Abstract
Background
Trabectedin and thioglitazones have been documented to induce adipocytic maturation in myxoid liposarcoma; we have noted this in our experience as well. Intriguingly, we have also encountered this same phenomenon in myxoid liposarcomas exposed to various combinations of neoadjuvant doxorubicin and ifosfamide systemic chemotherapy with preoperative radiation, where the pathological effects have been less characterized. We examined the histological changes, including adipocytic maturation, associated with this treatment in patients with myxoid liposarcoma and evaluated for prognostic significance.
Methods
Twenty-two patients were identified with histologically confirmed myxoid liposarcomas (9 with variable hypercellular areas) who were treated with neoadjuvant doxorubicin (75-90 mg/m2/continous infusion over 72h every 3 week) and ifosfamide (2.5 g/m2 daily x 4 every 3 weeks) for 4-6 cycles. Twenty-one patients received pre-operative radiation including 5 with concurrent gemcitabine. Pre- and post-treatment MRI studies were compared for changes in tumor area, fat content and contrast uptake, with the latter two estimated as: none, <25%, 25-49% and >50%. Post-treatment specimens were evaluated for hyalinization, necrosis and adipocytic maturation. Clinical follow-up was obtained.
Results
Median age was 45 (26-72) years with a median tumor size of 11 (2-18) cm. All occurred in the lower extremities except for one case in the neck. As is common in myxoid liposarcoma, all had extensive treatment changes (>90%) with extensive hyalinization (n = 16; >90%) or prominent adipocytic maturation (n = 6; >50%) including 2 cases composed almost entirely of mature-appearing adipose tissue. Variable necrosis was identified (5-30%). MRI revealed a decrease in tumor area in all but one tumor (median, 65%), an increase in fat content in 7 tumors (n = 2, >50%;n = 2, 25-50%;n = 3,<25%), and a decrease in contrast enhancement in most tumors (n = 5, >50%; n = 9, 25-49%; n = 7, <25%). Median follow-up was 57 (12-96) months with 17 alive with no disease/metastases, 3 alive with disease and 2 dead of disease. Six patients developed metastases with median interval of 26 (22-51) months post resection. Four of 6 tumors with increased adipocytic maturation >50% on histology had increased fat detected by MRI (>25%). All 6 are alive but 2 developed metastases. In the remaining patients, 4 developed metastases with 14 alive and 2 dead of disease.
Conclusion
Myxoid liposarcoma exposed to neoadjuvant doxorubicin and ifosfamide and pre-operative radiation can have prominent adipocytic maturation similar to trabectedin treatment. Myxoid liposarcomas exhibit extensive treatment changes with prominent hyalinization being the most common histological change. Despite this, patients develop metastases regardless of adipocytic maturation. While of unclear significance, no patient with fatty maturation died of disease.
Keywords: Adipocytic maturation, Myxoid liposarcoma, Treatment
Background
Myxoid liposarcoma is the second most common malignant lipomatous tumor and preferentially affects ages younger than most other liposarcomas (young adults), and typically occurs in the lower extremities. Classically, these tumors are characterized by monotonous small short spindle cells in myxoid stroma with abundant thin-walled capillaries. Scattered lipoblasts, typically univacuolar, are variably present sometimes accompanied by variable amounts of more mature-appearing adipocytes [1,2]. Despite their banal histological appearance, metastases are common, and often to peculiar sites such as to the retroperitoneum and bone [3,4]. Tumors with increased hypercellular areas composed of enlarged, more round tumor cells without intervening myxoid stroma have an increased risk for metastases and poor prognosis [2-6]. Those with a significant proportion of round cell change are termed round cell liposarcoma and considered to be a higher grade variant of myxoid liposarcoma [7].
The vast majority of myxoid liposarcomas are characterized by a recurrent translocation involving t(12;16) (q13;p11) fusing the 3′-end promoter region of FUS/TLS (16p11) with the 5′-end of the CHOP/DDIT3 locus (12q13) [8-14]. The resulting fusion protein is believed to play a critical role in tumorigenesis, blocking the PPARγ pathway which promotes adipocytic differentiation or maturation, offering insight into the primitive pre-adipocytic histological appearance of this tumor [15,16]. In rare situations, the alternative t(12;22) (q13;p11) resulting in a EWSR1-CHOP/DDIT3 fusion transcript is present [9,17-20]. Other genetic mutations including involvement of the p53, RET, MET, and PI-3 kinase pathways, have also been implicated in myxoid liposarcomas [6,15,16,21-23].
ET-743 (also known as trabectedin) is an alkaloid compound discovered from the sea squirt Ecteinascidia turbinata and found to show efficacy in the treatment of sarcoma patients including those with myxoid liposarcoma [24-27]. While the mechanism of action is not completely known, trabectedin may block transcription factor binding to the fusion gene FUS-CHOP/DDIT3[15]. It has also been well documented to induce differentiation in myxoid liposarcoma with prominent adipocytic maturation (Figure 1). In addition, PPARγ agonists, thioglitazones, have been reported to produce similar histological effects in myxoid liposarcomas [28]. These effects have been purported to be characteristic and perhaps specific and thus suggesting a particular mechanism of action leading to the release of a blockade of adipocytic maturation in the tumor.
Figure 1.
Myxoid liposarcoma metastasis in a patient being treated with trabectedin. Histological sections reveal irregularly-sized mature-appearing adipocytes in a myxoid stroma with thin-walled vessels. Adipocytic maturation is seen in patients receiving trabectedin therapy.
We have encountered this phenomenon of adipocytic maturation in myxoid liposarcomas exposed to neoadjuvant doxorubicin and ifosfamide and pre-operative radiation, where the pathological effects have been less characterized. We examined the histological changes, including adipocytic maturation, associated with this treatment in patients with myxoid liposarcoma and evaluated for any prognostic significance of this feature.
Methods
Under institutional board review approval, twenty-two patients were identified between 2000 and 2009 who had histologically confirmed (WLW, AJL) myxoid liposarcoma on pre-treatment biopsy and were not previously treated with chemotherapy. Nine of these patients had variable hypercellular/round cell change. Patients were treated with neoadjuvant doxorubicin (75-90 mg/m2/continous infusion over 72 hours every three weeks) and ifosfamide (2.5 g/m2 over three hours for four days, every three weeks) for 4-6 cycles. Twenty-one patients also received preoperative radiation including 5 with concurrent gemcitabine. Pre- and post-treatment MRI studies were compared (JM) for changes in tumor area, fat content and contrast uptake, with the latter two estimated as: none, <25%, 25-49% and >50%. Post-treatment specimens were compared with pre-treatment biopsies and evaluated for percentage of hyalinization, necrosis and adipocytic maturation (WLW, AJL). Clinical follow-up was obtained. Fluorescence in situ hybridization (FISH) on a selected post-treatment case for 12q13 to detect CHOP/DDIT3 rearrangement was performed using the LSI FUS dual-color, break-apart probe (Abbott Molecular/Vysis, Des Plaines, IL, USA) according to the manufacturer’s recommendations as previously described [14].
Results
Clinical, pathological and radiological findings are summarized in Table 1. The median age was 45 (26-72) years with a median size of 11 (2-18) cm. All occurred in the lower extremities except for one case in the neck.
Table 1.
Summary of clinical, pathological and imaging factors
|
Median age (range) years |
45 (26-72) |
|
Sex M:F |
13:9 |
|
Location (n, %) | |
| Thigh |
15 (68%) |
| Calf |
6 (27%) |
| Neck |
1 (4.5%) |
|
Round Cell Component (n,range of %) |
n = 9 (5-30%) |
|
Follow-up | |
| Median, range |
57 mos (12-96 mos) |
| Alive and Well (median, range) |
n = 17 (35 mos, 12-96 mos) |
| Alive with Disease (range) |
n = 3 (61,67, 72 mos) |
| Dead of Disease (range) |
n = 2 (82, 16 mos) |
| Metastases (median, range) |
n = 6 (26, 22-51 mos) |
|
Post-Treatment Pathological Findings | |
|
Median Size (range) |
11 (2-18) cm |
|
Tumor with Treatment Changes (median %, range) |
95% (90-95%) |
|
Hyalinization (median %, range) |
90% (10-95%) |
|
Fat Maturation | |
| Median (range)% |
10% (5-90%) |
| Prominent >50% (n) |
n = 6 with 2 >90% |
|
Necrosis (median %, range) |
5% (5-10%) |
|
Radiological Changes | |
|
Decrease in Tumor Size (median % decrease, range) |
65% (30 - 94%)* |
|
Fat Amount Change (n, % of cases with changes) | |
| Decrease <25% |
1 (4.5%) |
| No change |
14 (63.6%) |
| Increase <25% |
3 (13.6%) |
| Increase 25-49% |
2 (9.1%) |
| Increase >50% |
2 (9.1%) |
|
Contrast Media Change (n, % of cases with changes) | |
| Increase <25% |
1 (4.5%) |
| Decrease <25% |
7 (31.8%) |
| Decrease 25-49% |
9 (40.9%) |
| Decrease >50% | 5 (22.7%) |
All developed extensive post-treatment changes (>90%) with extensive hyalinization (>90%) being the most common change seen (16/22 patients, 72%) (Figure 2). Focal residual areas of characteristic vascular pattern were often identified. Prominent fatty maturation (>50%) was seen in 6/22 (27%) cases including 2 cases composed almost entirely of mature-appearing fat (Figure 3). Both cases received radiation therapy; one also received gemcitabine. One tumor with extensive post-treatment fatty maturation was tested and found to retain the rearrangement in CHOP/DDIT3 verifying that the mature adipocytes arose from the tumor cells. Variable necrosis was also identified (5-30%).
Figure 2.
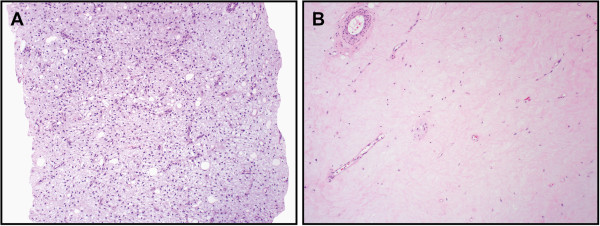
(A) Pre-treatment biopsy reveals characteristic bland spindle cells in myxoid stroma with branching thin walled vessels. (B) Post treatment specimen reveals extensive hyalinization that is essentially acellular. This was the most common pattern seen in treated tumors.
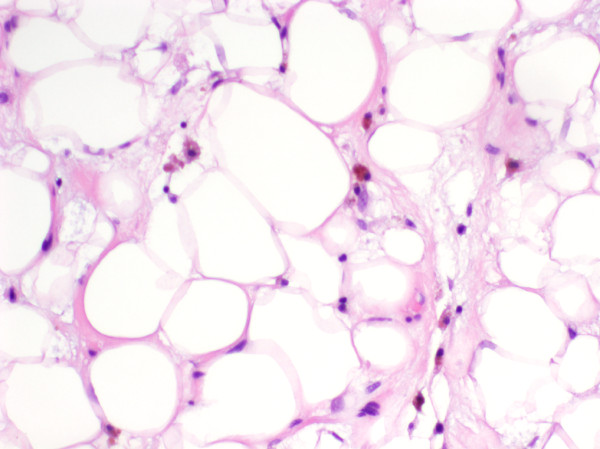
Figure 3.
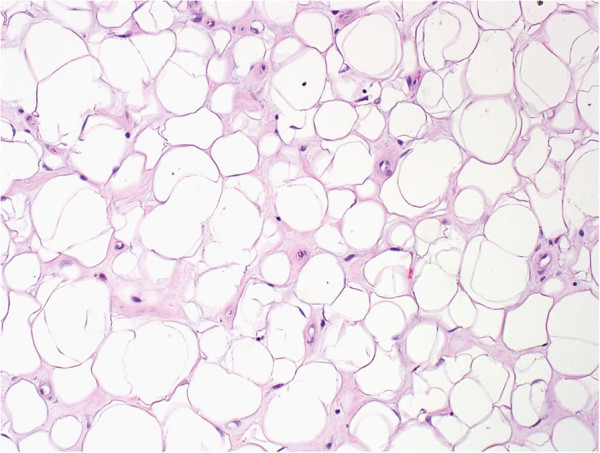
Histological example of extensive fatty maturation with variable sized adipocytes. Extensive fatty maturation was seen in one-third of cases; two of which had adipocytic maturation that was greater than 90%.
MRI revealed a decrease in tumor area in all but one tumor (median decrease in size was 65%). A decrease in contrast enhancement was found in most tumors (n = 5,>50%; n = 9, 25-49%; n = 7, <25%) (Figure 4), histologically corresponding to increased hyalinization, decreased cellularity and decreased vasculature. Seven tumors developed an increase in a register characteristic of mature adipose tissue (n = 2,>50%; n = 2, 25-49%; n = 3,<25%) (Figure 5). Four of 6 tumors with histological adipocytic maturation >50% also had increased adipose signal detected by MRI (>25%).
Figure 4.
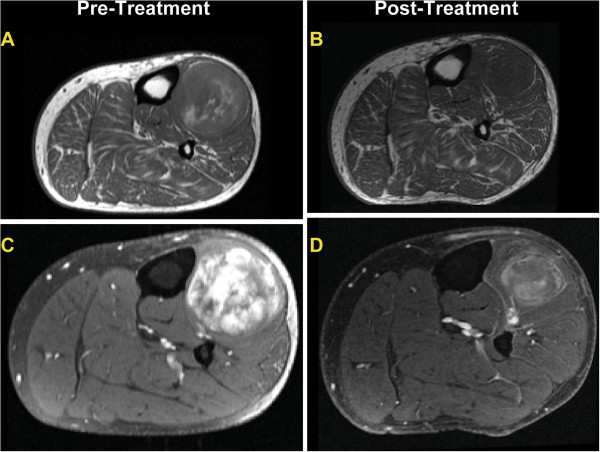
Example of MRI findings seen in the majority of myxoid liposarcomas undergoing treatment in our series. T1 weighted axial image of the calf with mass (A) pre-treatment and (B) post treatment showing a reduction in tumor size and decreased adipocytic content. T1 weighted post contrast media with fat saturation with (C) pre-treatment demonstrating an extensive heterogeneous enhancement, while (D) post treatment shows decreased tumor size and decreased enhancement greater than 50%. These findings correspond to increased hyalinization and decreased vascularity histologically.
Figure 5.
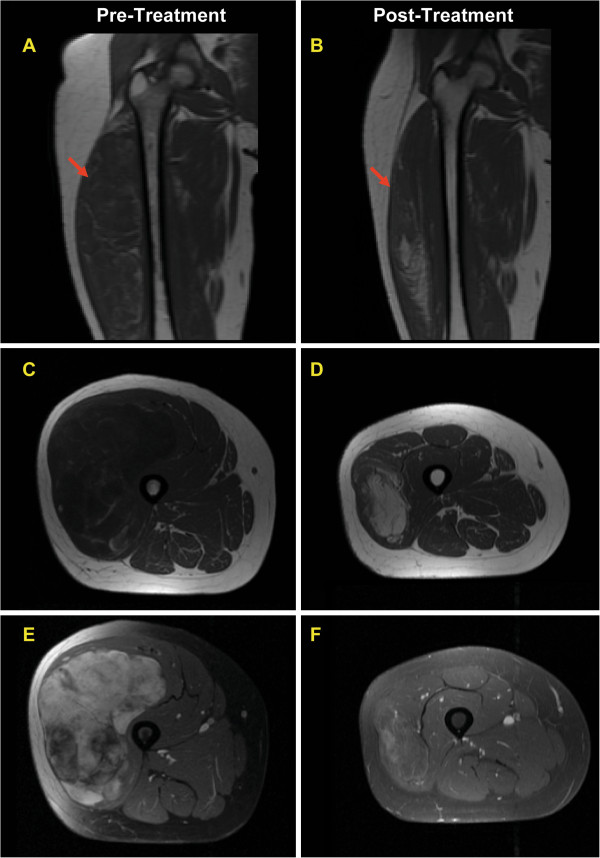
MRI findings in a case of myxoid liposarcoma which developed extensive adipocytic maturation during treatment. T1 weighted coronal right thigh (A) pre-treatment and (B) post treatment demonstrating a marked decrease in tumor size. T1 weighted axial image of right thigh (C) pre-treatment and (D) post treatment demonstrate an increase in signal characteristic of mature adipose tissue content (>50%) with reduction in size. T1 weighted axial with contrast media and fat saturation (E) pre-treatment showing large mass with extensive vascular enhancement. (F) Post treatment scans show a reduction in size of mass and a decrease in vascular enhancement greater than 50%.
Median follow-up was 57 (12-96) months with 17 patients alive with no disease/metastases, 3 patients alive with disease and 2 patients dead of disease. Six patients developed metastases with a median interval of 26 (22-51) months post resection. All 6 patients with adipocytic maturation are alive although 2 developed metastases.
Conclusions
Myxoid liposarcomas treated with neoadjuvant doxorubicin and ifosfamide and pre-operative radiation consistently develop extensive treatment effect that can include adipocytic maturation as demonstrated on MRI imaging and confirmed histologically at resection. In our series, the most common treatment change was extensive hyalinization (16/22, 72%) with decreased vasculature as seen in other studies [29]. In addition, approximately one-third of our patients also developed prominent adipocytic maturation, comprising more than 50% of the treated tumor. Two tumors had extensive adipocytic differentiation giving a misleading appearance of a well-differentiated liposarcoma or even a benign lipoma. Fortunately, most tumors also harbored areas of hyalinization, characteristic of treated myxoid liposarcoma. They also lacked the fibrous bands with scattered pleomorphic cells seen in most well-differentiated liposarcomas. Molecular testing, either for rearrangements involving CHOP/DDIT3 by fluorescence in-situ hybridization assay or reverse polymerase chain reaction for the specific fusion transcripts, can also be helpful in confirming the diagnosis of myxoid liposarcoma [14,30]. In our study, rearrangement was still identified in tumors with prominent fatty differentiation by fluorescence in-situ hybridization assay. We chose this modality since it allows one to determine the cellular component of the tissue showing the re-arrangement. Well-differentiated liposarcomas would lack this rearrangement and would instead demonstrate amplification of the 12q13-15 region [31].
The phenomenon of adipocytic maturation is comparable to myxoid liposarcomas in patients treated with trabectedin and the thioglitazone family of PPARγ agonists. In 2007, Grosso and colleagues described the development of “mature lipoblast-featuring cells” and “clear adipocytic differentiation” in myxoid liposarcomas treated with trabectedin [24]. Gronchi and colleagues reported maturation to lipoblasts, decreased cellularity and vascularity in the tumors of 16 of 23 patients who received neoadjuvant trabectedin for advanced localized myxoid liposarcomas [32]. Forni and colleagues saw similar findings in myxoid liposarcoma cell lines and two patients treated with trabectedin [33]. Similarly, Demetri and colleagues also saw adipocytic differentiation as early as six weeks in tumors from three patients treated with troglitazone, a PPARγ agonist. These changes coincided with an increased expression in mRNA associated with adipocytic differentiation including AP2, adipsin and PPARγ. Mild increase in adipose density signaling was also seen in these tumors on MRI imaging [28]. In our series, extensive treatment changes, predominantly hyalinization with decreased cellularity and vascularity, was seen in the tumors of our patients treated with doxorubicin and ifosfamide with pre-operative radiation therapy. However, adipocytic maturation was also seen in our tumors, histologically indistinguishable to what has been reported previously reported by others and also seen by us in myxoid liposarcoma tumors treated with trabectedin (WLW, AJL, PT). In most cases, MRI imaging correlated with evolution of treatment changes seen in the resection specimen. Decreased vascularity by contrast media enhanced imaging was seen in the majority of cases with extensive hyalinization and subsequent decrease of the thin-walled vessels in these areas. In addition, all four cases that had an increase of fat (>25%) by MRI also showed prominent adipocytic maturation on histological resection.
Myxoid liposarcoma can rarely have areas with mature-appearing adipocytes as well. Evans reported 29 cases of myxoid liposarcoma, some of which had areas that were reported to resemble mature, non-neoplastic adult adipose tissue [1]. In a more recent study by Fritchie and colleagues, 10 of 46 (22%) myxoid liposarcomas had a mature fat appearing lipomatous component that composed 5-79% of the tumor. Full information regarding treatment was not provided in either study. Some degree of stromal hyalinization was also observed in seven cases in the Fritchie study, six of which were known to be untreated. However, this was only focal, composing only 1-9% of these tumors, in contrast to our treated cases which had extensive hyalinization [7]. It is possible that some changes could have been present before treatment and not sampled in the pre-treatment biopsy, emphasizing the importance of comparing pre- and post-treatment imaging studies. One case with histological evidence of adipocytic maturation revealed no change in adipocytic content by MRI and adipocytic maturation was noted in the pre-treatment biopsy. However, in our experience (WLW, AJL) extensive fatty maturation and hyalinization are uncommon in untreated myxoid liposarcomas. The remaining pre-treatment biopsies lacked these changes and developed increased fat on MRI studies between pre- and post treatment tumors, which corresponded histologically to increased fatty maturation, increased hyalinization and decrease vascularity on the resection specimen.
The significance of adipocytic maturation is unclear. No patients with extensive adipocytic maturation died of disease, but patients did develop metastases regardless of adipocytic maturation. However, our series is small and additional larger studies are necessary. The mechanism of fatty maturation when exposed to doxorubicin and ifosfamide and radiation therapy is also not known. Doxorubicin is an anthracycline and proposed to have many mechanisms of actions among which include intercalcating DNA, formation of free radicals, inhibition of topoisomerase II, prohibiting DNA and RNA unwinding, and induction of cell death [34]. Ifosfamide is a DNA alkylating agent which prohibits DNA replication and results in cell death [35]. Interestingly, doxorubicin at low concentrations has been reported to induce differentiation in leukemia and breast carcinoma cell lines [34,36,37]. In breast cell lines, differentiation corresponded to the reduction in c-myc expression and growth arrest in response to doxorubicin induced DNA damage [37]. To our knowledge, ifosfamide has not been reported to induce differentiation. Perhaps a similar non-specific response to DNA damage with doxorubicin can occur in myxoid liposarcomas. Other sarcomas have also been reported to differentiate in response to chemotherapy and radiotherapy including rhabdomyosarcoma [38]. Our study would support the notion that these changes can be a general response to effective treatment in myxoid liposarcoma and may not necessarily be specific to a treatment modality such as trabectedin. In this context, we should be cautious in interpreting potential insights into the unknown mechanism of action of trabectedin from this phenomenon.
The fusion transcript characteristic in the vast majority of myxoid liposarcomas is composed of FUS, which encodes for a RNA/DNA binding domain, and CHOP/DDIT3, which encodes for a transcription factor which dimerizes with CAAT/enhancer binding proteins (C/EBP) and negatively regulates genes involved in adipocytic differentiation including PPARγ. Multiple fusion variants and types exist; however, none have been associated with increased adipogenesis [39]. Trabectedin binds to the minor groove of DNA and is theorized to sterically hinder the binding of the FUS-CHOP/DDIT3 fusion protein (probably mediated primarily by the CHOP/DDIT3-donated DNA binding domain) and thus allow adipocytic differentiation to continue by relieving repression. It could also effect the post transcriptional modification of the fusion transcript [15]. In addition, other mutations are known to exist including activation of the ERK/MAPK, PI-3-kinase/Akt pathways, alterations of p53, IGF1R and IGF2; some of these have been associated with round cell change and poor prognosis. [16,22] None are known to correlate with adipocytic differentiation.
In summary, we report the histological and radiological findings seen in myxoid liposarcomas in patients treated with doxorubicin and ifosfamide and pre-operative radiation therapy. The vast majority of these tumors demonstrated significant treatment effect in the form of hyalinization; however, adipocytic maturation, once thought to be exclusive to tumors treated with trabectedin and PPARγ agonists, were also seen in some of our cases. Pathologists and radiologists should be aware of this as it may cause diagnostic confusion if prior history is not available and in comparing histological treatment changes between trials.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WLW reviewed the histological findings and wrote the publication; AJL reviewed the histological findings and is the senior author on this publication; PT reviewed selected histological findings; JM reviewed the radiological studies; DL edited the manuscript; VOL, PL, and RP surgically removed the specimens; AG and GZ performed the radiation treatment; RB, SP, RV, DA, and DK administered the chemotherapy, DLT performed the fluorescence in-situ hybridization. All authors read and approved the final manuscript.
Contributor Information
Wei-Lien Wang, Email: wlwang@mdanderson.org.
Daniela Katz, Email: danielakatz@hadassah.org.il.
Dejka M Araujo, Email: daraujo@mdanderson.org.
Vinod Ravi, Email: vravi@mdanderson.org.
Joseph A Ludwig, Email: jaludwig@mdanderson.org.
Jonathan C Trent, Email: JTrent@med.miami.edu.
Shreyaskumar R Patel, Email: spatel@mdanderson.org.
Patrick P Lin, Email: plin@mdanderson.org.
Ashleigh Guadagnolo, Email: aguadagn@mdanderson.org.
Dolores Lòpez-Terrada, Email: DHterrad@texaschildren.org.
Angelo Paola dei Tos, Email: apdeitos@ulss.tv.it.
Valerie O Lewis, Email: volewis@mdanderson.org.
Dina Lev, Email: Dlev@mdnderson.org.
Raphael E Pollock, Email: rpollock@mdanderson.org.
Gunar K Zagars, Email: gzagars@mdanderson.org.
Robert S Benjamin, Email: rbenjami@mdanderson.org.
John E Madewell, Email: jmadewell@mdanderson.org.
Alexander J Lazar, Email: alazar@mdanderson.org.
Acknowledgements
We acknowledge Kim Vu for her expert assistance in the graphic arts. The Liddy Shriver Foundation is thanked for their generous support of our myxoid liposarcoma studies.
References
- Evans HL. Liposarcoma: a study of 55 cases with a reassessment of its classification. Am J Surg Pathol. 1979;3:507–523. doi: 10.1097/00000478-197912000-00004. [DOI] [PubMed] [Google Scholar]
- Kilpatrick SE, Doyon J, Choong PF, Sim FH, Nascimento AG. The clinicopathologic spectrum of myxoid and round cell liposarcoma. A study of 95 cases. Cancer. 1996;77:1450–1458. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1450::AID-CNCR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Antonescu C, Ladanyi M. In: Pathology and genetics of Tumuors of bone and soft tissue. Fletcher CDM, Unni KK, Mertens F, editor. Lyon: International Agency for Research on Cancer Press; 2002. Myxoid Liposarcoma. [Google Scholar]
- Weiss SW, Goldblum JR. Enzinger and Weiss’s soft tissue tumors. 5. Maryland Heights, MO: Mosby Elsevier; 2008. pp. 498–510. [Google Scholar]
- Haniball J, Sumathi VP, Kindblom LG, Prognostic factors and metastatic patterns in primary myxoid/round-cell liposarcoma. Sarcoma. 2011. 538085. [DOI] [PMC free article] [PubMed]
- Antonescu CR, Tschernyavsky SJ, Decuseara R. et al. Prognostic impact of P53 status, TLS-CHOP fusion transcript structure, and histological grade in myxoid liposarcoma: a molecular and clinicopathologic study of 82 cases. Clin Cancer Res. 2001;7:3977–3987. [PubMed] [Google Scholar]
- Fritchie KJ, Goldblum JR, Tubbs RR. et al. The expanded histologic spectrum of myxoid liposarcoma with an emphasis on newly described patterns: implications for diagnosis on small biopsy specimens. Am J Clin Pathol. 2012;137:229–239. doi: 10.1309/AJCP90YNOKBAGCDM. [DOI] [PubMed] [Google Scholar]
- Antonescu CR, Elahi A, Humphrey M. et al. Specificity of TLS-CHOP rearrangement for classic myxoid/round cell liposarcoma: absence in predominantly myxoid well-differentiated liposarcomas. J Mol Diagn. 2000;2:132–138. doi: 10.1016/S1525-1578(10)60628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode-Lesniewska B, Frigerio S, Exner U, Abdou MT, Moch H, Zimmermann DR. Relevance of translocation type in myxoid liposarcoma and identification of a novel EWSR1-DDIT3 fusion. Genes Chromosomes Cancer. 2007;46:961–971. doi: 10.1002/gcc.20478. [DOI] [PubMed] [Google Scholar]
- Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993;363:640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- Hisaoka M, Tsuji S, Morimitsu Y. et al. Detection of TLS/FUS-CHOP fusion transcripts in myxoid and round cell liposarcomas by nested reverse transcription-polymerase chain reaction using archival paraffin-embedded tissues. Diagn Mol Pathol. 1998;7:96–101. doi: 10.1097/00019606-199804000-00006. [DOI] [PubMed] [Google Scholar]
- Knight JC, Renwick PJ, Dal Cin P, Van den Berghe H, Fletcher CD. Translocation t(12;16)(q13;p11) in myxoid liposarcoma and round cell liposarcoma: molecular and cytogenetic analysis. Cancer Res. 1995;55:24–27. [PubMed] [Google Scholar]
- Panagopoulos I, Mandahl N, Ron D. et al. Characterization of the CHOP breakpoints and fusion transcripts in myxoid liposarcomas with the 12;16 translocation. Cancer Res. 1994;54:6500–6503. [PubMed] [Google Scholar]
- Powers MP, Wang WL, Hernandez VS. et al. Detection of myxoid liposarcoma-associated FUS-DDIT3 rearrangement variants including a newly identified breakpoint using an optimized RT-PCR assay. Mod Pathol. 2011;23:1307–1315. doi: 10.1038/modpathol.2010.118. [DOI] [PubMed] [Google Scholar]
- Charytonowicz E, Terry M, Coakley K. et al. PPARgamma agonists enhance ET-743-induced adipogenic differentiation in a transgenic mouse model of myxoid round cell liposarcoma. J Clin Invest. 2012;122:886–898. doi: 10.1172/JCI60015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Dodge J, Mehl E. et al. Validation of immature adipogenic status and identification of prognostic biomarkers in myxoid liposarcoma using tissue microarrays. Hum Pathol. 2009;40:1244–1251. doi: 10.1016/j.humpath.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Birch NC, Antonescu CR, Nelson M. et al. Inconspicuous insertion 22;12 in myxoid/round cell liposarcoma accompanied by the secondary structural abnormality der(16)t(1;16) J Mol Diagn. 2003;5:191–194. doi: 10.1016/S1525-1578(10)60472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Cin P Sciot R Panagopoulos I et al. Additional evidence of a variant translocation t(12;22) with EWS/CHOP fusion in myxoid liposarcoma: clinicopathological features J Pathol 1997182437–441.9306965 [DOI] [Google Scholar]
- Hosaka T, Nakashima Y, Kusuzaki K. et al. A novel type of EWS-CHOP fusion gene in two cases of myxoid liposarcoma. J Mol Diagn. 2002;4:164–171. doi: 10.1016/S1525-1578(10)60698-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrozek K, Szumigala J, Brooks JS, Crossland DM, Karakousis CP, Bloomfield CD. Round cell liposarcoma with the insertion (12;16)(q13;p11.2p13) Am J Clin Pathol. 1997;108:35–39. [PubMed] [Google Scholar]
- Negri T, Virdis E, Brich S. et al. Functional mapping of receptor tyrosine kinases in myxoid liposarcoma. Clin Cancer Res. 2010;16:3581–3593. doi: 10.1158/1078-0432.CCR-09-2912. [DOI] [PubMed] [Google Scholar]
- Demicco EG, Torres KE, Ghadimi MP. et al. Involvement of the PI3K/Akt pathway in myxoid/round cell liposarcoma. Mod Pathol. 2012;25:212–221. doi: 10.1038/modpathol.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barretina J, Taylor BS, Banerji S. et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010;42:715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso F, Jones RL, Demetri GD. et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007;8:595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- Casali PG, Sanfilippo R, D’Incalci M. Trabectedin therapy for sarcomas. Curr Opin Oncol. 2010;22:342–346. doi: 10.1097/CCO.0b013e32833aaac1. [DOI] [PubMed] [Google Scholar]
- Grohar PJ, Griffin LB, Yeung C. et al. Ecteinascidin 743 interferes with the activity of EWS-FLI1 in Ewing sarcoma cells. Neoplasia. 2011;13:145–153. doi: 10.1593/neo.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso F, Sanfilippo R, Virdis E. et al. Trabectedin in myxoid liposarcomas (MLS): a long-term analysis of a single-institution series. Ann Oncol. 2009;20:1439–1444. doi: 10.1093/annonc/mdp004. [DOI] [PubMed] [Google Scholar]
- Demetri GD, Fletcher CD, Mueller E. et al. Induction of solid tumor differentiation by the peroxisome proliferator-activated receptor-gamma ligand troglitazone in patients with liposarcoma. Proc Natl Acad Sci U S A. 1999;96:3951–3956. doi: 10.1073/pnas.96.7.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge D, Skamene T, Nahal A, Turcotte RE, Powell T, Freeman C. Radiological and pathological response following pre-operative radiotherapy for soft-tissue sarcoma. Radiother Oncol. 2010;97:404–407. doi: 10.1016/j.radonc.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Downs-Kelly E, Goldblum JR, Patel RM. et al. The utility of fluorescence in situ hybridization (FISH) in the diagnosis of myxoid soft tissue neoplasms. Am J Surg Pathol. 2008;32:8–13. doi: 10.1097/PAS.0b013e3181578d5a. [DOI] [PubMed] [Google Scholar]
- Weaver J, Downs-Kelly E, Goldblum JR. et al. Fluorescence in situ hybridization for MDM2 gene amplification as a diagnostic tool in lipomatous neoplasms. Mod Pathol. 2008;21:943–949. doi: 10.1038/modpathol.2008.84. [DOI] [PubMed] [Google Scholar]
- Gronchi A, Bui BN, Bonvalot S. et al. Phase II clinical trial of neoadjuvant trabectedin in patients with advanced localized myxoid liposarcoma. Ann Oncol. 2012;23:771–776. doi: 10.1093/annonc/mdr265. [DOI] [PubMed] [Google Scholar]
- Forni C, Minuzzo M, Virdis E. et al. Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol Cancer Ther. 2009;8:449–457. doi: 10.1158/1535-7163.MCT-08-0848. [DOI] [PubMed] [Google Scholar]
- Gerwirtz D. A Critical Evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/S0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Kerbusch T, de Kraker J, Keizer HJ. et al. Clinical pharmacokinetics and pharmacodynamics of ifosfamide and its metabolites. Clin Pharmacakinet. 2001;40:41–62. doi: 10.2165/00003088-200140010-00004. [DOI] [PubMed] [Google Scholar]
- Gamba-Vitalo C, Blair OC, Titton TR, Lane PA, Carbone R, Sartorelli AC. Cytotoxicity and differentiating actions of adriamycin in WEH1-3B D + leukemia cells. Leukemia. 1987;1:188–197. [PubMed] [Google Scholar]
- Fornari FA, Jarvis WD, Grant S. et al. Induction of differentiation and growth arrest associated with nascent (nonoliogsomal) DNA fragmentation and reduced c-myc expression in MCF-7 human breast tumor cells after continous exposure to a sublethal concentration of doxorubicin. Cell Growth & Differentiation. 1994;5:723–733. [PubMed] [Google Scholar]
- Smith LM, Anderson JR, Coffin CM. Cytodifferentiation and clinical outcome after chemotherapy and radiation therapy for rhabdomyosarcoma (RMS) Med Pediatr Oncol. 2002;38:398–404. doi: 10.1002/mpo.10060. [DOI] [PubMed] [Google Scholar]
- Huang HY, Antonescu CR. Molecular variability of TLS-CHOP structure shows no significant impact on the level of adipogenesis: a comparative ultrastructural and RT-PCR analysis of 14 cases of myxoid/round cell liposarcomas. Ultrastruct Pathol. 2003;27:217–226. doi: 10.1080/01913120309917. [DOI] [PubMed] [Google Scholar]