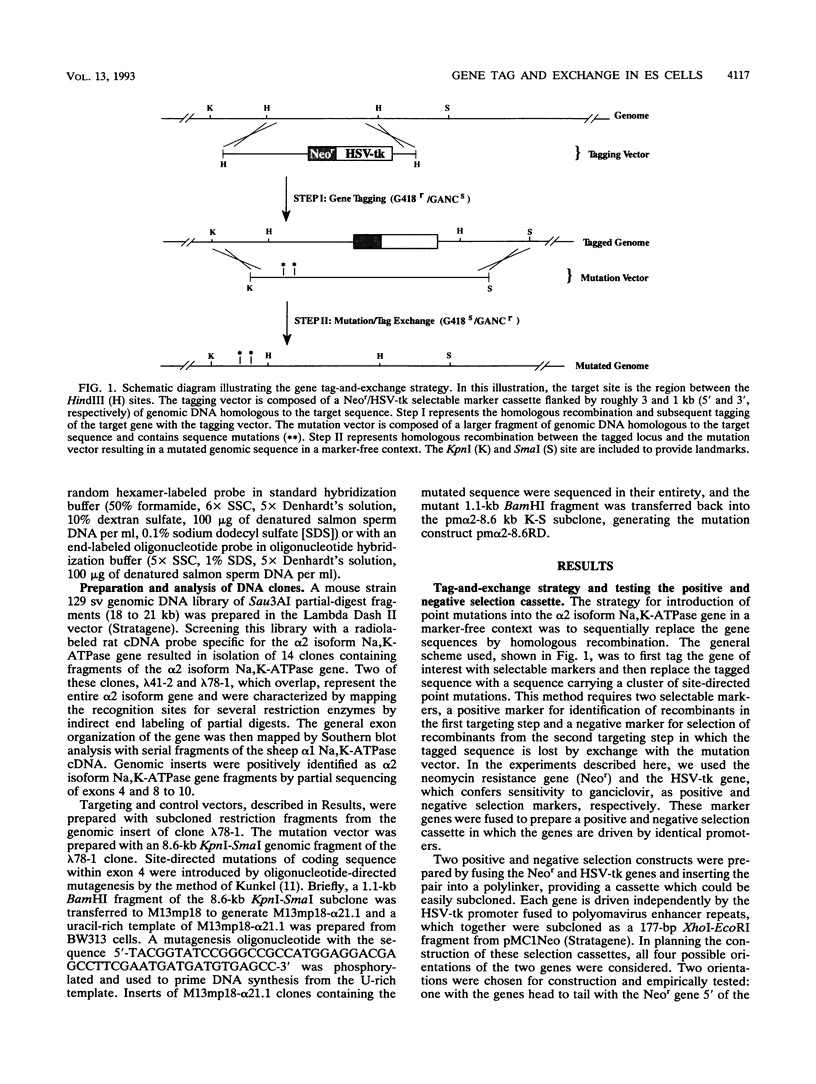
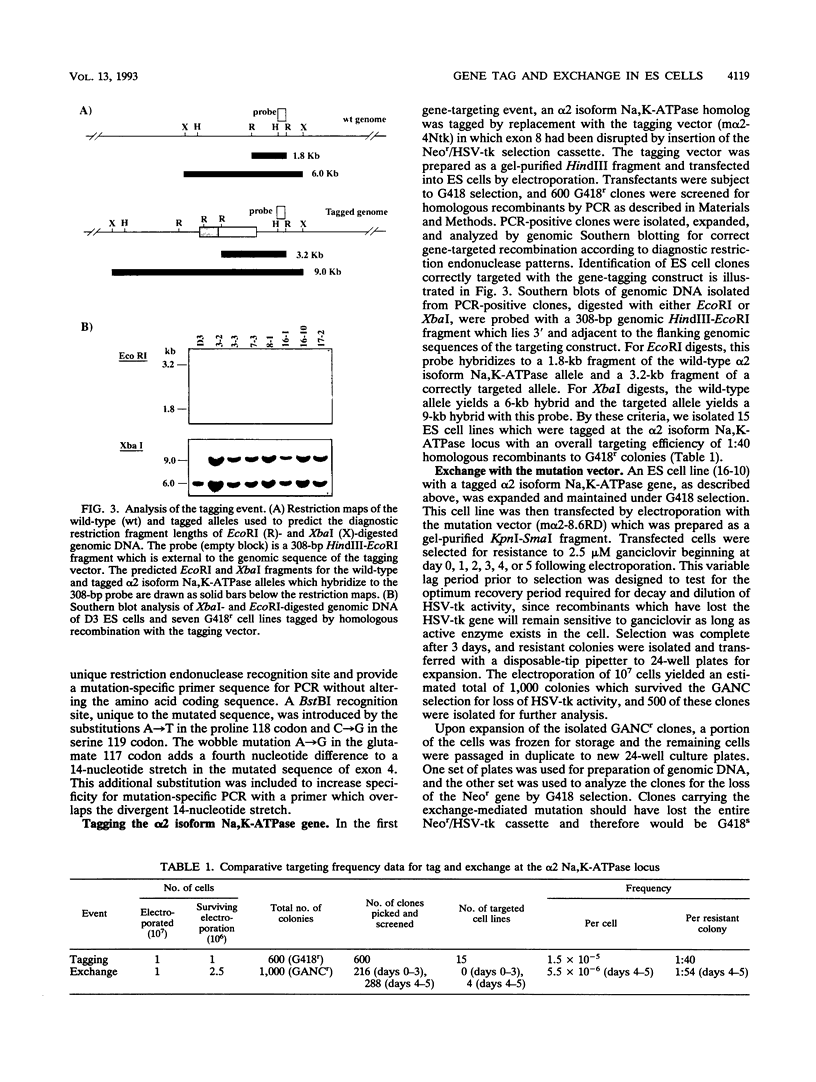
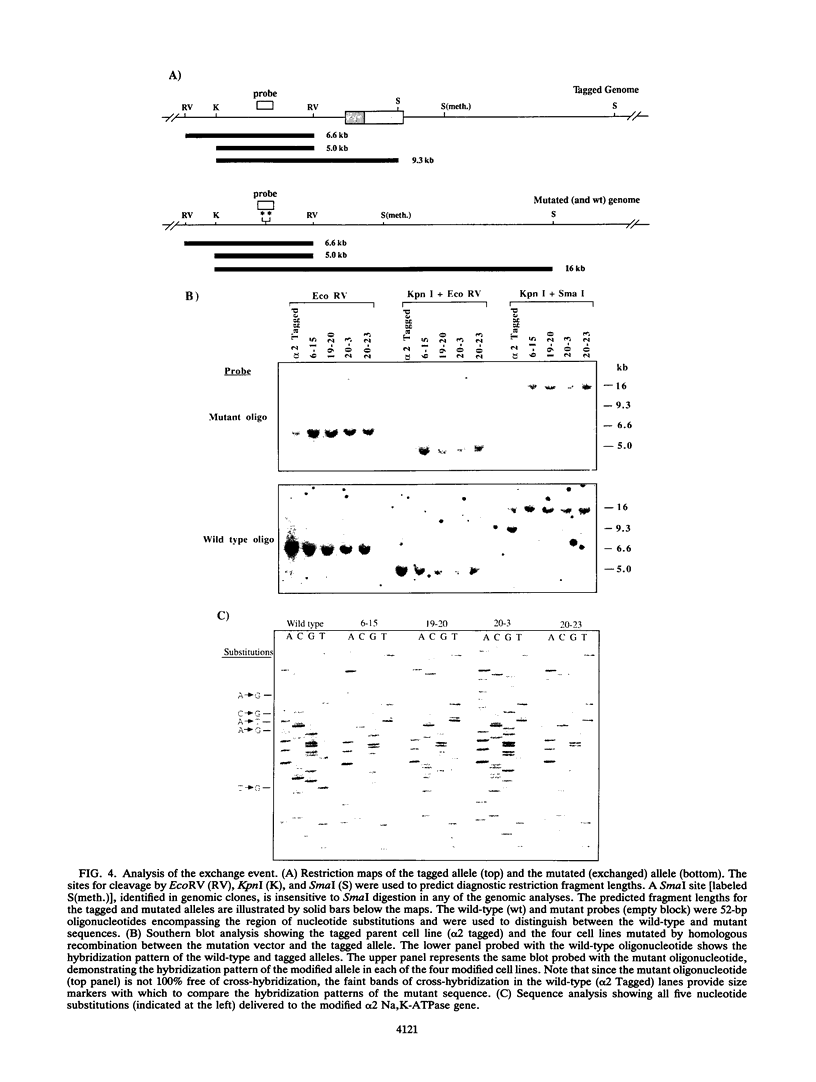
Abstract
Sequential gene targeting was used to introduce point mutations into one alpha 2 isoform Na,K-ATPase homolog in mouse embryonic stem (ES) cells. In the first round of targeted replacement, the gene was tagged with selectable markers by insertion of a Neor/HSV-tk gene cassette, and this event was selected for by gain of neomycin (G418) resistance. In the second targeted replacement event, the tagged genomic sequence was exchanged with a vector consisting of homologous genomic sequences carrying five site-directed nucleotide substitutions. Embryonic stem cell clones modified by exchange with the mutation vector were selected for loss of the HSV-tk gene by resistance to ganciclovir. Candidate clones were further screened and identified by polymerase chain reaction and Southern blot analysis. By this strategy, the endogenous alpha 2 isoform Na,K-ATPase gene was altered to encode two other amino acids so that the enzyme is resistant to inhibition by cardiac glycosides while maintaining its transmembrane ion-pumping function. Since the initial tagging event and the subsequent mutation-exchange event are independent of one another, a tagged cell line can be used to generate a variety of mutant lines by exchange with various mutation vectors at the tagged locus. This method should be useful for testing specific mutations introduced into the genomes of tissue culture cells and animals and for developing animal models encompassing the mutational variability of known genetic disorders.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun R. E., Lo D., Pinkert C. A., Widera G., Flavell R. A., Palmiter R. D., Brinster R. L. Infertility in male transgenic mice: disruption of sperm development by HSV-tk expression in postmeiotic germ cells. Biol Reprod. 1990 Oct;43(4):684–693. doi: 10.1095/biolreprod43.4.684. [DOI] [PubMed] [Google Scholar]
- Cole R. J., Paul J. The effects of erythropoietin on haem synthesis in mouse yolk sac and cultured foetal liver cells. J Embryol Exp Morphol. 1966 Apr;15(2):245–260. [PubMed] [Google Scholar]
- Davis A. C., Wims M., Bradley A. Investigation of coelectroporation as a method for introducing small mutations into embryonic stem cells. Mol Cell Biol. 1992 Jun;12(6):2769–2776. doi: 10.1128/mcb.12.6.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C., Capecchi M. R. Reexamination of gene targeting frequency as a function of the extent of homology between the targeting vector and the target locus. Mol Cell Biol. 1992 Aug;12(8):3365–3371. doi: 10.1128/mcb.12.8.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Evans M. J. Potential for genetic manipulation of mammals. Mol Biol Med. 1989 Dec;6(6):557–565. [PubMed] [Google Scholar]
- Gossler A., Doetschman T., Korn R., Serfling E., Kemler R. Transgenesis by means of blastocyst-derived embryonic stem cell lines. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9065–9069. doi: 10.1073/pnas.83.23.9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P., Ramírez-Solis R., Krumlauf R., Bradley A. Introduction of a subtle mutation into the Hox-2.6 locus in embryonic stem cells. Nature. 1991 Mar 21;350(6315):243–246. doi: 10.1038/350243a0. [DOI] [PubMed] [Google Scholar]
- Jiang L., Connor A., Shulman M. J. Effects of mutation position on frequency of marker rescue by homologous recombination. Mol Cell Biol. 1992 Aug;12(8):3609–3613. doi: 10.1128/mcb.12.8.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W., Zijderveld A., Linders K., Rudnicki M. A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991 Aug 11;19(15):4293–4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L. Gene targeting in murine embryonic stem cells: introduction of specific alterations into the mammalian genome. Genet Anal Tech Appl. 1990 Dec;7(8):219–227. doi: 10.1016/0735-0651(90)90004-y. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Price E. M., Lingrel J. B. Structure-function relationships in the Na,K-ATPase alpha subunit: site-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988 Nov 1;27(22):8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- Ratcliff R., Evans M. J., Doran J., Wainwright B. J., Williamson R., Colledge W. H. Disruption of the cystic fibrosis transmembrane conductance regulator gene in embryonic stem cells by gene targeting. Transgenic Res. 1992 Jul;1(4):177–181. doi: 10.1007/BF02522536. [DOI] [PubMed] [Google Scholar]
- Reid L. H., Shesely E. G., Kim H. S., Smithies O. Cotransformation and gene targeting in mouse embryonic stem cells. Mol Cell Biol. 1991 May;11(5):2769–2777. doi: 10.1128/mcb.11.5.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh I. M., Lau B. W., Siegfried B. A., Valdes R., Jr Isolation of digoxin-like immunoreactive factors from mammalian adrenal cortex. J Biol Chem. 1991 Jul 25;266(21):13672–13678. [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990 Aug 30;346(6287):847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Deng C., Capecchi M. R. High-fidelity gene targeting in embryonic stem cells by using sequence replacement vectors. Mol Cell Biol. 1992 Jul;12(7):2919–2923. doi: 10.1128/mcb.12.7.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valancius V., Smithies O. Testing an "in-out" targeting procedure for making subtle genomic modifications in mouse embryonic stem cells. Mol Cell Biol. 1991 Mar;11(3):1402–1408. doi: 10.1128/mcb.11.3.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Hasty P., Brenneman M. A., Grompe M., Gibbs R. A., Wilson J. H., Bradley A. Fidelity of targeted recombination in human fibroblasts and murine embryonic stem cells. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8067–8071. doi: 10.1073/pnas.88.18.8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]
- Zimmer A., Gruss P. Production of chimaeric mice containing embryonic stem (ES) cells carrying a homoeobox Hox 1.1 allele mutated by homologous recombination. Nature. 1989 Mar 9;338(6211):150–153. doi: 10.1038/338150a0. [DOI] [PubMed] [Google Scholar]
- te Riele H., Maandag E. R., Berns A. Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5128–5132. doi: 10.1073/pnas.89.11.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deursen J., Wieringa B. Targeting of the creatine kinase M gene in embryonic stem cells using isogenic and nonisogenic vectors. Nucleic Acids Res. 1992 Aug 11;20(15):3815–3820. doi: 10.1093/nar/20.15.3815. [DOI] [PMC free article] [PubMed] [Google Scholar]