Abstract
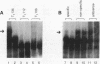
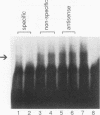
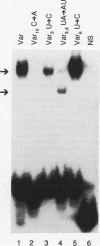
An activity from Saccharomyces cerevisiae mitochondria was identified that specifically bound to a 12-nucleotide sequence, AAUAA(U/C)AUUCUU, that is a site for processing of pre-mRNAs so as to generate the mature 3' ends of mRNAs. Because processing occurs 3' to the end of the dodecamer site, all mRNAs in yeast mitochondria terminate with that sequence. RNase T1 digestion fragments which terminated precisely at their 3' ends with the dodecamer sequence bound the activity, indicating that mRNAs in vivo would be capable of binding. Gel mobility shift analyses using RNA oligonucleotides showed that binding was reduced by a U-to-A substitution at position 3 of the dodecamer sequence; a C-to-A substitution at position 10 eliminated binding. UV cross-linking identified three polypeptides with approximate molecular masses of 19, 60, and 70 kDa as constituents of the binding activity. These estimates included the contribution of the 32P-labeled RNA oligonucleotide used to tag these polypeptides. An oligonucleotide with a UA-->AU substitution at positions 3 and 4 of the dodecamer site formed complexes deficient in the 19-kDa species, suggesting that binding specificity was inherent to the higher-molecular-weight polypeptides. Assembly of the complex at a dodecamer site on an RNA protected sequences located 5' to the dodecamer site from digestion by a nucleoside triphosphate-dependent 3' exoribonuclease found in yeast mitochondria. Since mitochondrial mRNAs terminate with an intact dodecamer sequence, the binding activity may function in the stabilization of mRNAs in addition to 3'-end formation of mRNAs.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein P., Ross J. Poly(A), poly(A) binding protein and the regulation of mRNA stability. Trends Biochem Sci. 1989 Sep;14(9):373–377. doi: 10.1016/0968-0004(89)90011-x. [DOI] [PubMed] [Google Scholar]
- Dake E., Hofmann T. J., McIntire S., Hudson A., Zassenhaus H. P. Purification and properties of the major nuclease from mitochondria of Saccharomyces cerevisiae. J Biol Chem. 1988 Jun 5;263(16):7691–7702. [PubMed] [Google Scholar]
- Dieckmann C. L., Mittelmeier T. M. Nuclearly-encoded CBP1 interacts with the 5' end of mitochondrial cytochrome b pre-mRNA. Curr Genet. 1987;12(6):391–397. doi: 10.1007/BF00434815. [DOI] [PubMed] [Google Scholar]
- Gillis P., Malter J. S. The adenosine-uridine binding factor recognizes the AU-rich elements of cytokine, lymphokine, and oncogene mRNAs. J Biol Chem. 1991 Feb 15;266(5):3172–3177. [PubMed] [Google Scholar]
- Grivell L. A. Nucleo-mitochondrial interactions in yeast mitochondrial biogenesis. Eur J Biochem. 1989 Jul 1;182(3):477–493. doi: 10.1111/j.1432-1033.1989.tb14854.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malter J. S. Identification of an AUUUA-specific messenger RNA binding protein. Science. 1989 Nov 3;246(4930):664–666. doi: 10.1126/science.2814487. [DOI] [PubMed] [Google Scholar]
- Min J., Heuertz R. M., Zassenhaus H. P. Isolation and characterization of an NTP-dependent 3'-exoribonuclease from mitochondria of Saccharomyces cerevisiae. J Biol Chem. 1993 Apr 5;268(10):7350–7357. [PubMed] [Google Scholar]
- Mueller D. M., Getz G. S. Steady state analysis of mitochondrial RNA after growth of yeast Saccharomyces cerevisiae under catabolite repression and derepression. J Biol Chem. 1986 Sep 5;261(25):11816–11822. [PubMed] [Google Scholar]
- Mueller D. M., Getz G. S. Transcriptional regulation of the mitochondrial genome of yeast Saccharomyces cerevisiae. J Biol Chem. 1986 Sep 5;261(25):11756–11764. [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Osinga K. A., De Vries E., Van der Horst G., Tabak H. F. Processing of yeast mitochondrial messenger RNAs at a conserved dodecamer sequence. EMBO J. 1984 Apr;3(4):829–834. doi: 10.1002/j.1460-2075.1984.tb01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs A. The role of poly(A) in the translation and stability of mRNA. Curr Opin Cell Biol. 1990 Dec;2(6):1092–1098. doi: 10.1016/0955-0674(90)90161-7. [DOI] [PubMed] [Google Scholar]
- Scaringe S. A., Francklyn C., Usman N. Chemical synthesis of biologically active oligoribonucleotides using beta-cyanoethyl protected ribonucleoside phosphoramidites. Nucleic Acids Res. 1990 Sep 25;18(18):5433–5441. doi: 10.1093/nar/18.18.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Stephens J. M., Carter B. Z., Pekala P. H., Malter J. S. Tumor necrosis factor alpha-induced glucose transporter (GLUT-1) mRNA stabilization in 3T3-L1 preadipocytes. Regulation by the adenosine-uridine binding factor. J Biol Chem. 1992 Apr 25;267(12):8336–8341. [PubMed] [Google Scholar]
- Zassenhaus H. P., Butow R. A., Hannon Y. P. Rapid electroelution of nucleic acids from agarose and acrylamide gels. Anal Biochem. 1982 Sep 1;125(1):125–130. doi: 10.1016/0003-2697(82)90392-x. [DOI] [PubMed] [Google Scholar]
- Zhu H., Macreadie I. G., Butow R. A. RNA processing and expression of an intron-encoded protein in yeast mitochondria: role of a conserved dodecamer sequence. Mol Cell Biol. 1987 Jul;7(7):2530–2537. doi: 10.1128/mcb.7.7.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]