Abstract
Background
CD133 was recently reported to be a cancer stem cell marker and a prognostic marker for several tumors. However, few studies have investigated CD133 expression in esophageal squamous cell carcinoma (ESCC). Therefore, we examined whether CD133 could serve as a prognostic marker of ESCC and investigated the correlation between CD133 expression and the clinicopathological findings of ESCC patients and several markers.
Methods
We studied 86 ESCC patients who underwent curative surgery without neoadjuvant treatment at Tohoku University Hospital (Sendai, Japan) between January 2000 and December 2005. We analyzed tissue specimens by immunohistochemical staining for CD133, p53, p16, p27, murine double minute 2 (MDM2), Ki-67, and epidermal growth factor receptor (EGFR).
Results
Pathological tumor depth and tumor stage were significantly more advanced among CD133-negative patients than among CD133-positive patients. A log-rank test showed that CD133 immunoreactivity was significantly correlated with the overall survival of the patients (P = 0.049). However, multivariate analysis showed that it was not significantly correlated (P = 0.078). Moreover, CD133 was significantly positively correlated with p27 immunoreactivity (P = 0.0013) and tended to be positively correlated with p16 immunoreactivity (P = 0.057). In addition, p16 immunoreactivity was correlated with smoking history (P = 0.018), pathological lymph node status (P = 0.033), and lymphatic invasion (P = 0.018).
Conclusions
This study indicated that CD133 immunoreactivity is a good predictor of prognosis in ESCC patients. In addition, CD133 may play a role in the regulation of tumor cell cycle through p27 and p16 in ESCC. At present, it thus remains controversial whether CD133 expression is a valid prognostic marker for ESCC. To elucidate this relationship, further investigations are required.
Keywords: AC133, Esophagus, Prominin-1, p16, p27, Stem cell marker
Background
Prognosis or clinical outcome of esophageal squamous cell carcinoma (ESCC) has markedly improved over the last several decades, owing to advancements in medical treatment. However, in Japan, 11,867 people succumbed to this disease in 2010, and esophageal cancer was the seventh most common cause of cancer mortality in men (3.4% of the total cancer deaths in Japan) [1]. Various prognostic markers have recently been evaluated, including the stem cell marker CD133 (Prominin-1), which was reported to be a cancer stem cell marker for cancers of the brain [2], colon [3,4], prostate [5], liver [6,7], lung [8], kidney [9], ovaries [10], and skin [11,12]. It was also reported to be a marker of poor prognosis for cancers of the brain and spinal cord [13], colon [14], rectum [15], pancreas [16], breast [17], and stomach [18]. In addition, a potent cytotoxic drug, monomethyl auristatin F, which acts as an anti-CD133 antibody-drug conjugate for hepatocellular and gastric cancer cells, may be utilized to treat CD133-positive tumors [19]. To date, there have been limited studies of CD133 in esophageal cancer, and thus, the significance of CD133 in this form of cancer remains unclear. Therefore, we examined whether CD133 could serve as a prognostic marker of ESCC. In addition, we explored the correlation between CD133 expression and the clinicopathological findings of ESCC patients and the correlation between CD133 expression and the immunolocalization of several markers, such as p53, p16, p27, murine double minute 2 (MDM2), Ki-67, and epidermal growth factor receptor (EGFR), which are known as prognostic markers or tumor proliferation factors in ESCC [20-27].
Methods
Patients and tissue samples
A total of 86 consecutive ESCC patients, who underwent curative surgery without neoadjuvant treatment at Tohoku University Hospital (Sendai, Japan) between January 2000 and December 2005, were selected. All patients underwent thoracoscopic esophagectomy with two- or three-field node dissection, except for four patients who underwent pharyngo-laryngo-esophagectomy with one-field node dissection, six patients who underwent transhiatal esophagectomy with one-field node dissection, and eight patients who underwent esophagectomy by right thoracotomy.
The resected specimens and lymph nodes were fixed in 10% formalin, and representative sections were embedded in paraffin wax. The sections were histologically examined according to the Union for International Cancer Control (UICC) TNM (tumor, node, metastasis) classification (7th edition) system [28]. Patient survival time was determined from the date of surgery until death, recurrence, or the last follow-up examination. This study was approved by the ethical committee of the Tohoku University Hospital (Accession number 2011–596).
Immunohistochemical staining and evaluation
Serial sections (4 μm thick), including the deepest area of the tumors, were deparaffinized in xylene, rehydrated in graded alcohol, and immersed in 3.0% hydrogen peroxide in methanol for 10 min at room temperature (RT) to inhibit endogenous peroxidase activity. For antigen retrieval, the slides for p53 were heated by microwave irradiation at 95°C for 15 min in 0.01 M citrate buffer (pH 6.0). The slides for p16, p27, MDM2, and Ki-67 were heated by autoclave at 121°C for 5 min in 0.01 M citrate buffer (pH 6.0). The slides for CD133 were autoclaved at 121°C for 5 min in Histofine antigen retrieval solution (pH 9.0, Nichirei Biosciences Inc., Tokyo, Japan). The slides for EGFR were incubated in 0.05% protease in Tris–HCl buffer (pH 7.6) at 37°C for 10 min. After washing three times for 5 min each in phosphate-buffered saline (PBS), the slides were incubated in 1% normal rabbit serum for 30 min at RT to reduce nonspecific antibody binding and were subsequently incubated at 4°C overnight with mouse monoclonal antibody against p53 (DO-7, Leica Microsystems, Bannockburn, IL, USA, diluted 1/100), p16 (G175-1239, BD Biosciences, Franklin Lakes. NJ, USA, diluted 1/100), p27 (SX53G8, Dako, Glostrup, Denmark, diluted 1/800), MDM2 (SMP14, Santa Cruz Biotechnology Inc., CA, USA, diluted 1/1000), Ki-67 (MIB-1, Dako, diluted 1/300), EGFR (31G7, Nichirei Biosciences Inc., dilution unknown, product code 413701), CD133 (AC133, Miltenyi Biotec, Auburn, CA, USA, diluted 1/10). The following day, the sections were washed three times for 5 min each in PBS, incubated with biotinylated anti-mouse immunoglobulin (Nichirei Biosciences Inc.), washed three times for 5 min each in PBS, and incubated with peroxidase-labeled streptavidin (Nichirei Biosciences Inc.) for 30 min at RT. The immunohistochemical signal was visualized with 3,3′-diaminobenzidine, and the slides were counterstained with Mayer’s hematoxylin, dehydrated in graded alcohol, and cleared in xylene. For CD133, omission of the primary antibody and substitution by nonspecific immunoglobulin (Mouse IgG1, Dako) at the same concentration were used as negative and isotype controls, respectively.
The sections were examined by two independent observers (HO and FF) who were blinded to patients’ clinical information. The proportion of positive nuclei in more than 1,000 tumor cells of more than three fields under a ×400 magnification microscope (Leica DM LB2) at the deepest area of each tumor was calculated for p53, p27, MDM2, and Ki-67. The proportion of nuclei and cytoplasm of tumor cells positive for p16 was evaluated. The proportion of membranes of tumor cells positive for EGFR was evaluated. The cut-off values for abnormal expression were as follows: p53, ≥10% [21]; p16, ≤5% [26]; p27, ≥10% [25]; MDM2, ≥20% [29]; and Ki-67, ≥30% [27]. An immunoreactive score (IRS) was used for the scoring of EGFR. The IRS is obtained by multiplying the intensity score (0, no staining; 1, faint staining; 2, moderate staining; 3, strong staining) by the extent score (0, none; 1, <10%; 2, 10 to 50%; 3, >50 to 80%; 4, >80%) and ranges from 1 to 12. An IRS of ≥6 was defined as positive for EGFR expression [20]. When evaluating CD133, the tumors were defined as positive and negative when >1% and ≤1% of the membranes and cytoplasm of all tumor cells were immunostained, respectively [13,30].
Statistical analyses
Statistical analyses were performed using JMP Pro Version 9.0.2 (SAS Institute Inc., Cary, NC, USA). The correlation of factors was evaluated by the chi-square test, Fisher’s exact test, or Wilcoxon test, as appropriate. Survival curves were determined by the Kaplan-Meier method, and differences in survival between groups were compared by the log-rank test. The Cox proportional hazard model was used for multivariate analysis. A P value of <0.05 was considered statistically significant.
Results
Correlation between CD133 and clinicopathological findings of patients
Table 1 summarizes the clinicopathological findings of the patients examined. The median follow-up time was 69.0 months (range, 1 to 149 months). The patients included 73 men and 13 women with a median age of 64 years (range, 37 to 81 years). The number of patients in each pathological stage was as follows: 20, pStageI; 28, pStageII; 33, pStageIII; and 5, pStageIV. There were five patients with M1 lymph nodes. Of the 86 patients, 38 (44.2%) were immunohistochemically positive for CD133 (Figure 1). pT and pStage were significantly more advanced among CD133-negative patients compared with CD133-positive patients (Table 1).
Table 1.
Correlation between clinicopathological findings and CD133 status
| |
|
Total (%) |
CD133 expression |
|
P |
|---|---|---|---|---|---|
| |
|
|
Positive |
Negative |
|
| n = 38 (44.2%) | n = 48 (55.8%) | ||||
|
Age (years) |
Mean ± SD |
63.9 ± 9.4 |
64.8 ± 10.9 |
63.2 ± 8.0 |
0.45 |
| |
(Range) |
(37–81) |
(37–9) |
(44–81) |
|
|
Sex |
Male |
73 (84.9) |
32 (84.2) |
41 (85.4) |
0.88 |
| Female |
13 (15.1) |
6 (15.8) |
7 (14.9) |
||
|
Smoking |
Absent |
13 (15.1) |
8 (21.1) |
5 (10.4) |
0.17 |
| Present |
73 (84.9) |
30 (79.0) |
43 (89.6) |
||
|
Location |
Cervix |
5 (5.8) |
3 (7.9) |
2 (4.2) |
0.59 |
| Upper |
5 (5.8) |
1 (2.6) |
4 (8.3) |
||
| Middle |
35 (40.7) |
17 (44.7) |
18 (37.5) |
||
| Lower |
41 (47.7) |
17 (44.7) |
24 (50.0) |
||
|
Histological type |
Well |
17 (19.8) |
9 (23.7) |
8 (16.7) |
0.68 |
| Moderate |
56 (65.1) |
23 (60.5) |
33 (68.8) |
||
| Poor |
13 (15.1) |
6 (15.8) |
7 (14.6) |
||
|
pT |
pT1 |
28 (32.6) |
19 (50.0) |
9 (18.8) |
0.017 |
| pT2 |
9 (10.5) |
3 (7.9) |
6 (12.5) |
||
| pT3 |
46 (53.5) |
15 (39.5) |
31 (64.6) |
||
| pT4 |
3 (3.5) |
1 (2.6) |
2 (4.2) |
||
|
pN |
pN0 |
36 (41.9) |
19 (50.0) |
17 (35.4) |
0.17 |
| pN1-3 |
50 (58.1) |
19 (50.0) |
31 (64.6) |
||
|
pM |
pM0 |
81 (94.2) |
35 (92.1) |
46 (95.8) |
0.65 |
| pM1 (LYM) |
5 (5.8) |
3 (7.9) |
2 (4.2) |
||
|
pStage |
pStageI |
20 (23.3) |
14 (36.8) |
6 (12.5) |
0.035 |
| pStageII |
28 (32.6) |
9 (23.7) |
19 (39.6) |
||
| pStageIII |
33 (38.4) |
12 (31.6) |
21 (43.8) |
||
| pStageIV |
5 (5.8) |
3 (7.9) |
2 (4.2) |
||
|
Lymphatic invasion |
Negative |
34 (39.5) |
13 (34.2) |
21 (43.8) |
0.37 |
| Positive |
52 (60.5) |
25 (65.8) |
27 (56.3) |
||
|
Venous invasion |
Negative |
31 (36.1) |
11 (29.0) |
20 (41.7) |
0.22 |
| Positive | 55 (64.0) | 27 (71.1) | 28 (58.3) |
SD, standard deviation.
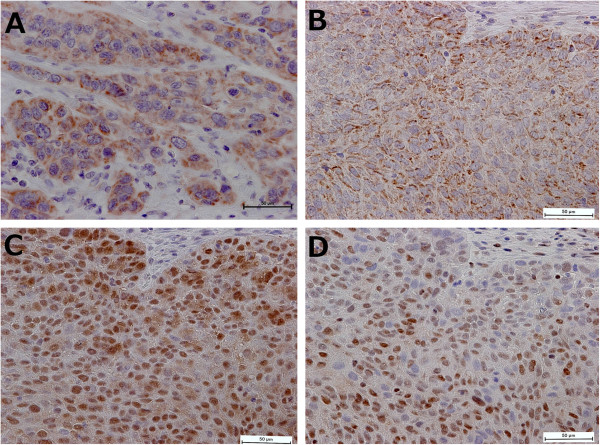
Figure 1.
Immunohistochemical staining of esophageal squamous cell carcinoma. Tumor cells positive for CD133 (A,B), p16 (C), and p27 (D) expression (×400 magnification). In addition, B, C, and D were at the same site of the same tumor.
Correlation between CD133 and other markers
Table 2 summarizes the correlation between expression of CD133 and expression of other molecular markers examined. CD133 and p27 expression were positively correlated (P = 0.0013), and CD133 and p16 expression tended to be positively correlated (P = 0.057) but did not reach statistical significance. No significant correlations were detected between expression of CD133 and expression of any other marker.
Table 2.
Correlation between expression of CD133 and expression of other molecular markers
| |
|
Total (%) |
CD133 expression |
|
P |
|---|---|---|---|---|---|
| |
|
|
Positive |
Negative |
|
| n = 38 (44.2%) | n = 48 (55.8%) | ||||
|
p53 |
Negative |
29 (33.7) |
13 (34.2) |
16 (33.3) |
0.93 |
| |
Positive |
57 (66.3) |
25 (65.8) |
32 (66.7) |
|
|
p16 |
Negative |
69 (80.2) |
27 (71.1) |
42 (87.5) |
0.057 |
| |
Positive |
17 (19.8) |
11 (29.0) |
6 (12.5) |
|
|
p27 |
Negative |
37 (43.0) |
9 (23.7) |
28 (58.3) |
0.0013 |
| |
Positive |
49 (57.0) |
29 (76.3) |
20 (41.7) |
|
|
MDM2 |
Negative |
66 (76.7) |
31 (81.6) |
35 (72.9) |
0.35 |
| |
Positive |
20 (23.3) |
7 (18.4) |
13 (27.1) |
|
|
Ki-67 |
<30 |
43 (50.0) |
17 (44.7) |
26 (54.2) |
0.39 |
| |
≥30 |
43 (50.0) |
21 (55.3) |
22 (45.8) |
|
|
EGFR |
Negative |
46 (53.5) |
23 (60.5) |
23 (47.9) |
0.24 |
| Positive | 40 (46.5) | 15 (39.5) | 25 (52.1) |
Correlations for other molecular markers
In terms of correlations between the other molecular markers and clinicopathological findings, p16 expression was correlated with smoking history (P = 0.018), pathological lymph node status (P = 0.033), and lymphatic invasion (P = 0.018) (Additional file 1). With regard to correlations among other molecular markers, p53 expression was positively correlated positively with Ki-67 expression (P = 0.0030) (Additional file 2).
Survival analysis
The 3- and 5-year survival rates of all patients examined were 65.0% and 61.5%, respectively. Results of univariate analysis of postoperative overall survival (OS) and disease-free survival (DFS) are summarized in Table 3. Overall survival was significantly correlated with pT, pN, pStage, and CD133 status, and was significantly longer in CD133-positive patients than in CD133-negative patients (P = 0.049) (Figure 2). No significant correlation between OS and the other markers was observed (Figure 3). Multivariate analysis demonstrated that pStage was a significant prognostic factor for OS and that pStage and tumor location were significant prognostic factors for DFS. Correlation between CD133 expression and patient survival did not reach statistical significance by multivariate analysis (Table 4).
Table 3.
Univariate survival analysis of clinicopathological findings and expression of molecular markers
| |
Variables |
Number |
Overall survival |
Disease-free survival |
||
|---|---|---|---|---|---|---|
| 5-year overall survival rate (%) | P | 5-year disease-free survival rate (%) | P | |||
| Age (years) |
≤60 |
29 |
72.4 |
0.11 |
62.1 |
0.21 |
| |
>60 |
57 |
55.9 |
52.5 |
||
| Sex |
Male |
73 |
58.7 |
0.13 |
51.9 |
0.080 |
| |
Female |
13 |
76.9 |
76.9 |
||
| Location |
Cervix/upper |
10 |
50.0 |
0.12 |
40.0 |
0.067 |
| |
Middle/lower |
76 |
63.0 |
57.8 |
||
| Histological type |
Well or moderate |
73 |
61.5 |
0.64 |
57.4 |
0.36 |
| |
Poor |
13 |
61.5 |
46.2 |
||
| pT |
pT1/pT2 |
37 |
78.1 |
0.0012 |
70.0 |
0.0033 |
| |
pT3/pT4 |
49 |
49.0 |
44.9 |
||
| pN |
pN0 |
36 |
80.6 |
0.0051 |
75.0 |
0.0078 |
| |
pN1-3 |
50 |
47.7 |
41.7 |
||
| pStage |
pStageI/II |
48 |
79.0 |
0.0002 |
72.8 |
0.0002 |
| |
pStageIII/VI |
38 |
39.5 |
34.2 |
||
| Lymphatic invasion |
Negative |
34 |
70.2 |
0.086 |
58.4 |
0.29 |
| |
Positive |
52 |
55.8 |
53.9 |
||
| Venous invasion |
Negative |
31 |
64.5 |
0.47 |
54.8 |
0.63 |
| |
Positive |
55 |
59.8 |
56.2 |
||
| p53 |
Negative |
29 |
62.1 |
0.30 |
55.2 |
0.55 |
| |
Positive |
57 |
61.3 |
56.1 |
||
| p16 |
Negative |
69 |
58.0 |
0.19 |
53.6 |
0.14 |
| |
Positive |
17 |
76.0 |
63.7 |
||
| p27 |
Negative |
37 |
56.8 |
0.30 |
54.0 |
0.49 |
| |
Positive |
49 |
65.0 |
56.9 |
||
| MDM2 |
Negative |
66 |
63.6 |
0.31 |
59.1 |
0.61 |
| |
Positive |
20 |
53.3 |
59.2 |
||
| Ki-67 |
<30 |
43 |
69.8 |
0.24 |
62.8 |
0.27 |
| |
≥30 |
43 |
53.2 |
48.6 |
||
| EGFR |
Negative |
46 |
69.6 |
0.22 |
63.0 |
0.16 |
| |
Positive |
40 |
52.0 |
47.1 |
||
| CD133 |
Negative |
48 |
54.2 |
0.049 |
47.9 |
0.059 |
| Positive | 38 | 70.8 | 65.5 | |||
Figure 2.
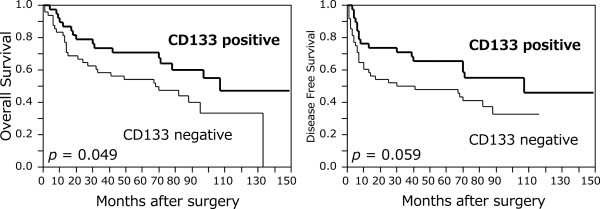
Kaplan-Meier curves of patients with esophageal squamous cell carcinoma according to CD133 expression. Overall survival was significantly longer in CD133-positive patients than in CD133-negative patients (P = 0.049). There was no significant correlation between disease-free survival and CD133 status (P = 0.059).
Figure 3.
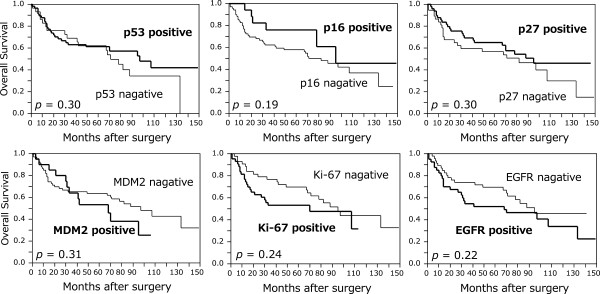
Kaplan-Meier curves of patients with esophageal squamous cell carcinoma according to expression of the other markers. No significant correlation between overall survival and the other markers was observed.
Table 4.
Multivariate survival analysis of clinicopathological findings and expression of molecular markers
| Variables | Hazard ratio | 95% confidence interval | P | |
|---|---|---|---|---|
|
Overall survival |
pStageIII/IV |
2.64 |
1.42-5.03 |
0.0020 |
| Lymphatic invasion positive |
1.48 |
0.79-2.89 |
0.23 |
|
| CD133 negative |
1.74 |
0.94-3.35 |
0.078 |
|
|
Disease-free survival |
Male |
2.09 |
0.84-6.99 |
0.12 |
| |
Cervix/upper |
2.51 |
1.07-5.21 |
0.035 |
| |
pStageIII/IV |
2.93 |
1.62-5.42 |
0.0004 |
| CD133 negative | 1.73 | 0.96-3.24 | 0.071 |
Discussion
CD133 was originally identified as a transmembrane glycoprotein in normal hematopoietic stem and progenitor cells [31] that participated in proliferation, self-renewal, and multilineage differentiation [32]. Furthermore, CD133 was recently used to identify putative cancer stem cells of several tumors [33]. According to several studies, CD133 was associated with tumor differentiation in several organs [16,34-36]. For example, Jiang et al. [36] reported that CD133 expression was increased in diffuse-type gastric cancers compared with intestinal-type cancers and was increased more so in poorly differentiated than in moderately or well differentiated gastric cancers. In addition, Feng et al. [35] reported that CD133 was negatively correlated with the cellular differentiation status of colon cancer cells. Finally, Fan et al. [34] reported that CD133 expression was correlated with well differentiated or moderately differentiated cholangiocarcinomas and that subcellular CD133 localization was correlated with the tumor differentiation status. In terms of ESCC, Hang et al. reported that CD133 expression was increased in well differentiated and moderately differentiated ESCCs compared with poorly differentiated ESCCs [30]. However, in our study, no correlation was detected between CD133 expression and tumor differentiation of carcinoma cells. We think that this was because there is a difference among pathologists or facilities regarding the histological evaluation of tumor differentiation status, and in addition, our study was small. On the other hand, CD133 expression correlated with p27 expression (P = 0.0013) and tended to correlate with the status of p16 immunoreactivity (P = 0.057). The relationship between CD133 and cell cycle regulators has remained unclear in esophageal cancer. There may be a correlation between CD133 and cell cycle pathways associated with the INK4 family or the CIP/KIP family of cyclin-dependent kinase inhibitors [37], but this possibility requires further investigation.
To the best of our knowledge, there are few reports that have investigated the effect of CD133 expression on survival of ESCC patients. Nakajima et al. [38] reported that CD133 expression in resected ESCC specimens following neoadjuvant chemoradiotherapy tended to be correlated with poor prognosis, but multivariate analysis did not produce a significant correlation. In contrast, CD133 expression was significantly correlated with poor response to neoadjuvant chemoradiotherapy. Hang et al. [30] reported that CD133 expression in resected ESCC specimens without preoperative treatment was not significantly correlated with prognosis. Our study revealed that OS was significantly longer in CD133-positive patients than in CD133-negative patients, as determined by log-rank test. One reason for this was that the tumors were significantly more advanced (according to their pStage classification) in CD133-negative patients than in CD133-positive patients. Although correlation between CD133 expression and patient survival did not reach statistical significance, as determined by multivariate analysis, CD133 immunoreactivity may have the potential to be a good predictor of prognosis in ESCC patients. With regard to other tumors, CD133 expression in non-small cell lung cancers [39,40], hepatocellular carcinomas [18], and pancreatic cancers [41] was not correlated with patient survival. Moreover, CD133-negative expression in cholangiocarcinomas was correlated with poor prognosis [34], which is similar to that revealed in our study. Further studies are needed to clarify this issue.
Conclusions
In conclusion, this study demonstrated that CD133 immunoreactivity may have the potential to be a good predictor of prognosis in ESCC patients, and that CD133 may play a role in the regulation of tumor cell cycle through p27 and p16 in ESCC. At present, whether CD133 expression is a valid prognostic marker for ESCC remains controversial. To elucidate this relationship, further investigations are required, including verification of an evaluation method for CD133 immunoreactivity in ESCC.
Consent
Written informed consent concerning the procedure of this study was obtained from all patients prior to study enrollment.
Abbreviations
DFS: Disease-free survival; EGFR: Epidermal growth factor receptor; ESCC: Esophageal squamous cell carcinoma; IRS: Immunoreactive score; MDM2: Murine double minute 2; OS: Overall survival; PBS: Phosphate-buffered saline; RT: Room temperature; TNM: Tumor, node, metastasis; UICC: Union for International Cancer Control.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HO is the main author of this article. HO and FF conceived this study. FF and YN supervised the manuscript writing. MZ, YO, GM, TK, TN, YT, and JT contributed to the collection of clinical information and data analysis. HO and YT performed the experiments. HO and FF performed the pathological examination and immunohistochemical evaluation. MW, AS, NO, and HS reviewed the manuscript and revised it thoroughly. All authors have read and approved the final manuscript.
Supplementary Material
Correlation between clinicopathological findings and p16 status. p16 expression was correlated with smoking history (P = 0.018), advanced pN (P = 0.033), and lymphatic invasion (P = 0.018).
Correlation between p53 status and Ki-67 status, MDM2 status. p53 expression was positively correlated with Ki-67 expression (P = 0.0030).
Contributor Information
Hiroshi Okamoto, Email: hi_ok_0531@yahoo.co.jp.
Fumiyoshi Fujishima, Email: ffujishima@patholo2.med.tohoku.ac.jp.
Yasuhiro Nakamura, Email: yasu-naka@patholo2.med.tohoku.ac.jp.
Masashi Zuguchi, Email: zugumasa@yahoo.co.jp.
Yohei Ozawa, Email: ozawa.youhei@opal.plala.or.jp.
Yayoi Takahashi, Email: ytakahashi@patholo2.med.tohoku.ac.jp.
Go Miyata, Email: miyata5@med.tohoku.ac.jp.
Takashi Kamei, Email: tkamei@med.tohoku.ac.jp.
Toru Nakano, Email: torun@med.tohoku.ac.jp.
Yusuke Taniyama, Email: yusuketaniyama@med.tohoku.ac.jp.
Jin Teshima, Email: jintesshy@yahoo.co.jp.
Mika Watanabe, Email: mkawatan@patholo2.med.tohoku.ac.jp.
Akira Sato, Email: attkas@med.tohoku.ac.jp.
Noriaki Ohuchi, Email: noriaki-ohuchi@med.tohoku.ac.jp.
Hironobu Sasano, Email: hsasano@patholo2.med.tohoku.ac.jp.
Acknowledgements
We thank the staff of the Department of Pathology, Tohoku University Hospital for their excellent technical assistance and Enago ( http://www.enago.jp) for an English language review.
References
- Cancer Statistics in Japan 2011. http://ganjoho.jp/public/statistics/backnumber/2011_en.html.
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, De Maria R. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820–824. doi: 10.1016/j.bbrc.2006.10.128. [DOI] [PubMed] [Google Scholar]
- Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D, Yang S, Zheng S, Gu J. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444–1450. doi: 10.1002/ijc.22476. [DOI] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- Florek M, Haase M, Marzesco AM, Freund D, Ehninger G, Huttner WB, Corbeil D. Prominin-1/CD133, a neural and hematopoietic stem cell marker, is expressed in adult human differentiated cells and certain types of kidney cancer. Cell Tissue Res. 2005;319:15–26. doi: 10.1007/s00441-004-1018-z. [DOI] [PubMed] [Google Scholar]
- Ferrandina G, Bonanno G, Pierelli L, Perillo A, Procoli A, Mariotti A, Corallo M, Martinelli E, Rutella S, Paglia A, Zannoni G, Mancuso S, Scambia G. Expression of CD133-1 and CD133-2 in ovarian cancer. Int J Gynecol Cancer. 2008;18:506–514. doi: 10.1111/j.1525-1438.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- Klein WM, Wu BP, Zhao S, Wu H, Klein-Szanto AJ, Tahan SR. Increased expression of stem cell markers in malignant melanoma. Mod Pathol. 2007;20:102–107. doi: 10.1038/modpathol.3800720. [DOI] [PubMed] [Google Scholar]
- Monzani E, Facchetti F, Galmozzi E, Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D, Santinami M, Invernici G, Parati E, Alessandri G, La Porta CA. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur J Cancer. 2007;43:935–946. doi: 10.1016/j.ejca.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Zeppernick F, Ahmadi R, Campos B, Dictus C, Helmke BM, Becker N, Lichter P, Unterberg A, Radlwimmer B, Herold-Mende CC. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14:123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- Horst D, Kriegl L, Engel J, Kirchner T, Jung A. CD133 expression is an independent prognostic marker for low survival in colorectal cancer. Br J Cancer. 2008;99:1285–1289. doi: 10.1038/sj.bjc.6604664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Sakakura C, Komiyama S, Miyashita A, Nishio M, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Ichikawa D, Fujiwara H, Okamoto K, Ochiai T, Kokuba Y, Sonoyama T, Otsuji E. Expression of cancer stem cell markers CD133 and CD44 in locoregional recurrence of rectal cancer. Anticancer Res. 2011;31:495–500. [PubMed] [Google Scholar]
- Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, Natsugoe S, Aikou T, Takao S. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008;98:1389–1397. doi: 10.1038/sj.bjc.6604307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieni A, Giuffre G, Adamo V, Tuccari G. Prognostic impact of CD133 immunoexpression in node-negative invasive breast carcinomas. Anticancer Res. 2011;31:1315–1320. [PubMed] [Google Scholar]
- Salnikov AV, Kusumawidjaja G, Rausch V, Bruns H, Gross W, Khamidjanov A, Ryschich E, Gebhard MM, Moldenhauer G, Büchler MW, Schemmer P, Herr I. Cancer stem cell marker expression in hepatocellular carcinoma and liver metastases is not sufficient as single prognostic parameter. Cancer Lett. 2009;275:185–193. doi: 10.1016/j.canlet.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Smith LM, Nesterova A, Ryan MC, Duniho S, Jonas M, Anderson M, Zabinski RF, Sutherland MK, Gerber HP, Van Orden KL, Moore PA, Ruben SM, Carter PJ. CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. Br J Cancer. 2008;99:100–109. doi: 10.1038/sj.bjc.6604437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson MK, Abraham SC, Wu TT, Burtness B, Heitmiller RF, Heath E, Forastiere A. Epidermal growth factor receptor, p53 mutation, and pathological response predict survival in patients with locally advanced esophageal cancer treated with preoperative chemoradiotherapy. Clin Cancer Res. 2003;9:6461–6468. [PubMed] [Google Scholar]
- Inada S, Koto T, Futami K, Arima S, Iwashita A. Evaluation of malignancy and the prognosis of esophageal cancer based on an immunohistochemical study (p53, E-cadherin, epidermal growth factor receptor) Surg Today. 1999;29:493–503. doi: 10.1007/BF02482343. [DOI] [PubMed] [Google Scholar]
- Inoue K, Ozeki Y, Suganuma T, Sugiura Y, Tanaka S. Vascular endothelial growth factor expression in primary esophageal squamous cell carcinoma. Association with angiogenesis and tumor progression. Cancer. 1997;79:206–213. doi: 10.1002/(SICI)1097-0142(19970115)79:2<206::AID-CNCR2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Research Committee on Malignancy of Esophageal Cancer, Japanese Society for Esophageal Diseases. Prognostic significance of CyclinD1 and E-Cadherin in patients with esophageal squamous cell carcinoma: multiinstitutional retrospective analysis. J Am Coll Surg. 2001;192:708–718. [PubMed] [Google Scholar]
- Saito H, Tsujitani S, Oka S, Ikeguchi M, Maeta M, Kaibara N. The expression of murine double minute 2 is a favorable prognostic marker in esophageal squamous cell carcinoma without p53 protein accumulation. Ann Surg Oncol. 2002;9:450–456. doi: 10.1007/BF02557267. [DOI] [PubMed] [Google Scholar]
- Shiozaki H, Doki Y, Kawanishi K, Shamma A, Yano M, Inoue M, Monden M. Clinical application of malignancy potential grading as a prognostic factor of human esophageal cancers. Surgery. 2000;127:552–561. doi: 10.1067/msy.2000.105028. [DOI] [PubMed] [Google Scholar]
- Taghavi N, Biramijamal F, Sotoudeh M, Khademi H, Malekzadeh R, Moaven O, Memar B, A'Rabi A, Abbaszadegan MR. p16INK4a hypermethylation and p53, p16 and MDM2 protein expression in esophageal squamous cell carcinoma. BMC Cancer. 2010;10:138. doi: 10.1186/1471-2407-10-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef EM, Matsuda T, Takada N, Osugi H, Higashino M, Kinoshita H, Watanabe T, Katsura Y, Wanibuchi H, Fukushima S. Prognostic significance of the MIB-1 proliferation index for patients with squamous cell carcinoma of the esophagus. Cancer. 1995;76:358–366. doi: 10.1002/1097-0142(19950801)76:3<358::AID-CNCR2820760303>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wittekind C, Yamasaki S. In: International Union Against Cancer TNM Classiication of Malignant Tumours. 7. Sobin LH, Gospodarowicz MK, Wittekind C, editor. New York: Wiley-Blackwell; 2009. Oesophagus including oesophagogastric junction; pp. 66–72. [Google Scholar]
- Osman I, Sherman E, Singh B, Venkatraman E, Zelefsky M, Bosl G, Scher H, Shah J, Shaha A, Kraus D, Cordon-Cardo C, Pfister DG. Alteration of p53 pathway in squamous cell carcinoma of the head and neck: impact on treatment outcome in patients treated with larynx preservation intent. J Clin Oncol. 2002;20:2980–2987. doi: 10.1200/JCO.2002.06.161. [DOI] [PubMed] [Google Scholar]
- Hang D, Dong HC, Ning T, Dong B, Hou DL, Xu WG. Prognostic value of the stem cell markers CD133 and ABCG2 expression in esophageal squamous cell carcinoma. Dis Esophagus. 2012;25:638–644. doi: 10.1111/j.1442-2050.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, Almeida-Porada G, Ogawa M, Leary AG, Olweus J, Kearney J, Buck DW. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- Bidlingmaier S, Zhu X, Liu B. The utility and limitations of glycosylated human CD133 epitopes in defining cancer stem cells. J Mol Med. 2008;86:1025–1032. doi: 10.1007/s00109-008-0357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrak D, Brittan M, Alison MR. CD133: molecule of the moment. J Pathol. 2008;214:3–9. doi: 10.1002/path.2283. [DOI] [PubMed] [Google Scholar]
- Fan L, He F, Liu H, Zhu J, Liu Y, Yin Z, Wang L, Guo Y, Wang Z, Yan Q, Huang G. CD133: a potential indicator for differentiation and prognosis of human cholangiocarcinoma. BMC Cancer. 2011;11:320. doi: 10.1186/1471-2407-11-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HL, Liu YQ, Yang LJ, Bian XC, Yang ZL, Gu B, Zhang H, Wang CJ, Su XL, Zhao XM. Expression of CD133 correlates with differentiation of human colon cancer cells. Cancer Biol Ther. 2010;9:216–223. doi: 10.4161/cbt.9.3.10664. [DOI] [PubMed] [Google Scholar]
- Jiang Y, He Y, Li H, Li HN, Zhang L, Hu W, Sun YM, Chen FL, Jin XM. Expressions of putative cancer stem cell markers ABCB1, ABCG2, and CD133 are correlated with the degree of differentiation of gastric cancer. Gastric Cancer. 2012;15:440–450. doi: 10.1007/s10120-012-0140-y. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 2000;60:3689–3695. [PubMed] [Google Scholar]
- Nakajima TE, Yoshida H, Okamoto N, Nagashima K, Taniguchi H, Yamada Y, Shimoda T, Masutomi K. Nucleostemin and TWIST as predictive markers for recurrence after neoadjuvant chemotherapy for esophageal carcinoma. Cancer Sci. 2012;103:233–238. doi: 10.1111/j.1349-7006.2011.02142.x. [DOI] [PubMed] [Google Scholar]
- Herpel E, Jensen K, Muley T, Warth A, Schnabel PA, Meister M, Herth FJ, Dienemann H, Thomas M, Gottschling S. The cancer stem cell antigens CD133, BCRP1/ABCG2 and CD117/c-KIT are not associated with prognosis in resected early-stage non-small cell lung cancer. Anticancer Res. 2011;31:4491–4500. [PubMed] [Google Scholar]
- Salnikov AV, Gladkich J, Moldenhauer G, Volm M, Mattern J, Herr I. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer. 2010;126:950–958. doi: 10.1002/ijc.24822. [DOI] [PubMed] [Google Scholar]
- Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. Expression of the ‘stem cell marker’ CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between clinicopathological findings and p16 status. p16 expression was correlated with smoking history (P = 0.018), advanced pN (P = 0.033), and lymphatic invasion (P = 0.018).
Correlation between p53 status and Ki-67 status, MDM2 status. p53 expression was positively correlated with Ki-67 expression (P = 0.0030).