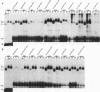
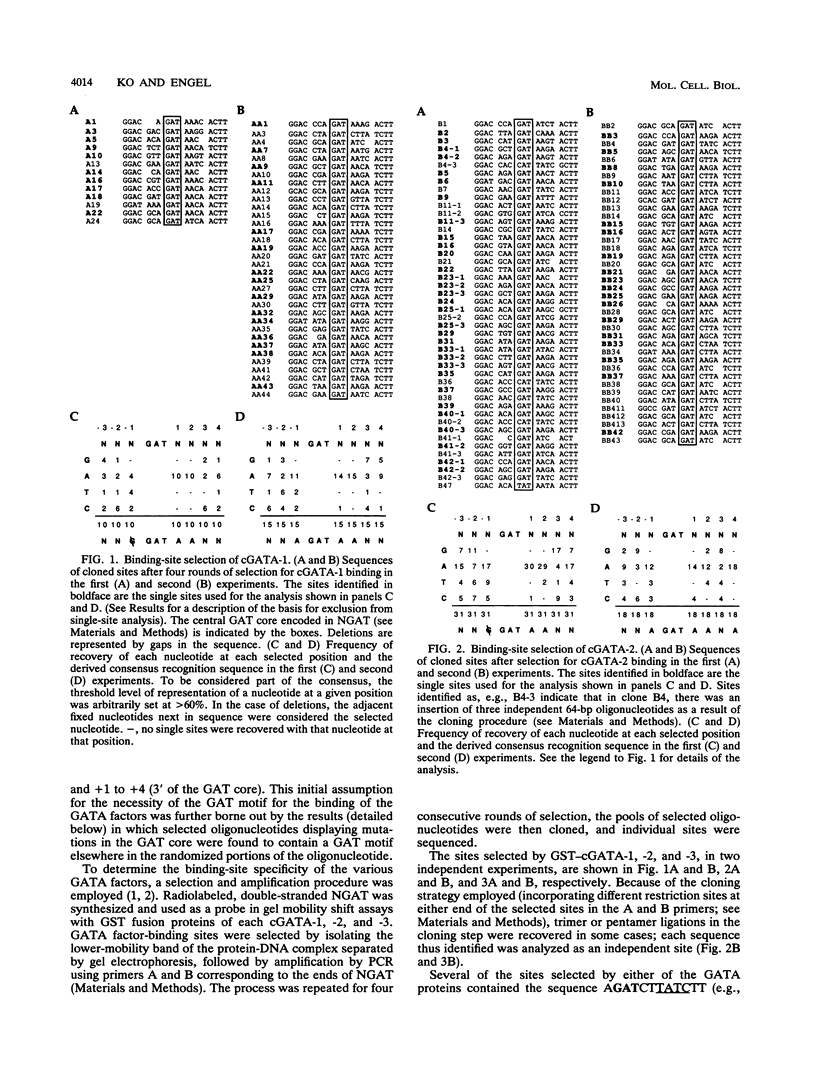
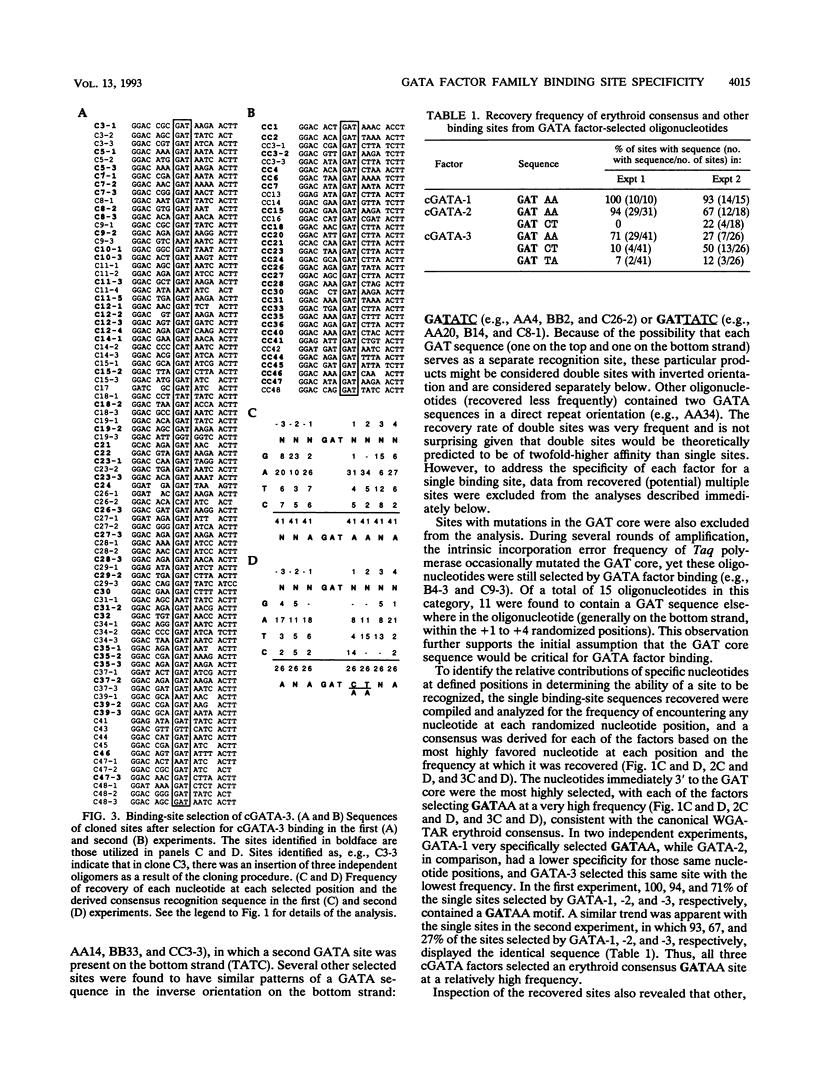
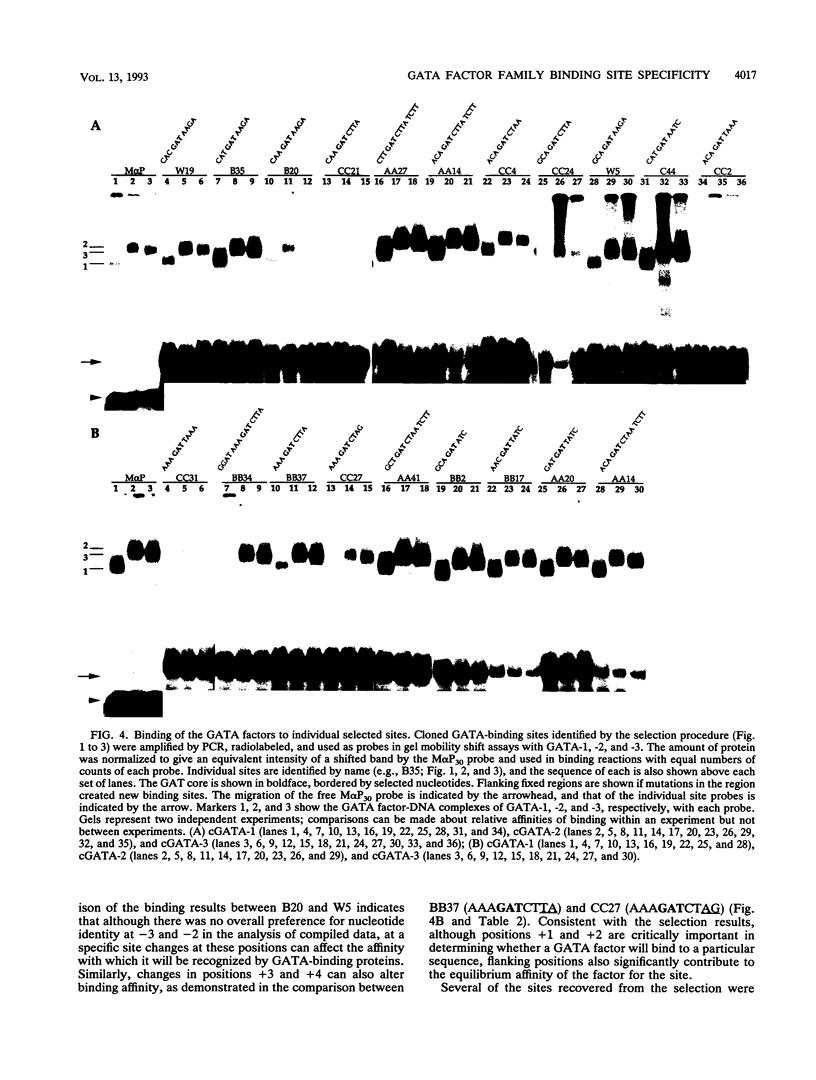
Abstract
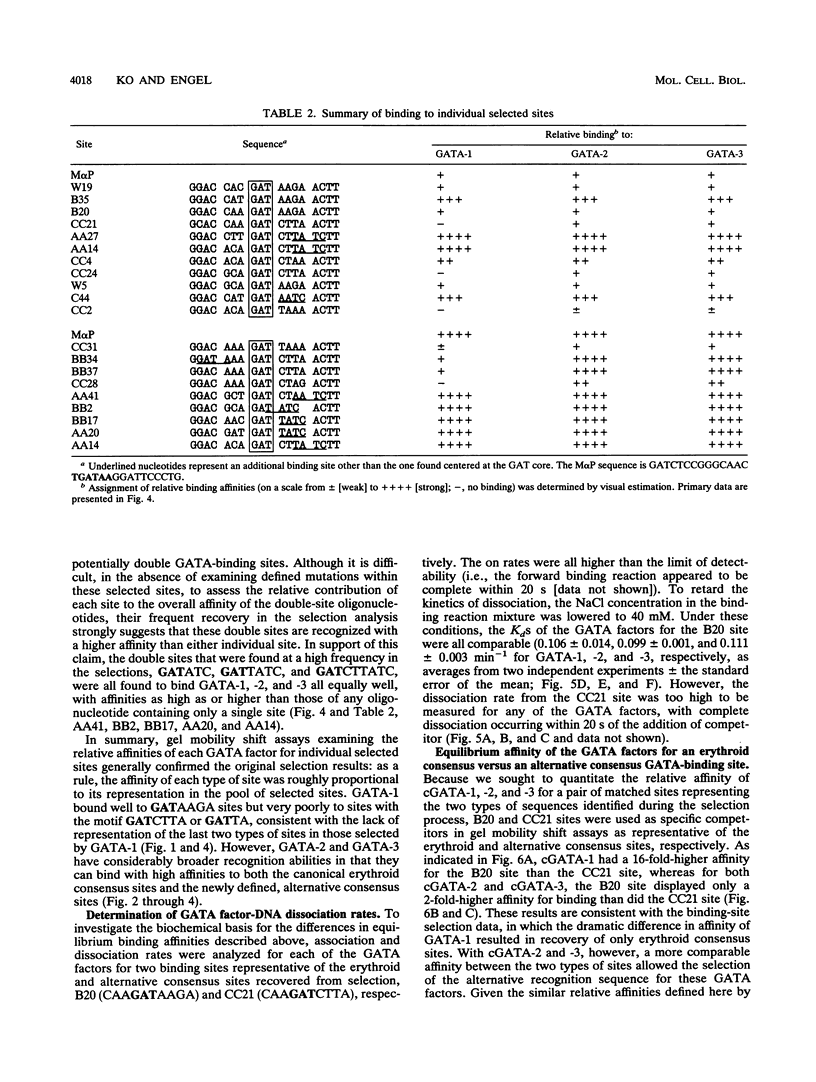
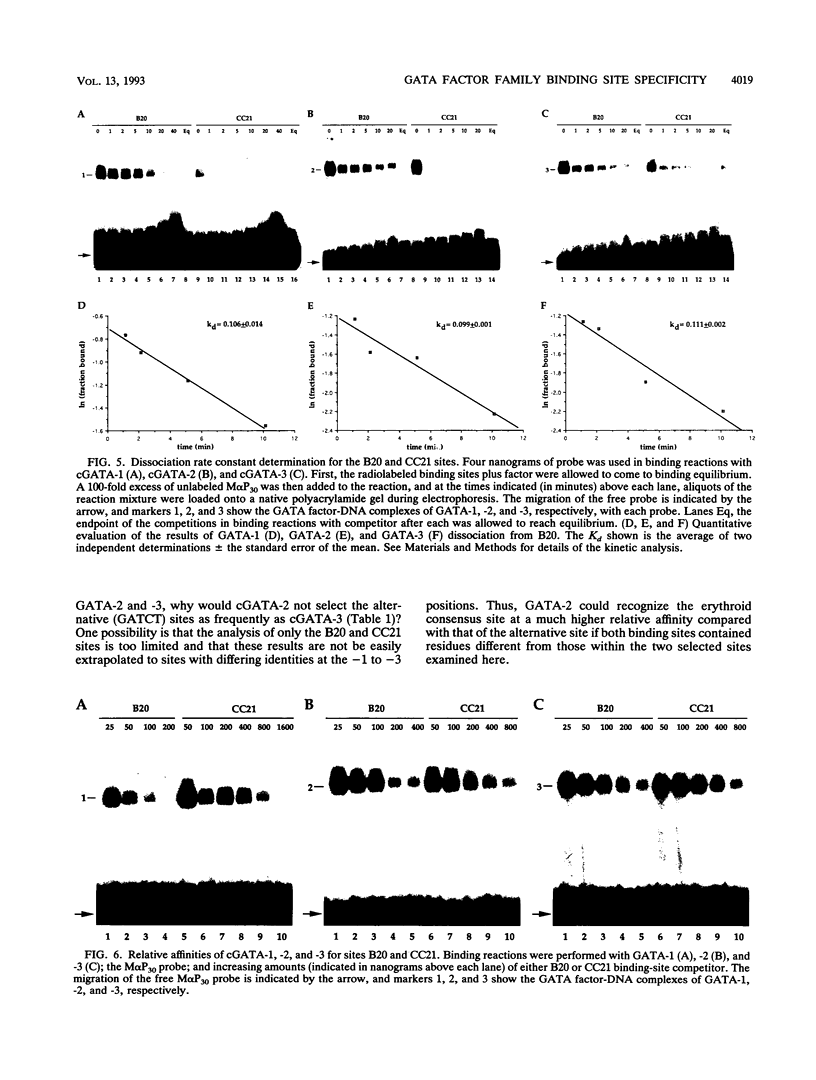
Members of the GATA family of transcription factors, which are related by a high degree of amino acid sequence identity within their zinc finger DNA-binding domains, each show distinct but overlapping patterns of tissue-restricted expression. Although GATA-1, -2, and -3 have been shown to recognize a consensus sequence derived from regulatory elements in erythroid cell-specific genes, WGATAR (in which W indicates A/T and R indicates A/G), the potential for more subtle differences in the binding preferences of each factor has not been previously addressed. By employing a binding selection and polymerase chain reaction amplification scheme with randomized oligonucleotides, we have determined the binding-site specificities of bacterially expressed chicken GATA-1, -2, and -3 transcription factors. Whereas all three GATA factors bind an AGATAA erythroid consensus motif with high affinity, a second, alternative consensus DNA sequence, AGATCTTA, is also recognized well by GATA-2 and GATA-3 but only poorly by GATA-1. These studies suggest that all three GATA factors are capable of mediating transcriptional effects via a common erythroid consensus DNA-binding motif. Furthermore, GATA-2 and GATA-3, because of their distinct expression patterns and broader DNA recognition properties, may be involved in additional regulatory processes beyond those of GATA-1. The definition of an alternative GATA-2-GATA-3 consensus sequence may facilitate the identification of new target genes in the further elucidation of the roles that these transcription factors play during development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Catala F., deBoer E., Habets G., Grosveld F. Nuclear protein factors and erythroid transcription of the human A gamma-globin gene. Nucleic Acids Res. 1989 May 25;17(10):3811–3827. doi: 10.1093/nar/17.10.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty T., Olson E. N. Domains outside of the DNA-binding domain impart target gene specificity to myogenin and MRF4. Mol Cell Biol. 1991 Dec;11(12):6103–6108. doi: 10.1128/mcb.11.12.6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. A 3' enhancer is required for temporal and tissue-specific transcriptional activation of the chicken adult beta-globin gene. Nature. 1986 Oct 23;323(6090):731–734. doi: 10.1038/323731a0. [DOI] [PubMed] [Google Scholar]
- Danielsen M., Hinck L., Ringold G. M. Two amino acids within the knuckle of the first zinc finger specify DNA response element activation by the glucocorticoid receptor. Cell. 1989 Jun 30;57(7):1131–1138. doi: 10.1016/0092-8674(89)90050-0. [DOI] [PubMed] [Google Scholar]
- Dessain S., Gross C. T., Kuziora M. A., McGinnis W. Antp-type homeodomains have distinct DNA binding specificities that correlate with their different regulatory functions in embryos. EMBO J. 1992 Mar;11(3):991–1002. doi: 10.1002/j.1460-2075.1992.tb05138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker S. C., von Kessler D. P., Beachy P. A. Differential DNA sequence recognition is a determinant of specificity in homeotic gene action. EMBO J. 1992 Nov;11(11):4059–4072. doi: 10.1002/j.1460-2075.1992.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson B. M., Nickol J. M., Jackson P. D., Felsenfeld G. Analysis of the tissue-specific enhancer at the 3' end of the chicken adult beta-globin gene. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4786–4790. doi: 10.1073/pnas.84.14.4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. The erythroid-specific transcription factor Eryf1: a new finger protein. Cell. 1989 Sep 8;58(5):877–885. doi: 10.1016/0092-8674(89)90940-9. [DOI] [PubMed] [Google Scholar]
- Evans T., Felsenfeld G. trans-Activation of a globin promoter in nonerythroid cells. Mol Cell Biol. 1991 Feb;11(2):843–853. doi: 10.1128/mcb.11.2.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Reitman M., Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. E., Parent L. A., Sharp P. A. High affinity DNA-binding Myc analogs: recognition by an alpha helix. Cell. 1993 Feb 12;72(3):467–476. doi: 10.1016/0092-8674(93)90122-7. [DOI] [PubMed] [Google Scholar]
- Gallarda J. L., Foley K. P., Yang Z. Y., Engel J. D. The beta-globin stage selector element factor is erythroid-specific promoter/enhancer binding protein NF-E4. Genes Dev. 1989 Dec;3(12A):1845–1859. doi: 10.1101/gad.3.12a.1845. [DOI] [PubMed] [Google Scholar]
- Hartzog G. A., Myers R. M. Discrimination among potential activators of the beta-globin CACCC element by correlation of binding and transcriptional properties. Mol Cell Biol. 1993 Jan;13(1):44–56. doi: 10.1128/mcb.13.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho I. C., Yang L. H., Morle G., Leiden J. M. A T-cell-specific transcriptional enhancer element 3' of C alpha in the human T-cell receptor alpha locus. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6714–6718. doi: 10.1073/pnas.86.17.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E., Toki T., Ishihara H., Ohtani H., Gu L., Yokoyama M., Engel J. D., Yamamoto M. Erythroid transcription factor GATA-1 is abundantly transcribed in mouse testis. Nature. 1993 Apr 1;362(6419):466–468. doi: 10.1038/362466a0. [DOI] [PubMed] [Google Scholar]
- Joulin V., Bories D., Eléouet J. F., Labastie M. C., Chrétien S., Mattéi M. G., Roméo P. H. A T-cell specific TCR delta DNA binding protein is a member of the human GATA family. EMBO J. 1991 Jul;10(7):1809–1816. doi: 10.1002/j.1460-2075.1991.tb07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezetic J. A., Felsenfeld G. Identification and characterization of a chicken alpha-globin enhancer. Mol Cell Biol. 1989 Mar;9(3):893–901. doi: 10.1128/mcb.9.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko L. J., Yamamoto M., Leonard M. W., George K. M., Ting P., Engel J. D. Murine and human T-lymphocyte GATA-3 factors mediate transcription through a cis-regulatory element within the human T-cell receptor delta gene enhancer. Mol Cell Biol. 1991 May;11(5):2778–2784. doi: 10.1128/mcb.11.5.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. I., Orkin S. H. Transcriptional activation and DNA binding by the erythroid factor GF-1/NF-E1/Eryf 1. Genes Dev. 1990 Nov;4(11):1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Tsai S. F., Orkin S. H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989 Mar 30;338(6214):435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- Martin D. I., Zon L. I., Mutter G., Orkin S. H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990 Mar 29;344(6265):444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- Minie M. E., Kimura T., Felsenfeld G. The developmental switch in embryonic rho-globin expression is correlated with erythroid lineage-specific differences in transcription factor levels. Development. 1992 Aug;115(4):1149–1164. doi: 10.1242/dev.115.4.1149. [DOI] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Plumb M., Frampton J., Wainwright H., Walker M., Macleod K., Goodwin G., Harrison P. GATAAG; a cis-control region binding an erythroid-specific nuclear factor with a role in globin and non-globin gene expression. Nucleic Acids Res. 1989 Jan 11;17(1):73–92. doi: 10.1093/nar/17.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo J. M., Hata S., Brocklehurst C., Krangel M. S. A T cell-specific transcriptional enhancer within the human T cell receptor delta locus. Science. 1990 Mar 9;247(4947):1225–1229. doi: 10.1126/science.2156339. [DOI] [PubMed] [Google Scholar]
- Redondo J. M., Pfohl J. L., Krangel M. S. Identification of an essential site for transcriptional activation within the human T-cell receptor delta enhancer. Mol Cell Biol. 1991 Nov;11(11):5671–5680. doi: 10.1128/mcb.11.11.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M., Felsenfeld G. Mutational analysis of the chicken beta-globin enhancer reveals two positive-acting domains. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6267–6271. doi: 10.1073/pnas.85.17.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M., Lee E., Westphal H., Felsenfeld G. Site-independent expression of the chicken beta A-globin gene in transgenic mice. Nature. 1990 Dec 20;348(6303):749–752. doi: 10.1038/348749a0. [DOI] [PubMed] [Google Scholar]
- Romeo P. H., Prandini M. H., Joulin V., Mignotte V., Prenant M., Vainchenker W., Marguerie G., Uzan G. Megakaryocytic and erythrocytic lineages share specific transcription factors. Nature. 1990 Mar 29;344(6265):447–449. doi: 10.1038/344447a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzbauer G., Schlesinger K., Evans T. Interaction of the erythroid transcription factor cGATA-1 with a critical auto-regulatory element. Nucleic Acids Res. 1992 Sep 11;20(17):4429–4436. doi: 10.1093/nar/20.17.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Pevny L., Wiles M. V., Keller G., Costantini F., Orkin S. H. Rescue of erythroid development in gene targeted GATA-1- mouse embryonic stem cells. Nat Genet. 1992 May;1(2):92–98. doi: 10.1038/ng0592-92. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Lai J. S., Herr W. Promoter-selective activation domains in Oct-1 and Oct-2 direct differential activation of an snRNA and mRNA promoter. Cell. 1992 Feb 21;68(4):755–767. doi: 10.1016/0092-8674(92)90150-b. [DOI] [PubMed] [Google Scholar]
- Trainor C. D., Stamler S. J., Engel J. D. Erythroid-specific transcription of the chicken histone H5 gene is directed by a 3' enhancer. 1987 Aug 27-Sep 2Nature. 328(6133):827–830. doi: 10.1038/328827a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Strauss E., Orkin S. H. Functional analysis and in vivo footprinting implicate the erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Genes Dev. 1991 Jun;5(6):919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- Umesono K., Evans R. M. Determinants of target gene specificity for steroid/thyroid hormone receptors. Cell. 1989 Jun 30;57(7):1139–1146. doi: 10.1016/0092-8674(89)90051-2. [DOI] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall L., deBoer E., Grosveld F. The human beta-globin gene 3' enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988 Sep;2(9):1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Tsai S. F., Hogben P., Orkin S. H. Regulated expression of globin chains and the erythroid transcription factor GATA-1 during erythropoiesis in the developing mouse. Mol Cell Biol. 1990 Dec;10(12):6596–6606. doi: 10.1128/mcb.10.12.6596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Ko L. J., Leonard M. W., Beug H., Orkin S. H., Engel J. D. Activity and tissue-specific expression of the transcription factor NF-E1 multigene family. Genes Dev. 1990 Oct;4(10):1650–1662. doi: 10.1101/gad.4.10.1650. [DOI] [PubMed] [Google Scholar]
- Yang H. Y., Evans T. Distinct roles for the two cGATA-1 finger domains. Mol Cell Biol. 1992 Oct;12(10):4562–4570. doi: 10.1128/mcb.12.10.4562. [DOI] [PMC free article] [PubMed] [Google Scholar]