Abstract
Whether premating isolation is achieved by male-specific, female-specific or sex-independent assortative preferences often depends on the underlying evolutionary processes. Here we test mate preferences of males presented with females of different allopatric colour variants of the cichlid fish Tropheus sp., a Lake Tanganyika endemic with rich geographical colour pattern variation, in which the strength of sexual isolation varies between populations. We conducted two-way mate choice experiments to compare behaviour of males of a red-bodied morph (population Moliro) towards females from their own population with behaviour towards females from four allopatric populations at different stages of phylogenetic and phenotypic divergence. Males courted same-population females significantly more intensely than females of other populations, and reduced their heteromorphic courtship efforts both with increasing genetic and increasing phenotypic distinctness of the females. In particular, females of a closely related red-bodied population received significantly more courtship than either genetically distinct, similarly coloured females (‘Kirschfleck’ morph) or genetically related, differently coloured females (‘yellow-blotch’ morph), both of which were courted similarly. Genetically and phenotypically distinct females (Tropheus polli) were not courted at all. Consistent with previous female-choice experiments, female courtship activity also decreased with increasing genetic distance from the males’ population. Given successful experimental and natural introgression between colour morphs and the pervasive allopatry of related variants, we consider it unlikely that assortative preferences of both sexes were driven by direct selection during periods of secondary contact or, in turn, drove colour pattern differentiation in allopatry. Rather, we suggest that sexual isolation evolved as by-product of allopatric divergence.
Keywords: allopatric divergence, Cichlidae, Lake Tanganyika, male preference, mate choice, reproductive isolation, Tropheus
Introduction
Assortative and conspecific mate preferences play an important role in maintaining reproductive isolation between species or among morphs within a species (Ritchie, 2007). It has been argued that the pressure to avoid hybridization should be stronger on the sex paying the higher costs for mating mistakes, which is frequently assumed to be the female (Parker & Partridge, 1998; Wirtz, 1999; but see Servedio, 2007). Several empirical examples of female biases in the strengths of assortative preferences support this hypothesis (Saetre et al., 1997; Russell et al., 2006; Hochkirch et al., 2007; Kozak & Boughman, 2009; Verzijden et al., 2009; O'Rourke & Mendelson, 2010). Nonetheless, a fairly large proportion of studies comparing male and female mate preferences between sympatric species and in hybrid zones found that male mate choice contributes equally or even more than female choice to sexual isolation (Knight & Turner, 1999; Seehausen et al., 1999; Jiggins et al., 2001; Shine et al., 2002, 2004; Peterson et al., 2005; Svensson et al., 2007; Pryke & Griffith, 2007; Pierotti et al., 2008; Espinedo et al., 2010), either because males incur high costs by courtship and by mating mistakes (Albert & Schluter, 2004; Peterson et al., 2005; Svensson et al., 2007) or because of processes other than reinforcement and direct selection on mating preferences (Jiggins et al., 2004; Nosil et al., 2007; Pierotti et al., 2008; Espinedo et al., 2010; Mendelson & Shaw, 2012). For example, sexual isolation can arise as a by-product of allopatric divergence (Coyne & Orr, 2004), in which case a sex bias in the strength of assortative preferences is not necessarily expected. Accordingly, assortative preferences were strong in both males and females of a Lake Malawi cichlid fish species when given the choice between individuals from their own and from an allopatric population (Pauers et al., 2010).
The endemic Lake Tanganyika genus Tropheus comprises another cichlid species complex with rich allopatric colour pattern variation (Konings, 1998; Schupke, 2003). Most Tropheus populations are sexually monomorphic or display only subtle sexual differences in size and colour pattern, and males and females each defend their individual territories. Both sexes employ colour signals to communicate in social and sexual interactions (Nelissen, 1976). For mating, females give up their territories and move into a chosen male's territory for several days to weeks until spawning, upon which they leave the male and mouthbrood their offspring alone for about 4 weeks (Yanagisawa & Nishida, 1991). Genetic analyses indicated that broods are fertilized by a single male each, that is presumably by the females’ temporary social mates (Egger et al., 2006). Dispersal of the stenotopic rock-dwellers is restricted by habitat barriers, and significant genetic differentiation as well as slight colour pattern differentiation exists among many adjacent populations, whereas large and longstanding barriers such as river estuaries often separate highly distinct colour variants (Egger et al., 2007; Koblmüller et al., 2011). The classification of the geographically separated, phenotypically diverse but closely related colour variants into the nominal species is not completely resolved, and populations are typically specified by cheironyms indicating their geographical origin or colour pattern. The most recent common ancestor of the species complex (the genus Tropheus excepting the clearly divergent Tropheus duboisi) dates back to approximately 1.5 million years ago (Ma; Koblmüller et al., 2010). Divergence times between individual populations range between this onset of diversification and the population fragmentation associated with the latest major lake level rise between 10 000 and 20 000 years ago (Egger et al., 2007; Koblmüller et al., 2011), providing opportunities to examine reproductive isolation at different levels of phenotypic and genetic distance.
Studies of mate choice in laboratory experiments and in an artificially admixed field population suggested that reproductive isolation among allopatric morphs depends (with exceptions) on the extent of phenotypic and genetic differentiation (Salzburger et al., 2006; Egger et al., 2008, 2010, 2012). Where present, assortative preferences may either have evolved in allopatry or may have been promoted during periods of secondary contact with other morphs precipitated by lake level fluctuations (Sturmbauer et al., 2005; Egger et al., 2007). With uniparental maternal brood care and a mating system leading to a male-biased operational sex ratio (Sefc, 2008), avoidance of potentially disadvantageous inter-morph matings would be expected to evolve primarily in females. However, inter-morph fertility in experimental crosses (K.M. Sefc, personal observation) as well as natural introgression between morphs and the putative hybrid origin of some colour morphs (Egger et al., 2007, 2012) cast doubt on whether heteromorphic matings necessarily incur severe costs on either of the sexes.
In the previous mate choice experiments, females were offered a two-way choice between a homomorphic and a heteromorphic male, and interactions of females with the males were scored in categories representing aggression and mate preference. Although designed as female choice experiments, both male and female preferences could have contributed to the observed patterns, for instance because the intensity of male courtship apparently influences female preferences in within-population interactions (Steinwender et al., 2012). In this study, we quantified behaviour in homomorphic and heteromorphic intersexual interactions to examine whether males discriminate against heteromorphic females and modify their behaviour according to their phenotypic and phylogenetic distance from the heteromorphic stimulus females. A two-way male choice design was selected to assess male mate preferences in situations when both homomorphic and heteromorphic choices are available. Behaviours of females, for which the experiment presented a no-choice situation, were scored to assess congruency with male behaviour.
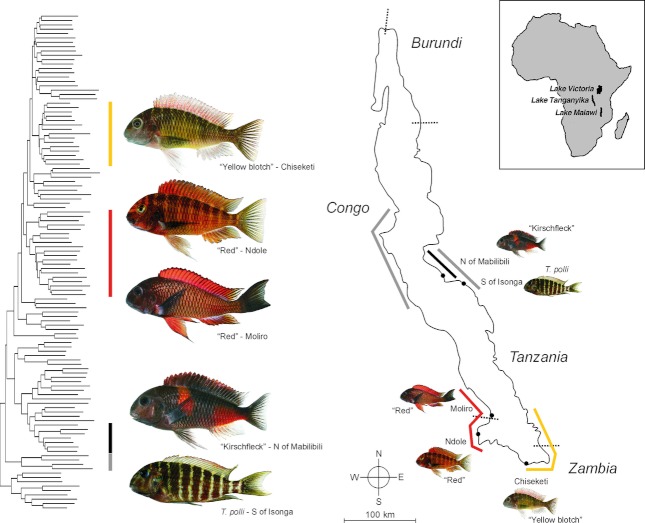
The tested males belong to a red-bodied morph occurring in southern Lake Tanganyika (population Moliro, Fig. 1), proposed alternatively to represent its own species Tropheus sp. ‘red’ (Konings, 1998) or belong to Tropheus moorii (Egger et al., 2007). Females of the red-bodied morph displayed strictly assortative mating preferences vis-à-vis several distinct morphs in previous studies (Egger et al., 2008; Hermann, 2011; H. Brindl & K. M. Sefc unpublished), but did not discriminate against a closely related and similarly coloured population (Egger et al., 2010). For this study, the heteromorphic stimulus females were chosen from populations with different degrees of genetic and colour pattern differentiation from the Moliro population, namely a genetically closely related and similarly coloured population of the same red-bodied lineage (Ndole), a related population with a distinct yellow-blotch colouration (population Chiseketi, T. moorii), a genetically distinct population with a red ‘Kirschfleck’ (cherry-blotch) colour pattern (assigned to Tropheus sp. ‘black’ by Konings, 1998) and a genetically and phenotypically distinct population assigned to Tropheus polli (Fig. 1).
Fig. 1.
Phylogenetic relationships, colour pattern variation and geographical distribution of the Tropheus populations used in this study. The phylogenetic tree of Tropheus populations is based on AFLP data and analyses by Egger et al. (2007). The coloured bars next to the tree indicate the clades in which the tested populations are placed. Coloured lines along the lake shore represent the distribution of the Tropheus morphs (grey lines: striped Tropheus polli; black lines: ‘Kirschfleck’ morph; yellow lines: the yellow-blotch morph; red lines: the red-bodied morph). Photographs courtesy of Wolfgang Gessl (www.pisces.at).
Materials and methods
The fish were wild caught adults imported by an ornamental fish trader. Prior to the experiments, the fish were weighed, measured and transferred into individual aquaria (60 × 30 × 30 cm) equipped with internal box filters, heaters and hollow bricks and illuminated with overhead white light in a 12:12 h light:dark cycle.
Experimental setup was analogous to the female-choice design developed for Tropheus by Egger et al. (2008, 2010). Experimental tanks (150 × 70 × 50 cm) were divided into three compartments for two stimulus females (outer compartments, 45 cm) and the test male (centre compartment, 60 cm) by mesh partitions (mesh size 13 mm). The tanks were equipped with internal box filters, heaters and overhead lights, and each compartment was provided with identical hollow bricks serving as hiding place and territory focus. The fish were allowed an acclimatization period of 2 days after introduction into the experimental tank, during which males and females remained separated by the partitions but were able to communicate by visual, olfactory and acoustic signals. This part of the trial corresponds to the ‘decision phase’ of the design by Egger et al. (2008), during which the test fish can assess the two stimulus fish under standardized conditions. Next, males were allowed to interact freely with each of the two stimulus females at a time in ‘sequential access’ test sessions (Egger et al., 2008), for which the partition between the male and one of the females was removed, whereas a nontransparent panel was positioned between the male and the other female to prevent distraction of the tested individuals and manipulation of the by-standing female. In the next session, the male was given access to the other female. Access sessions lasted for 20 min after the first interaction between the pair. Intervals between access sessions were at least 1 h. Each trial consisted of three access sessions per stimulus female, and the order of access (beginning with either the homomorphic or the heteromorphic female) was alternated between trials. The scoring of unobstructed interactions between pairs had proven necessary in previous experiments, which had shown that association time and interactions through a partition did not predict mating preferences, but could represent territorial behaviour as well (Egger et al., 2008). Furthermore, the repetitions of the access sessions take into account that males and females interact over a longer period of time during their pre-spawning pair-bonding phase.
Males of the Moliro population (n = 15 males) were given a choice of one female from the Moliro population (the ‘homomorphic’ female, n = 19 females) and one female from one of the four other populations (the ‘heteromorphic’ female), resulting in four experiments with the four different heteromorphic populations. Heteromorphic stimulus females were from Ndole (n = 13 trials, five females), Chiseketi (n = 20 trials, eight females), a location north of Mabilibili (‘Kirschfleck’; n = 20 trials, eight females) and a location south of Isonga (T. polli; n = 14 trials, eight females). Logistic constraints limited the numbers of stimulus females that could be used in the experiments. Figure 1 illustrates the geographical distribution, genetic relatedness and colour patterns of the different morphs. Individuals were used in one to three trials (once, in four trials) per experiment (Table S1). Body sizes (standard length, SL) of individuals are reported as supplementary information (Table S1). Relative body size differences between males and females were calculated as RSD = (male SL − female SL)/(male SL + female SL).
Fish were observed continuously during the access sessions and components of their behaviour were scored with EthoLog 2.2 (Ottoni, 2000). Males and females court (among other behaviours) by quiver displays, and mutual courtship involves reciprocal T-positions, in which one of the pair quivers and the other one nuzzles the genital papilla of the partner (Nelissen, 1976). Courtship quivers of males and aggressive displays (charges and displays with spread fins) of males and females were tallied to provide measures of male courtship and male and female aggression respectively. Female sexual behaviour was quantified as the sum of the number of courtship quivers by the female and the number of times the female was involved in a ‘T-position’ display, that is, either presented herself to the male or let him nuzzle her genital papilla, or, with roles reversed, nuzzled the papilla of her courtship partner. Tallies were summed across the three access sessions to obtain one data point for each male-female pair.
To account for the repeated use of individual males and females, we employed generalized linear mixed models (GLMM) with male and female identities as crossed random factors. As male behaviour towards homomorphic females did not differ between experiments (see Results), data from all four experiments were combined to build models testing for morph-dependent differences in male and female behaviour. As the response variables were tallies of events, models were fitted with negative binomial error distributions (NB1 or NB2, dependent on which model achieved the higher AIC value) and log link functions, and included total observation time as offset. Analyses were conducted in R v. 2.15.0 (R Development Core Team, http://www.R-project.org) with package glmmADMB.
Results
Behaviour of males towards homomorphic females did not differ between experiments, and there was no effect of body size asymmetry between males and females on the amounts of male aggression displays and male courtship quivers towards homomorphic females (Table S2). Courtship quivers towards homomorphic females occurred in all trials (minimum rate of 0.1 quivers per minute). In five trials (7.5% of all trials), homomorphic females received no male aggression.
Homomorphic male behaviours were compared with behaviours towards females of the four different heteromorphic populations. The intensity of male courtship, scored as number of courtship quivers, towards homomorphic females was significantly stronger than towards heteromorphic females (Table 1). Moreover, the courtship intensities towards the different heteromorphic populations differed significantly from each other except for the similar quiver rates towards the Chiseketi and the ‘Kirschfleck’ females (Table 1). In particular, males courted the phenotypically and genetically similar Ndole females more intensely than both, the phenotypically similar, genetically distinct ‘Kirschfleck’ females and the phenotypically distinct, genetically related Chiseketi females. Males did not court females of the phenotypically and genetically distinct T. polli population (Fig. 2).
Table 1.
Generalized linear mixed models (GLMM) estimates (intercepts and effect estimates β with standard errors SE) of the effects of female population on the rates of male courtship quivers. The model was fitted using a negative binomial error distribution (NB2) with a log link function, and male and female identity as crossed random factors. Pairwise comparisons between female populations were carried out by re-running models and alternating the population used as reference. Negative signs of the β values indicate that courtship towards the focus population was less vigorous than towards the reference population
| Reference population (intercept estimate ± SE) | ||||
|---|---|---|---|---|
| Focus population | Moliro (0.58 ± 0.25) | Ndole (−0.65 ± 0.41) | Chiseketi (−2.04 ± 0.36) | ‘Kirschfleck’ (−2.39 ± 0.37) |
| Ndole | β = −1.22 ± 0.39 | |||
| P = 0.0015 | ||||
| Chiseketi | β = −2.62 ± 0.33 | β = −1.39 ± 0.44 | ||
| P = 3.8 × 10−15 | P = 0.0015 | |||
| ‘Kirschfleck’ | β = −2.94 ± 0.33 | β = −1.72 ± 0.44 | β = −0.33 ± 0.39 | |
| P = < 2 × 10−16 | P = 9.0 × 10−5 | P = 0.40 | ||
| Tropheus polli | β = −7.60 ± 1.07 | β = −6.38 ± 1.11 | β = −4.98 ± 1.09 | β = −4.65 ± 1.09 |
| P = 1.3 × 10−12 | P = 9.2 × 10−9 | P = 5.1 × 10−6 | P = 2.1 × 10−5 | |
Fig. 2.
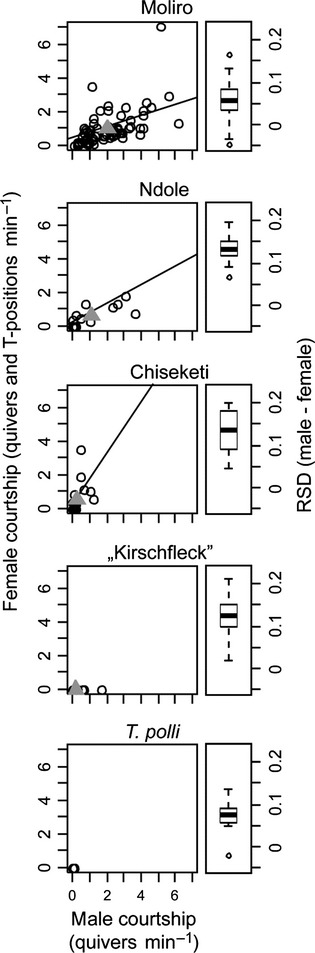
Rates of male and female courtship and body size asymmetry (RSD) between males and females. The regression lines are drawn using the intercepts and slopes estimated by the generalized linear mixed models (GLMM) reported in Table S3. Grey triangles mark the average courtship rates of males and females (omitted in the panel for Tropheus polli).
Female courtship was observed only in encounters between the Moliro males and females from Moliro, Ndole and Chiseketi, and there was a positive correlation between male and female courtship activity (Fig. 2, Table S3). The genetically and phenotypically similar Ndole females courted Moliro males as frequently as did the homomorphic Moliro females, and the rate, at which female courtship vigour increased with increasing male quiver rates, did not differ between the two morphs. In contrast, this rate was significantly higher in females from the genetically related but phenotypically distinct Chiseketi population (Fig. 2, Table S3). Females of the genetically and phenotypically distinct T. polli and the genetically different but similarly coloured ‘Kirschfleck’ did not court the males at all (Fig. 2).
Males directed significantly more aggressive behaviour against heteromorphic females than against females of their own population, but did not vary their levels of aggression between the different heteromorphic populations (Fig. 3; Table S4). Female aggression was displayed almost exclusively by Moliro females (Fig. 3; Table S5).
Fig. 3.
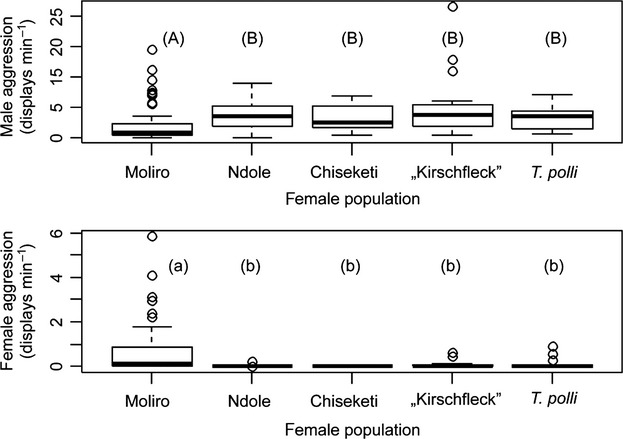
Rates of aggressive displays by Moliro males against females of different populations (upper panel), and by females of the different populations against Moliro males (lower panel). Different letters above boxplots indicate significant pairwise contrasts within panels [generalized linear mixed models (GLMM) in Tables S4 and S5].
Discussion
We found that (i) aggressive and courtship behaviour of males towards homomorphic females was independent of the morph of the alternative stimulus female, (ii) males behaved differently towards homomorphic and heteromorphic females in the contexts of both courtship and aggression, and addressed significantly more courtship and significantly less aggression to homomorphic females, and (iii) the intensity of male heteromorphic courtship, but not of aggression, depended on the females’ population, as genetically and phenotypically similar females received significantly more courtship than females of genetically and/or phenotypically distant populations. Females from genetically distant populations also showed reduced courtship activity towards Moliro males, but an opposite trend in the allocation of aggression, which they only displayed in homomorphic encounters. Aggression between males and females reflects competition for feeding territories (Yanagisawa & Nishida, 1991), and may have been determined by the interplay of body size asymmetries, which were in favour of the Moliro males especially in the heteromorphic encounters (Fig. 2), and sexual attraction between the pair, which interferes with agonistic behaviour.
Observations of different courtship intensities between different morphs are consistent with previous study on reproductive isolation in Tropheus, which demonstrated that discrimination against foreign populations is not universal and amongst other possible factors dependent on the similarity between the involved morphs (Salzburger et al., 2006; Egger et al., 2008, 2010, 2012). In particular, in female choice experiments with the Moliro population and another red-coloured population (Chimba; closely related to and resembling the Ndole population tested in this study), courtship was equally likely to occur within and between populations (Egger et al., 2010). In contrast, Moliro females exhibited strictly assortative mate choice in two-way laboratory trials and in mixed-morph breeding populations, when the alternative populations had distinct colour patterns (bluish and yellow-blotched populations), whereas Moliro males occasionally courted or mated with the heteromorphic females (Egger et al., 2008; Hermann, 2011; H. Brindl & K. M. Sefc, unpublished). The present data suggest that male courtship behaviour can contribute to sexual isolation between Moliro and other populations, provided that male courtship affects the probability of mating (Forsgren, 1997; Oliveira et al., 2000; Shamble et al., 2009; Lehtonen, 2012). Asymmetric courtship between Moliro males and ‘Kirschfleck’ females and between Chiseketi females and Moliro males further suggest that courtship can be displayed by one sex independent of the behaviour of the other sex. Hence, the population-dependent courtship intensities on the part of males are not necessarily reflections of female behaviour and preferences (Takahashi et al., 2008), but may rather result from male mating preferences and the ability of males to discriminate between populations.
The importance of male mate choice for species isolation in general is difficult to assess. The number of taxa, in which male preferences have been examined, is still rather small and the studied taxa differ distinctly in conditions known or assumed to influence the evolution of mating preferences (Parker & Partridge, 1998; Servedio, 2007), such as mating and breeding systems, patterns of current and past geographical distributions, natural and sexual selection regimes and hybrid fitness. In several taxa, only the females displayed strong assortative preferences (Tetrix ground hoppers, Hochkirch et al., 2007; darters, O'Rourke & Mendelson, 2010; guppies, Russell et al., 2006; Ficedula flycatchers, Saetre et al., 1997), whereas in others, males contributed significantly or exclusively to the maintenance of assortative mating (damselflies, Svensson et al., 2007; Heliconius butterflies, Jiggins et al., 2001, 2004; mosquito fish, Espinedo et al., 2010; garter snakes, Shine et al., 2004; cichlid fishes, Pauers et al., 2010; Egger et al., 2010; present study). Frequently, assortative male preferences were found in studies of sympatric taxa, where costs of nonassortative mating could explain the evolution of mate choice in males, for example to avoid egg predation by allomorphic females led to a stickleback male's nest (Albert & Schluter, 2004). Moreover, low hybrid fitness favours assortative mate choice in males when mating opportunities are limited (Peterson et al., 2005) or when courtship is associated with mortality risks, as for example due to predation on conspicuous, courting male damselflies (Svensson et al., 2007).
During allopatric divergence, assortative preferences can arise in both sexes for example when the social system promotes sexual imprinting and learning (Verzijden et al., 2012) or as a consequence of the correlated divergence of sensory systems and signals (Boughman, 2002). Indeed, assortative mate preferences of cichlids have been shown to be influenced by sexual imprinting (Verzijden & ten Cate, 2007; Verzijden et al., 2008) and by associations between sensory systems, signals and environmental variation (Maan & Seehausen, 2010). Both processes could theoretically be at work in Tropheus, as their territorial system, involving social interactions with numerous conspecifics, may be conducive to learning and individual recognition, and as divergent selection implied in colour pattern differentiation (Mattersdorfer et al., 2012) could also have acted on the sensory system. Mate discrimination by both sexes and the weak isolation between similar phenotypes are consistent with both sexual imprinting on and a sensory bias for homomorphic phenotypes, but discrimination between the similarly coloured but differently patterned Moliro and ‘Kirschfleck’ might be expected to be weaker if preferences were based on biased colour perception. Selection could also have targeted mate preferences directly or by reinforcement in periods when population displacements associated with lake level fluctuations resulted in secondary contact of differentiated morphs (Egger et al., 2007). It is certainly unlikely that each of the different pairs of morphs in this study have been interacting with each other in their past, but it has been proposed that single bouts of reinforcement may give rise to general choosiness (Coyne & Orr, 2004). Nonetheless, offspring viability and fertility in experimental crosses between some morphs (K.M. Sefc, personal observation), introgression and the potential hybrid origin of morphs (Sturmbauer et al., 2005; Egger et al., 2007), and the link between sexual isolation and genetic divergence provide no support for selection against heteromorphic mating. Furthermore, while genetically distinct lineages of Tropheus co-occur at some locations, it is striking that there is no sympatry of related colour variants (Konings, 1998; Egger et al., 2007), which would be expected if premating isolation was a product of secondary contact. Finally, a Fisherian run-away process driven by female preferences has been implied in the allopatric colour differentiation in a cichlid species complex from Lake Malawi, consistent with assortative mating in experimental situations (Knight & Turner, 2004). If mate preferences were driving colour pattern differentiation in Tropheus, we would expect strong discrimination already in the earliest stage of divergence, which is, however, not the case between the genetically related morphs studied here (Moliro and Ndole) and previously (Moliro and Chimba, Egger et al., 2010).
In previous studies of mate choice between Tropheus populations, the extent of colour pattern differentiation between populations covaried with their genetic distance, and it was not possible to discern whether body colour differences affected sexual isolation independently of divergence time (Egger et al., 2010). In this study, we compared sexual isolation in four different combinations of genetic and colour pattern differentiation, and presented Moliro males with heteromorphic choices of genetically and phenotypically similar females (Ndole), genetically and phenotypically distinct females (T. polli), genetically related females with distinct colour pattern (Chiseketi) and genetically distinct but similarly coloured females (‘Kirschfleck’). Males did not differentiate in their courtship rates between the latter two heteromorphic populations (Chiseketi and ‘Kirschfleck’), whereas they displayed significantly more courtship quivers to the genetically and phenotypically similar population (Ndole), and significantly less courtship towards the genetically and phenotypically distinct T. polli. Apparently, male discrimination was influenced not only by colour pattern differences but also by divergence in other, for example olfactory or acoustic, cues (Plenderleith et al., 2005; Verzijden et al., 2010).
Although males behaved similarly to Chiseketi and ‘Kirschfleck’ females, the females of the two populations differed in their courtship behaviour towards Moliro males. ‘Kirschfleck’ females did not respond to male courtship at all, whereas Chiseketi females showed a higher courtship rate relative to male courtship activity than for example the homomorphic Moliro females. Although the exaggerated heteromorphic courtship of Chiseketi females could be a consequence of a lack of choice offered to the females in our experiment, it is also consistent with previous observations made with females pertaining to the same yellow-blotched morph (a population from Mbita Island), which did not discriminate between homo- and heteromorphic males in laboratory two-way female choice tests against a bluish morph (Nakaku; Egger et al., 2010), and spawned preferentially with the bluish males in experimental ponds stocked with yellow-blotch and bluish Tropheus (Hermann, 2011).
Overall, mate choice data of Tropheus suggest that sexual isolation among populations increases with increasing genetic distance. Positive correlations between premating isolation and genetic distance have also been observed in other allopatric species pairs, including Drosophila flies (Coyne & Orr, 1997) and haplochromine cichlids (Stelkens et al., 2009). In the haplochromines, which is the cichlid clade comprising the genus Tropheus (Koblmüller et al., 2008), premating isolation was estimated to accumulate fastest during initial divergence and reach completion after 4.8/10/22 million years (Myr), depending on the underlying molecular clock (Stelkens et al., 2009). In our experiment, the deficiency in reproductively motivated interactions between males of the Moliro population and females of T. polli and the ‘Kirschfleck’ population is consistent with the proposed species delineations (Konings, 1998; Egger et al., 2007), provided that Moliro females do not successfully solicit matings from the foreign males, which has not been tested here. So far, however, red-morph females exerted strictly assortative mate choice in two-way and multiple choice experiments with two other distinct morphs (Egger et al., 2008; Hermann, 2011; H. Brindl & K. M. Sefc unpublished). Using a molecular clock calibration corresponding to the one yielding the 4.8 Myr estimate for the completion of premating isolation in the above haplochromine study, the most recent common ancestor of the Tropheus species complex (excluding T. duboisi), and hence the maximum divergence between populations, dates to approximately 1.5 Ma (Koblmüller et al., 2010). This does not necessarily imply that isolation evolved exceptionally fast in Tropheus, as premating isolation among the haplochromine species was estimated in no-choice tests and discrimination against heterospecifics in the presence of conspecific females, as in our experiments, may be achieved earlier during divergence. Accounting further for the imprecision of molecular clock dating and the different measures of premating isolation (spawning in Stelkens et al., 2009 vs. courtship in the Tropheus studies), the time span within which sexual isolation evolved among Tropheus populations fits well with the haplochromine model.
In conclusion, males of the Moliro population discriminate between females of different populations, and even vary their courtship behaviour between females of the same population and a phenotypically and genetically similar population. Discrimination among populations appears to increase with both, genetic distance and colour pattern dissimilarity. On the basis of current evidence, we consider it likely that assortative mate preferences evolved in both sexes as a by-product of allopatric divergence under natural or social selection. Experiments testing the effects of learning and sensory environments on mate choice are warranted to address the mechanisms underlying sexual isolation within this species complex. Understanding assortative mate preferences among Tropheus populations at their various stages of phylogenetic and phenotypic divergence has the potential to improve our general understanding of how allopatric populations evolve premating isolation on their way to speciation.
Acknowledgments
We thank Wolfgang Gessl for allowing us to use his photographs of fish used in this study, and Caroline Hermann for help with the experiments. Many thanks to Karen Grace-Martin for her accessible statistical tutoring made available through ‘The Analysis Factor’ website. Three anonymous reviewers provided valuable comments. This work was supported by the Austrian Science Fund (FWF; grant P20883-B16 to KMS). Data deposited in the Dryad repository: doi:10.5061/dryad.b78g9.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1 Body size (standard length SL) of females and males, and the number of times each individual was used in the experiments.
Table S2 Generalized linear mixed model estimating effects of experiment (heteromorphic female population) and RSD (relative body size difference between the male and the homomorphic female) on aggressive and courtship behaviour of Moliro males towards the homomorphic Moliro females.
Table S3 Generalized linear mixed model estimating effects (β ± SE) of female population (Moliro, Ndole, Chiseketi) and male courtship intensity (quiver rate) on female courtship.
Table S4 Generalized linear mixed model estimating effects (β ± SE) of female population on male aggression.
Table S5 Generalized linear mixed model estimating effects (β ± SE) of female population on female aggression.
References
- Albert AYK, Schluter D. Reproductive character displaecment of male stickleback mate preference: reinforcement or direct selection? Evolution. 2004;58:1099–1107. doi: 10.1111/j.0014-3820.2004.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Boughman JW. How sensory drive can promote speciation. Trends Ecol. Evol. 2002;17:571–577. [Google Scholar]
- Coyne JA, Orr HA. “Patterns of speciation in Drosophila” revisited. Evolution. 1997;51:295–303. doi: 10.1111/j.1558-5646.1997.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, Masschusetts, MA: Sinauer Associates, Inc; 2004. [Google Scholar]
- Egger B, Obermüller B, Phiri H, Sturmbauer C, Sefc KM. Monogamy in the maternally mouthbrooding Lake Tanganyika cichlid fish Tropheus moorii. Proc. R. Soc. Lond. B. 2006;273:1797–1802. doi: 10.1098/rspb.2006.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Koblmüller S, Sturmbauer C, Sefc KM. Nuclear and mitochondrial data reveal different evolutionary processes in the Lake Tanganyika cichlid genus Tropheus. BMC Evol. Biol. 2007;7:137. doi: 10.1186/1471-2148-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B, Obermüller E, Eigner E, Sturmbauer C, Sefc KM. Assortative mating preferences between colour morphs of the endemic Lake Tanganyika cichlid genus Tropheus. Hydrobiologia. 2008;615:37–48. [Google Scholar]
- Egger B, Mattersdorfer K, Sefc KM. Variable discrimination and asymmetric preferences in laboratory tests of reproductive isolation between cichlid colour morphs. J. Evol. Biol. 2010;23:433–439. doi: 10.1111/j.1420-9101.2009.01906.x. [DOI] [PubMed] [Google Scholar]
- Egger B, Sefc KM, Makasa L, Sturmbauer C, Salzburger W. Introgressive hybridization between color morphs in a population of cichlid fishes twelve years after human-induced secondary admixis. J. Hered. 2012;103:515–522. doi: 10.1093/jhered/ess013. [DOI] [PubMed] [Google Scholar]
- Espinedo CM, Gabor CR, Aspbury AS. Males, but not females, contribute to sexual isolation between two sympatric species of Gambusia. Evol. Ecol. 2010;24:865–878. [Google Scholar]
- Forsgren E. Mate sampling in a population of sand gobies. Anim. Behav. 1997;53:267–276. [Google Scholar]
- Hermann C. Mate choice in a cichlid fish. Graz: University of Graz; 2011. PhD thesis. [Google Scholar]
- Hochkirch A, Gröning J, Bücker A. Sympatry with the devil: reproductive interference could hamper species coexistence. J. Anim. Ecol. 2007;76:633–642. doi: 10.1111/j.1365-2656.2007.01241.x. [DOI] [PubMed] [Google Scholar]
- Jiggins CD, Naisbit RE, coe RL, Mallet J. Reproductive isolation caused by colour pattern mimicry. Nature. 2001;411:302–305. doi: 10.1038/35077075. [DOI] [PubMed] [Google Scholar]
- Jiggins CD, Estrada C, Rodrigues A. Mimicry and the evolution of premating isolation in Heliconius melpomene Linnaeus. J. Evol. Biol. 2004;17:680–691. doi: 10.1111/j.1420-9101.2004.00675.x. [DOI] [PubMed] [Google Scholar]
- Knight ME, Turner GF. Reproductive isolation among closely related Lake Malawi cichlids: can males recognize conspecific females by visual cues? Anim. Behav. 1999;58:761–768. doi: 10.1006/anbe.1999.1206. [DOI] [PubMed] [Google Scholar]
- Knight ME, Turner GF. Laboratory mating trials indicate incipient speciation by sexual selection among populations of the cichlid fish Pseudotropheus zebra from Lake Malawi. Proc. R. Soc. Lond. B. 2004;271:675–680. doi: 10.1098/rspb.2003.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koblmüller S, Schliewen UK, Duftner N, Sefc KM, Katongo C, Sturmbauer C. Age and spread of the haplochromine cichlid fishes in Africa. Mol. Phylogenet. Evol. 2008;49:153–169. doi: 10.1016/j.ympev.2008.05.045. [DOI] [PubMed] [Google Scholar]
- Koblmüller S, Egger B, Sturmbauer C, Sefc KM. Rapid radiation, ancient incomplete lineage sorting and ancient hybridization in the endemic Lake Tanganyika cichlid tribe Tropheini. Mol. Phylogenet. Evol. 2010;55:318–334. doi: 10.1016/j.ympev.2009.09.032. [DOI] [PubMed] [Google Scholar]
- Koblmüller S, Salzburger W, Obermüller B, Eigner E, Sturmbauer C, Sefc KM. Separated by sand, fused by dropping water: fragmented habitat and fluctuating water levels steer the evolution of rock-dwelling cichlid populations in Lake Tanganyika. Mol. Ecol. 2011;20:2272–2290. doi: 10.1111/j.1365-294X.2011.05088.x. [DOI] [PubMed] [Google Scholar]
- Konings A. Tanganyika Cichlids in their Natural Habitat. El Paso, Texas: Cichlid Press; 1998. [Google Scholar]
- Kozak GM, Boughman JW. Learned conspecific mate preference in a species pair of sticklebacks. Behav. Ecol. 2009;20:1282–1288. [Google Scholar]
- Lehtonen TK. Signal value of male courtship effort in a fish with parental care. Anim. Behav. 2012;83:1153–1161. [Google Scholar]
- Maan ME, Seehausen O. Mechanisms of species divergence through visual adaptation and sexual selection: perspectives from a cichlid model system. Curr. Zool. 2010;56:285–299. [Google Scholar]
- Mattersdorfer K, Koblmüller S, Sefc KM. AFLP genome scans suggest divergent selection on colour patterning in allopatric colour morphs of a cichlid fish. Mol. Ecol. 2012;21:3531–3544. doi: 10.1111/j.1365-294X.2012.05634.x. [DOI] [PubMed] [Google Scholar]
- Mendelson TC, Shaw KL. The (mis)concept of species recognition. Trends Ecol. Evol. 2012;27:421–427. doi: 10.1016/j.tree.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Nelissen M. Contribution to the ethology of Tropheus moorii Boulenger (Pisces, Cichlidae) and a discussion of the significance of its colour patterns. Rev. Zool. Afr. 1976;90:17–29. [Google Scholar]
- Nosil P, Crespi BJ, Gries R, Gries G. Natural selection and divergence in mate preference during speciation. Genetica. 2007;129:309–327. doi: 10.1007/s10709-006-0013-6. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Miranda JA, Carvalho N, Goncalves EJ, Grober MS, Santos RS. Male mating success in the Azorean rock-pool blenny: the effects of body size, male behaviour and nest characteristics. J. Fish Biol. 2000;57:1416–1428. [Google Scholar]
- O'Rourke C, Mendelson TC. Male and female preference for conspecifics in a fish with male parental care (Percidae: Catonotus. Behav. Process. 2010;85:157–162. doi: 10.1016/j.beproc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Ottoni EB. EthoLog 2.2 - a tool for the transcription and timing of behavior observation sessions. Behav. Res. Methods Instrum. Comput. 2000;32:446–449. doi: 10.3758/bf03200814. [DOI] [PubMed] [Google Scholar]
- Parker GA, Partridge L. Sexual conflict and speciation. Philos. Trans. R. Soc. Lond. B. 1998;353:261–274. doi: 10.1098/rstb.1998.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauers MJ, Ehlinger TJ, McKinnon JS. Female and male visually based mate preferences are consistent with reproductive isolation between populations of the Lake Malawi endemic Labeotropheus fuelleborni. Curr. Zool. 2010;56:65–72. [Google Scholar]
- Peterson MA, Honchak BM, Locke SE, Beeman TE, Mendoza J, Green J, et al. Relative abundance and the species-specific reinforcement of male mating preferences in the Chrysochus (Coleoptera: Chrysomelidae) hybrid zone. Evolution. 2005;59:2639–2655. [PubMed] [Google Scholar]
- Pierotti MER, Knight ME, Immler S, Barson NJ, Turner GF, Seehausen O. Individual variation in male mating preferences for female coloration in a polymorphic cichlid fish. Behav. Ecol. 2008;19:483–488. [Google Scholar]
- Plenderleith M, van Oosterhout C, Robinson RL, Turner GF. Female preference for conspecific males based on olfactory cues in a Lake Malawi cichlid fish. Biol. Lett. 2005;1:411–414. doi: 10.1098/rsbl.2005.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryke SR, Griffith SC. The relative role of male vs. female mate choice in maintaining assortative pairing among discrete colour morphs. J. Evol. Biol. 2007;20:1512–1521. doi: 10.1111/j.1420-9101.2007.01332.x. [DOI] [PubMed] [Google Scholar]
- Ritchie MG. Sexual selection and speciation. Annu. Rev. Ecol. Evol. Syst. 2007;38:79–102. [Google Scholar]
- Russell ST, Ramnarine IW, Mahabir R, Magurran AE. Genetic detection of sperm from forced copulations between sympatric populations of Poecilia reticulata and Poecilia picta. Biol. J. Linn. Soc. 2006;88:397–402. [Google Scholar]
- Saetre G-P, Kral M, Bures S. Differential species recognition abilities of males and females in a flycatcher hybrid zone. J. Avian Biol. 1997;28:259–263. [Google Scholar]
- Salzburger W, Niederstätter H, Brandstätter A, Berger B, Parson W, Snoeks J, et al. Colour-assortative mating among populations of Tropheus moorii, a cichlid fish from Lake Tanganyika, East Africa. Proc. R. Soc. Lond. B. 2006;273:257–266. doi: 10.1098/rspb.2005.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupke P. Buntbarsche des Tanganjikasees. Die Arten der Gattung Tropheus. Germany: Aqualog, ACS GmbH, Rodgau; 2003. [Google Scholar]
- Seehausen O, van Alphen JJM, Lande R. Color polymorphism and sex ratio distortion in a cichlid fish as an incipient stage of sympatric speciation by sexual selection. Ecol. Lett. 1999;2:367–378. [Google Scholar]
- Sefc KM. Variance in reproductive success and the opportunity for selection in a serially monogamous species: simulations of the mating system of Tropheus (Teleostei: Cichlidae) Hydrobiologia. 2008;615:21–35. [Google Scholar]
- Servedio MR. Male versus female mate choice: sexual selection and the evolution of species recognition via reinforcement. Evolution. 2007;61:2772–2789. doi: 10.1111/j.1558-5646.2007.00247.x. [DOI] [PubMed] [Google Scholar]
- Shamble PS, Wilgers DJ, Swoboda KA, Hebets EA. Courtship effort is a better predictor of mating success than ornamentation for male wolf spiders. Behav. Ecol. 2009;20:1242–1251. [Google Scholar]
- Shine R, Reed RN, Shetty S, Lemaster M, Mason RT. Reproductive isolating mechanisms between two sympatric sibling species of sea snakes. Evolution. 2002;56:1655–1662. doi: 10.1111/j.0014-3820.2002.tb01477.x. [DOI] [PubMed] [Google Scholar]
- Shine R, Phillips B:, Waye H, Lemaster M, Mason RT. Species-isolating mechanisms in a mating system with male mate choice (garter snakes, thamnophis spp.) Can. J. Zool. 2004;82:1091–1098. [Google Scholar]
- Steinwender B, Koblmüller S, Sefc KM. Concordant female mate preferences in the cichlid Tropheus moorii. Hydrobiologia. 2012;682:121–130. doi: 10.1007/s10750-011-0766-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelkens RB, Young KA, Seehausen O. The accumulation of reproductive incompatibilities in African cichlid fish. Evolution. 2009;64:617–633. doi: 10.1111/j.1558-5646.2009.00849.x. [DOI] [PubMed] [Google Scholar]
- Sturmbauer C, Koblmüller S, Sefc KM, Duftner N. Phylogeographic history of the genus Tropheus, a lineage of rock-dwelling cichlid fishes endemic to Lake Tanganyika. Hydrobiologia. 2005;542:335–366. [Google Scholar]
- Svensson EI, Karlsson K, Friberg M, Eroukhmanoff F. Gender differences in species recognition and the evolution of asymmetric sexual isolation. Curr. Biol. 2007;17:1943–1947. doi: 10.1016/j.cub.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Arita H, Hiraiwa-Hasegawa M, Hasegawa T. Peahens do not prefer peacocks with more elaborate traits. Anim. Behav. 2008;75:1209–1219. [Google Scholar]
- Verzijden MN, ten Cate C. Early learning influences species assortative mating preferences in Lake Victoria cichlid fish. Biol. Lett. 2007;3:134–136. doi: 10.1098/rsbl.2006.0601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verzijden MN, Korthof REM, ten Cate C. Females learn from mothers and males learn from others. The effect of mother and siblings on the development of female mate preferences and male aggression biases in Lake Victoria cichlids, genus Mbipia. Behav. Ecol. Sociobiol. 2008;62:1359–1368. [Google Scholar]
- Verzijden MN, Zwinkels J, ten Cate C. Cross-fostering does not influence the mate preferences and territorial behaviour of males in Lake Victoria cichlids. Ethology. 2009;115:39–48. [Google Scholar]
- Verzijden MN, van Heusden J, Bouton N, Witte F, ten Cate C, Slabbekoorn H. Sounds of male Lake Victoria cichlids vary within and between species and affect female mate preferences. Behav. Ecol. 2010;21:548–555. [Google Scholar]
- Verzijden MN, ten Cate C, Servedio MR, Kozak GM, Boughman JW, Svensson EI. The impact of learning on sexual selection and speciation. Trends Ecol. Evol. 2012;27:511–519. doi: 10.1016/j.tree.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Wirtz P. Mother species – father species: unidirectional hybridization in animals with female choice. Anim. Behav. 1999;58:1–12. doi: 10.1006/anbe.1999.1144. [DOI] [PubMed] [Google Scholar]
- Yanagisawa Y, Nishida M. The social and mating system of the maternal mouthbrooder Tropheus moorii (Cichlidae) in Lake Tanganyika. Japan. J. Ichthyol. 1991;38:271–282. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.