. doi: 10.1074/jbc.M611167200. [DOI] [PubMed] [Google Scholar]
- Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2 + −signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]
- Ito K, Miyashita Y, Kasai H. Micromolar and submicromolar Ca2+ spikes regulating distinct cellular functions in pancreatic acinar cells. EMBO J. 1997;16:242–251. doi: 10.1093/emboj/16.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arantes LA, Aguiar CJ, Amaya MJ, Figueiro NC, Andrade LM, Rocha-Resende C. Nuclear inositol 1,4,5-trisphosphate is a necessary and conserved signal for the induction of both pathological and physiological cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2012;53:475–486. doi: 10.1016/j.yjmcc.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Spitzer NC. In vivo regulation of axon extension and pathfinding by growth-cone calcium transients. Nature. 1999;397:350–355. doi: 10.1038/16927. [DOI] [PubMed] [Google Scholar]
- Zheng JQ. Turning of nerve growth cones induced by localized increases in intracellular calcium ions. Nature. 2000;403:89–93. doi: 10.1038/47501. [DOI] [PubMed] [Google Scholar]
- Wiegert JS, Bading H. Activity-dependent calcium signaling and ERK-MAP kinases in neurons: a link to structural plasticity of the nucleus and gene transcription regulation. Cell Calcium. 2011;49:296–305. doi: 10.1016/j.ceca.2010.11.009. [DOI] [PubMed] [Google Scholar]
- Resende RR, Adhikari A, da Costa JL, Lorencon E, Ladeira MS, Guatimosim S. Influence of spontaneous calcium events on cell-cycle progression in embryonal carcinoma and adult stem cells. Biochim Biophys Acta. 1803;2010:246–260. doi: 10.1016/j.bbamcr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Resende RR, Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal. 2009;7:20. doi: 10.1186/1478-811X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende RR, da Costa JL, Kihara AH, Adhikari A, Lorencon E. Intracellular Ca2+ regulation during neuronal differentiation of murine embryonal carcinoma and mesenchymal stem cells. Stem Cells Dev. 2010;19:379–394. doi: 10.1089/scd.2008.0289. [DOI] [PubMed] [Google Scholar]
- Tonelli FM, Santos AK, Gomes DA, Da Silva SL, Gomes KN, Ladeira LO. Stem cells and calcium signaling. Adv Exp Med Biol. 2012;740:891–916. doi: 10.1007/978-94-007-2888-2_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MA, Gomes DA, Nathanson MH, Leite MF. Nuclear calcium signaling: a cell within a cell. Braz J Med Biol Res. 2009;42:17–20. doi: 10.1590/S0100-879X2008005000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat Cell Biol. 2003;5:440–446. doi: 10.1038/ncb980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marius P, Guerra MT, Nathanson MH, Ehrlich BE, Leite MF. Calcium release from ryanodine receptors in the nucleoplasmic reticulum. Cell Calcium. 2006;39:65–73. doi: 10.1016/j.ceca.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Malhas A, Goulbourne C, Vaux DJ. The nucleoplasmic reticulum: form and function. Trends Cell Biol. 2011;21:362–373. doi: 10.1016/j.tcb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Fricker M, Hollinshead M, White N, Vaux D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J Cell Biol. 1997;136:531–544. doi: 10.1083/jcb.136.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes DA, Rodrigues MA, Leite MF, Gomez MV, Varnai P, Balla T. c-Met must translocate to the nucleus to initiate calcium signals. J Biol Chem. 2008;283:4344–4351. doi: 10.1074/jbc.M706550200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MA, Gomes DA, Andrade VA, Leite MF, Nathanson MH. Insulin induces calcium signals in the nucleus of rat hepatocytes. Hepatology. 2008;48:1621–1631. doi: 10.1002/hep.22424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehno-Bittel L, Luckhoff A, Clapham DE. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Gerace L, Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Brini M, Murgia M, Pasti L, Picard D, Pozzan T, Rizzuto R. Nuclear Ca2+ concentration measured with specifically targeted recombinant aequorin. EMBO J. 1993;12:4813–4819. doi: 10.1002/j.1460-2075.1993.tb06170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allbritton NL, Oancea E, Kuhn MA, Meyer T. Source of nuclear calcium signals. Proc Natl Acad Sci U S A. 1994;91:12458–12462. doi: 10.1073/pnas.91.26.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Hajnoczky G, Thomas AP. Propagation of cytosolic calcium waves into the nuclei of hepatocytes. Cell Calcium. 1994;16:247–258. doi: 10.1016/0143-4160(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Santella L, Kyozuka K. Effects of 1-methyladenine on nuclear Ca2+ transients and meiosis resumption in starfish oocytes are mimicked by the nuclear injection of inositol 1,4,5-trisphosphate and cADP-ribose. Cell Calcium. 1997;22:11–20. doi: 10.1016/S0143-4160(97)90085-3. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- Perez-Terzic C, Jaconi M, Clapham DE. Nuclear calcium and the regulation of the nuclear pore complex. Bioessays. 1997;19:787–792. doi: 10.1002/bies.950190908. [DOI] [PubMed] [Google Scholar]
- Lanini L, Bachs O, Carafoli E. The calcium pump of the liver nuclear membrane is identical to that of endoplasmic reticulum. J Biol Chem. 1992;267:11548–11552. [PubMed] [Google Scholar]
- Nicotera P, McConkey DJ, Jones DP, Orrenius S. ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci U S A. 1989;86:453–457. doi: 10.1073/pnas.86.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wu G, Lu ZH, Ledeen RW. Potentiation of a sodium-calcium exchanger in the nuclear envelope by nuclear GM1 ganglioside. J Neurochem. 2002;81:1185–1195. doi: 10.1046/j.1471-4159.2002.00917.x. [DOI] [PubMed] [Google Scholar]
- Gerasimenko OV, Gerasimenko JV, Tepikin AV, Petersen OH. ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell. 1995;80:439–444. doi: 10.1016/0092-8674(95)90494-8. [DOI] [PubMed] [Google Scholar]
- Adebanjo OA, Anandatheerthavarada HK, Koval AP, Moonga BS, Biswas G, Sun L. A new function for CD38/ADP-ribosyl cyclase in nuclear Ca2+ homeostasis. Nat Cell Biol. 1999;1:409–414. doi: 10.1038/15640. [DOI] [PubMed] [Google Scholar]
- Gerasimenko JV, Maruyama Y, Yano K, Dolman NJ, Tepikin AV, Petersen OH. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol. 2003;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Xie X, Lu ZH, Ledeen RW. Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc Natl Acad Sci U S A. 2009;106:10829–10834. doi: 10.1073/pnas.0903408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella L, Carafoli E. Calcium signaling in the cell nucleus. FASEB J. 1997;11:1091–1109. [PubMed] [Google Scholar]
- Humbert JP, Matter N, Artault JC, Koppler P, Malviya AN. Inositol 1,4,5-trisphosphate receptor is located to the inner nuclear membrane vindicating regulation of nuclear calcium signaling by inositol 1,4,5-trisphosphate. Discrete distribution of inositol phosphate receptors to inner and outer nuclear membranes. J Biol Chem. 1996;271:478–485. doi: 10.1074/jbc.271.1.478. [DOI] [PubMed] [Google Scholar]
- Cardenas C, Escobar M, Garcia A, Osorio-Reich M, Hartel S, Foskett JK. Visualization of inositol 1,4,5-trisphosphate receptors on the nuclear envelope outer membrane by freeze-drying and rotary shadowing for electron microscopy. J Struct Biol. 2010;171:372–381. doi: 10.1016/j.jsb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezin S, Charpentier G, Lee HC, Baux G, Fossier P, Cancela JM. Regulation of nuclear Ca2+ signaling by translocation of the Ca2+ messenger synthesizing enzyme ADP-ribosyl cyclase during neuronal depolarization. J Biol Chem. 2008;283:27859–27870. doi: 10.1074/jbc.M804701200. [DOI] [PubMed] [Google Scholar]
- Divecha N, Rhee SG, Letcher AJ, Irvine RF. Phosphoinositide signalling enzymes in rat liver nuclei: phosphoinositidase C isoform beta 1 is specifically, but not predominantly, located in the nucleus. Biochem J. 1993;289(Pt 3):617–620. doi: 10.1042/bj2890617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- Gerasimenko O, Gerasimenko J. New aspects of nuclear calcium signalling. J Cell Sci. 2004;117:3087–3094. doi: 10.1242/jcs.01295. [DOI] [PubMed] [Google Scholar]
- Collado-Hilly M, Shirvani H, Jaillard D, Mauger JP. Differential redistribution of Ca2 + −handling proteins during polarisation of MDCK cells: effects on Ca2+ signalling. Cell Calcium. 2010;48:215–224. doi: 10.1016/j.ceca.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Guatimosim S, Amaya MJ, Guerra MT, Aguiar CJ, Goes AM, Gomez-Viquez NL. Nuclear Ca2+ regulates cardiomyocyte function. Cell Calcium. 2008;44:230–242. doi: 10.1016/j.ceca.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Soliman EM, Rodrigues MA, Gomes DA, Sheung N, Yu J, Amaya MJ. Intracellular calcium signals regulate growth of hepatic stellate cells via specific effects on cell cycle progression. Cell Calcium. 2009;45:284–292. doi: 10.1016/j.ceca.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennager DJ, Welsh MJ, DeLisle S. Changes in either cytosolic or nucleoplasmic inositol 1,4,5-trisphosphate levels can control nuclear Ca2+ concentration. J Biol Chem. 1995;270:4959–4962. doi: 10.1074/jbc.270.10.4959. [DOI] [PubMed] [Google Scholar]
- Malviya AN, Rogue P, Vincendon G. Stereospecific inositol 1,4,5-[32P]trisphosphate binding to isolated rat liver nuclei: evidence for inositol trisphosphate receptor-mediated calcium release from the nucleus. Proc Natl Acad Sci U S A. 1990;87:9270–9274. doi: 10.1073/pnas.87.23.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leite MF, Thrower EC, Echevarria W, Koulen P, Hirata K, Bennett AM. Nuclear and cytosolic calcium are regulated independently. Proc Natl Acad Sci U S A. 2003;100:2975–2980. doi: 10.1073/pnas.0536590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature. 1998;392:198–202. doi: 10.1038/32448. [DOI] [PubMed] [Google Scholar]
- Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- Pusl T, Wu JJ, Zimmerman TL, Zhang L, Ehrlich BE, Berchtold MW. Epidermal growth factor-mediated activation of the ETS domain transcription factor Elk-1 requires nuclear calcium. J Biol Chem. 2002;277:27517–27527. doi: 10.1074/jbc.M203002200. [DOI] [PubMed] [Google Scholar]
- Thompson M, Andrade VA, Andrade SJ, Pusl T, Ortega JM, Goes AM. Inhibition of the TEF/TEAD transcription factor activity by nuclear calcium and distinct kinase pathways. Biochem Biophys Res Commun. 2003;301:267–274. doi: 10.1016/S0006-291X(02)03024-3. [DOI] [PubMed] [Google Scholar]
- Dobi A, Agoston D. Submillimolar levels of calcium regulates DNA structure at the dinucleotide repeat (TG/AC)n. Proc Natl Acad Sci U S A. 1998;95:5981–5986. doi: 10.1073/pnas.95.11.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MA, Gomes DA, Leite MF, Grant W, Zhang L, Lam W. Nucleoplasmic calcium is required for cell proliferation. J Biol Chem. 2007;282:17061–17068. doi: 10.1074/jbc.M700490200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8:361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- Groigno L, Whitaker M. An anaphase calcium signal controls chromosome disjunction in early sea urchin embryos. Cell. 1998;92:193–204. doi: 10.1016/S0092-8674(00)80914-9. [DOI] [PubMed] [Google Scholar]
- Taylor JT, Zeng XB, Pottle JE, Lee K, Wang AR, Yi SG. Calcium signaling and T-type calcium channels in cancer cell cycling. World J Gastroenterol. 2008;14:4984–4991. doi: 10.3748/wjg.14.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB J. 1995;9:219–228. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]
- Patel R, Holt M, Philipova R, Moss S, Schulman H, Hidaka H. Calcium/calmodulin-dependent phosphorylation and activation of human Cdc25-C at the G2/M phase transition in HeLa cells. J Biol Chem. 1999;274:7958–7968. doi: 10.1074/jbc.274.12.7958. [DOI] [PubMed] [Google Scholar]
- Rasmussen CD, Means AR. Calmodulin is required for cell-cycle progression during G1 and mitosis. EMBO J. 1989;8:73–82. doi: 10.1002/j.1460-2075.1989.tb03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berchtold MW, Celio MR, Heizmann CW. Parvalbumin in non-muscle tissues of the rat. Quantitation and immunohistochemical localization. J Biol Chem. 1984;259:5189–5196. [PubMed] [Google Scholar]
- Andressen C, Gotzos V, Berchtold MW, Pauls TL, Schwaller B, Fellay B. Changes in shape and motility of cells transfected with parvalbumin cDNA. Exp Cell Res. 1995;219:420–426. doi: 10.1006/excr.1995.1248. [DOI] [PubMed] [Google Scholar]
- Guerra MT, Fonseca EA, Melo FM, Andrade VA, Aguiar CJ, Andrade LM. Mitochondrial calcium regulates rat liver regeneration through the modulation of apoptosis. Hepatology. 2011;54:296–306. doi: 10.1002/hep.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagoudakis L, Garcin I, Julien B, Nahum K, Gomes DA, Combettes L. Cytosolic calcium regulates liver regeneration in the rat. Hepatology. 2010;52:602–611. doi: 10.1002/hep.23673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicou A, Serriere V, Prigent S, Boucherie S, Combettes L, Guillon G. Hypothalamic vasopressin release and hepatocyte Ca2+ signaling during liver regeneration: an interplay stimulating liver growth and bile flow. FASEB J. 2003;17:1901–1903. doi: 10.1096/fj.03-0082fje. [DOI] [PubMed] [Google Scholar]
- Kahl CR, Means AR. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr Rev. 2003;24:719–736. doi: 10.1210/er.2003-0008. [DOI] [PubMed] [Google Scholar]
- Andrade V, Guerra M, Jardim C, Melo F, Silva W, Ortega JM. Nucleoplasmic calcium regulates cell proliferation through legumain. J Hepatol. 2011;55:626–635. doi: 10.1016/j.jhep.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kembhavi AA, Buttle DJ, Knight CG, Barrett AJ. The two cysteine endopeptidases of legume seeds: purification and characterization by use of specific fluorometric assays. Arch Biochem Biophys. 1993;303:208–213. doi: 10.1006/abbi.1993.1274. [DOI] [PubMed] [Google Scholar]
- Chen JM, Dando PM, Rawlings ND, Brown MA, Young NE, Stevens RA. Cloning, isolation, and characterization of mammalian legumain, an asparaginyl endopeptidase. J Biol Chem. 1997;272:8090–8098. doi: 10.1074/jbc.272.12.8090. [DOI] [PubMed] [Google Scholar]
- Gawenda J, Traub F, Luck HJ, Kreipe H, von Wasielewski R. Legumain expression as a prognostic factor in breast cancer patients. Breast Cancer Res Treat. 2007;102:1–6. doi: 10.1007/s10549-006-9311-z. [DOI] [PubMed] [Google Scholar]
- Loak K, Li DN, Manoury B, Billson J, Morton F, Hewitt E. Novel cell-permeable acyloxymethylketone inhibitors of asparaginyl endopeptidase. Biol Chem. 2003;384:1239–1246. doi: 10.1515/BC.2003.136. [DOI] [PubMed] [Google Scholar]
- Batistatou A, Scopa CD. Pathogenesis and diagnostic significance of nuclear grooves in thyroid and other sites. Int J Surg Pathol. 2009;17:107–110. doi: 10.1177/1066896908316071. [DOI] [PubMed] [Google Scholar]
- Andrade LM, Geraldo JM, Gonçalves OX, Leite MTT, Catarina AM, Guimarães MM. Nucleoplasmic calcium buffering sensitizes human squamous cell carcinoma to anticancer therapy. J Cancer Sci Ther. 2012;4:131–139. [Google Scholar]