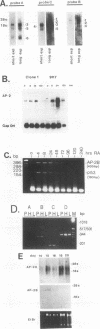
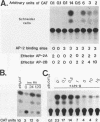
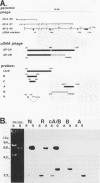
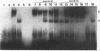
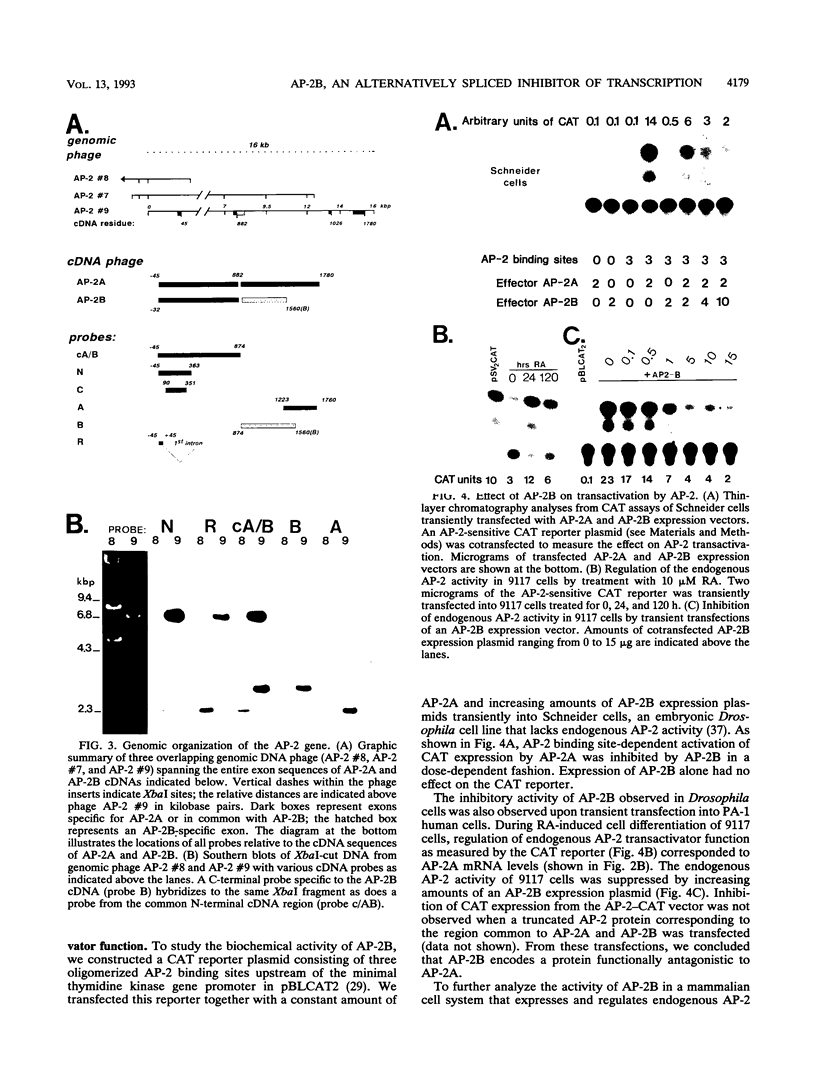
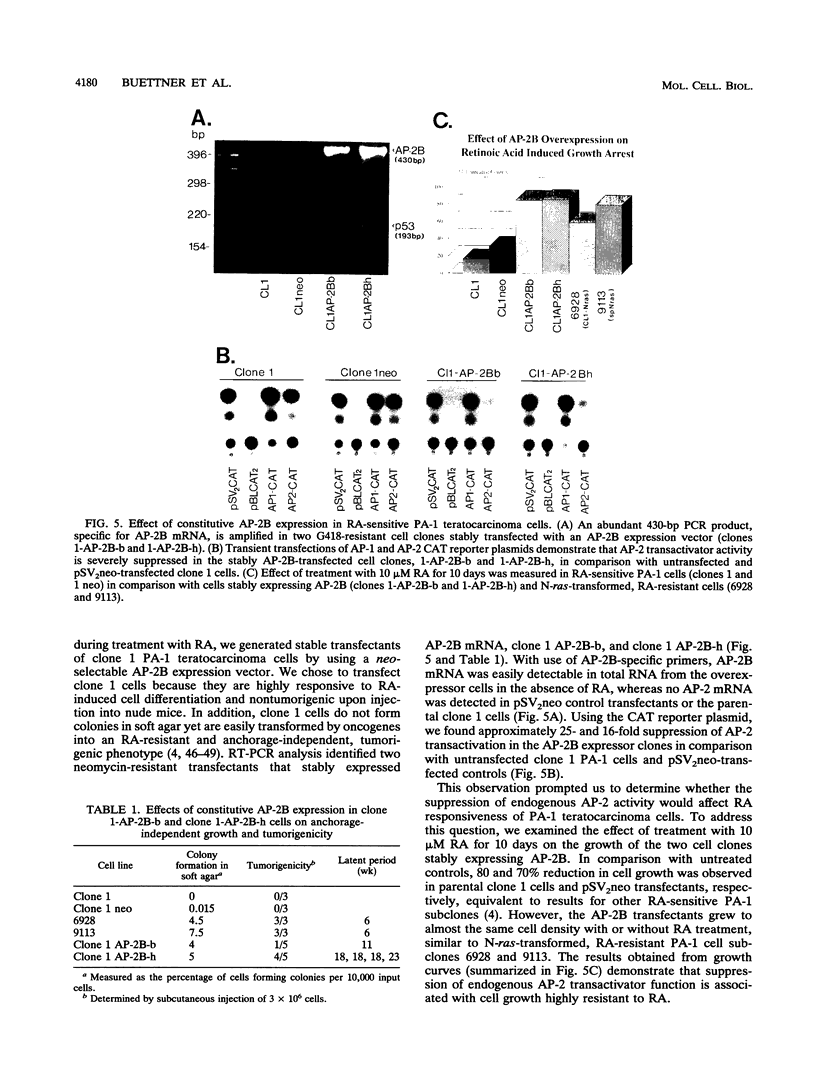
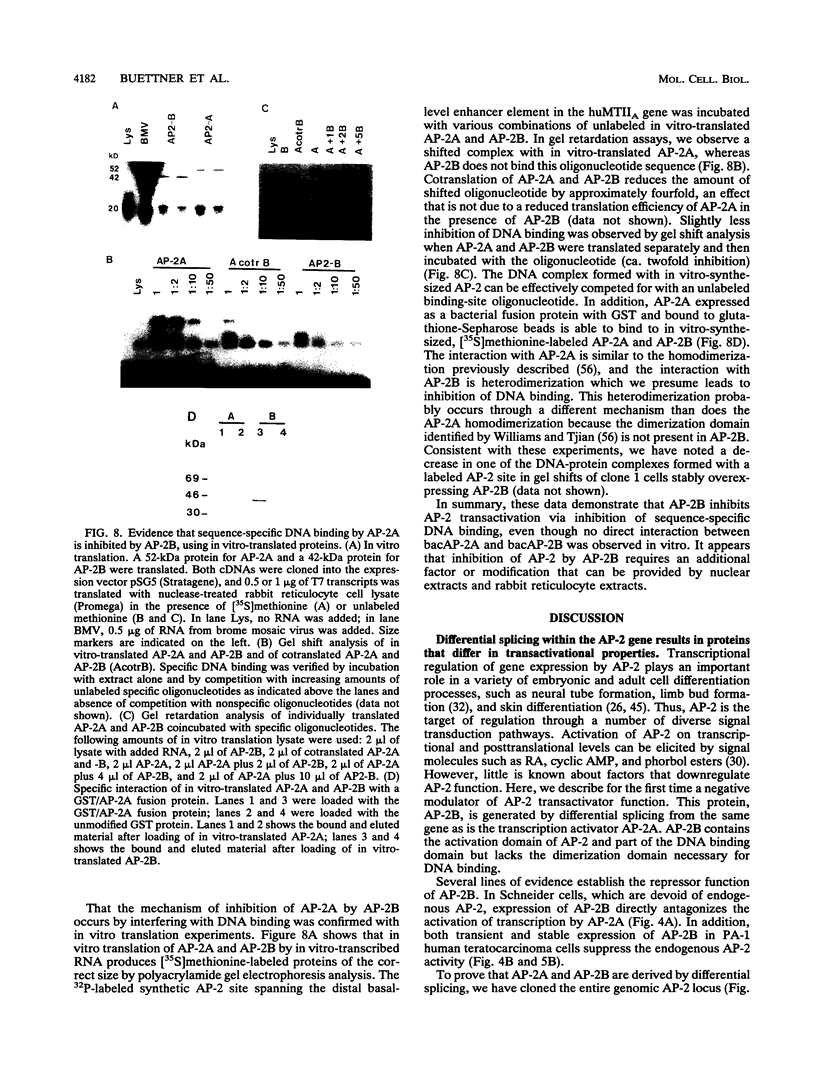
Abstract
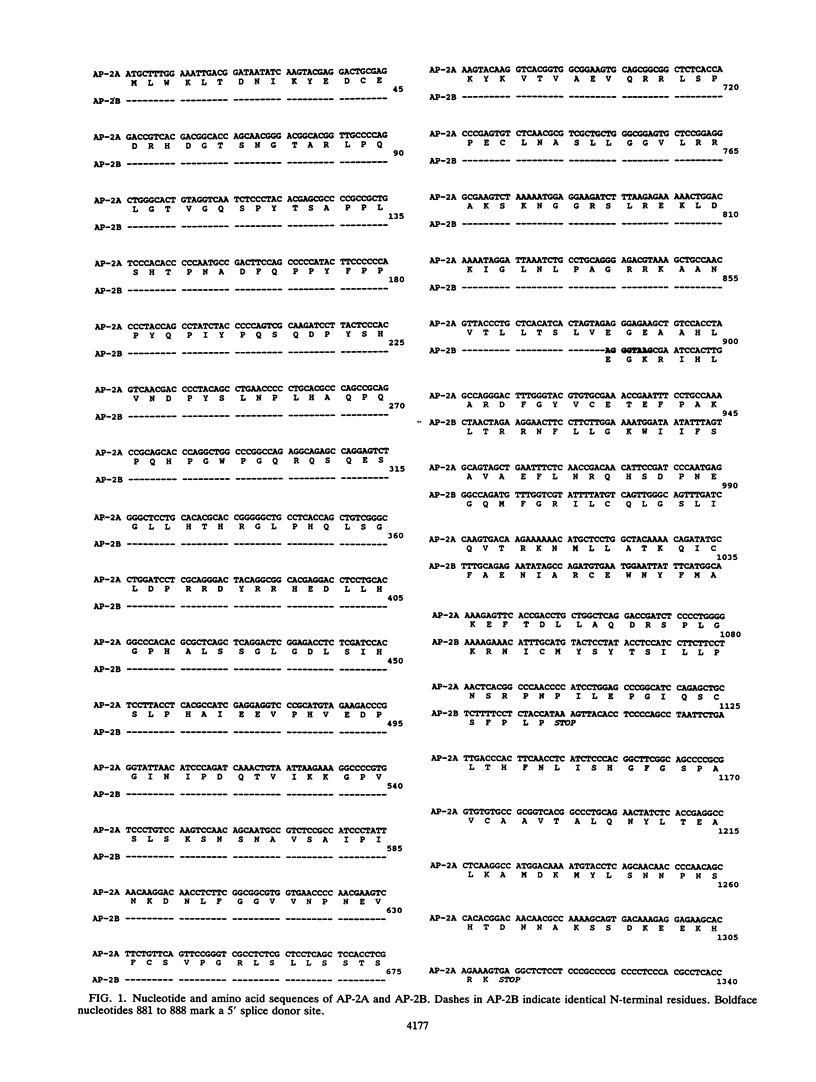
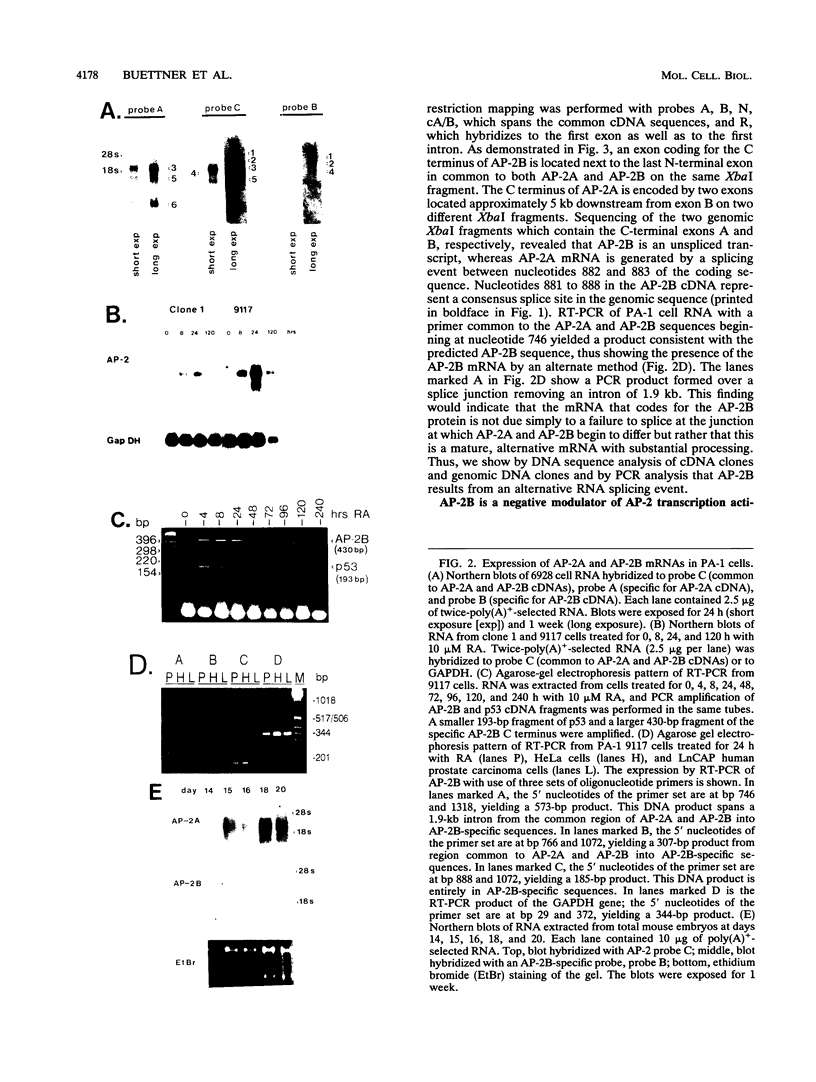
AP-2 is a retinoic acid-inducible and developmentally regulated activator of transcription. We have cloned an alternative AP-2 transcript (AP-2B) from the human teratocarcinoma cell line PA-1, which encodes a protein differing in the C terminus from the previously isolated AP-2 protein (AP-2A). This protein contains the activation domain of AP-2 and part of the DNA binding domain but lacks the dimerization domain which is necessary for DNA binding. Analysis of overlapping genomic clones spanning the entire AP-2 gene proves that AP-2A and AP-2B transcripts are alternatively spliced from the same gene. Both transient and stable transfection experiments show that AP-2B inhibits AP-2 transactivator function, as measured by an AP-2-responsive chloramphenicol acetyltransferase reporter plasmid. Furthermore, constitutive AP-2B expression in PA-1 cells causes a retinoic acid-resistant phenotype, anchorage-independent growth in soft agar, and tumorigenicity in nude mice, in a fashion similar to transformation of these cells by oncogenes. To determine the mechanism by which AP-2B exerts its inhibitory function, we purified bacterially expressed AP-2A and AP-2B proteins. While bacterial AP-2B does not bind an AP-2 consensus site, it strongly inhibits binding of the endogenous AP-2 present in PA-1 cell nuclear extracts. However, DNA sequence-specific binding of bacterially expressed AP-2A cannot be inhibited by bacterially expressed AP-2B. Therefore, inhibition of AP-2 activity by the protein AP-2B may require an additional factor or modification supplied by nuclear extracts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. W. Human teratocarcinomas. Biochim Biophys Acta. 1988 Aug 3;948(1):17–36. doi: 10.1016/0304-419x(88)90003-0. [DOI] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Bickmore W. A., Oghene K., Little M. H., Seawright A., van Heyningen V., Hastie N. D. Modulation of DNA binding specificity by alternative splicing of the Wilms tumor wt1 gene transcript. Science. 1992 Jul 10;257(5067):235–237. doi: 10.1126/science.1321494. [DOI] [PubMed] [Google Scholar]
- Buettner R., Yim S. O., Hong Y. S., Boncinelli E., Tainsky M. A. Alteration of homeobox gene expression by N-ras transformation of PA-1 human teratocarcinoma cells. Mol Cell Biol. 1991 Jul;11(7):3573–3583. doi: 10.1128/mcb.11.7.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao P. J., Kannan P., Yim S. O., Krizman D. B., Wu T. A., Gallick G. E., Tainsky M. A. Susceptibility to ras oncogene transformation is coregulated with signal transduction through growth factor receptors. Oncogene. 1991 May;6(5):713–720. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Luca L. M. Retinoids and their receptors in differentiation, embryogenesis, and neoplasia. FASEB J. 1991 Nov;5(14):2924–2933. [PubMed] [Google Scholar]
- Descombes P., Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991 Nov 1;67(3):569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichele G. Retinoids and vertebrate limb pattern formation. Trends Genet. 1989 Aug;5(8):246–251. doi: 10.1016/0168-9525(89)90096-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Borrelli E., Sassone-Corsi P. CREM gene: use of alternative DNA-binding domains generates multiple antagonists of cAMP-induced transcription. Cell. 1991 Feb 22;64(4):739–749. doi: 10.1016/0092-8674(91)90503-q. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Sassone-Corsi P. More is better: activators and repressors from the same gene. Cell. 1992 Feb 7;68(3):411–414. doi: 10.1016/0092-8674(92)90178-f. [DOI] [PubMed] [Google Scholar]
- Gaynor R. B., Muchardt C., Xia Y. R., Klisak I., Mohandas T., Sparkes R. S., Lusis A. J. Localization of the gene for the DNA-binding protein AP-2 to human chromosome 6p22.3-pter. Genomics. 1991 Aug;10(4):1100–1102. doi: 10.1016/0888-7543(91)90209-w. [DOI] [PubMed] [Google Scholar]
- Gogos J. A., Hsu T., Bolton J., Kafatos F. C. Sequence discrimination by alternatively spliced isoforms of a DNA binding zinc finger domain. Science. 1992 Sep 25;257(5078):1951–1955. doi: 10.1126/science.1290524. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haslinger A., Karin M. Upstream promoter element of the human metallothionein-IIA gene can act like an enhancer element. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8572–8576. doi: 10.1073/pnas.82.24.8572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzopoulos A. K., Stoykova A. S., Erselius J. R., Goulding M., Neuman T., Gruss P. Structure and expression of the mouse Oct2a and Oct2b, two differentially spliced products of the same gene. Development. 1990 Jun;109(2):349–362. doi: 10.1242/dev.109.2.349. [DOI] [PubMed] [Google Scholar]
- Hsu T., Gogos J. A., Kirsh S. A., Kafatos F. C. Multiple zinc finger forms resulting from developmentally regulated alternative splicing of a transcription factor gene. Science. 1992 Sep 25;257(5078):1946–1950. doi: 10.1126/science.1411512. [DOI] [PubMed] [Google Scholar]
- Hyman S. E., Comb M., Pearlberg J., Goodman H. M. An AP-2 element acts synergistically with the cyclic AMP- and phorbol ester-inducible enhancer of the human proenkephalin gene. Mol Cell Biol. 1989 Jan;9(1):321–324. doi: 10.1128/mcb.9.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Koenig R. J., Lazar M. A., Hodin R. A., Brent G. A., Larsen P. R., Chin W. W., Moore D. D. Inhibition of thyroid hormone action by a non-hormone binding c-erbA protein generated by alternative mRNA splicing. Nature. 1989 Feb 16;337(6208):659–661. doi: 10.1038/337659a0. [DOI] [PubMed] [Google Scholar]
- Le-Ruppert K., Masters J. R., Knuechel R., Seegers S., Tainsky M. A., Hofstaedter F., Buettner R. The effect of retinoic acid on chemosensitivity of PA-1 human teratocarcinoma cells and its modulation by an activated N-ras oncogene. Int J Cancer. 1992 Jun 19;51(4):646–651. doi: 10.1002/ijc.2910510423. [DOI] [PubMed] [Google Scholar]
- Leask A., Byrne C., Fuchs E. Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):7948–7952. doi: 10.1073/pnas.88.18.7948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Haslinger A., Karin M., Tjian R. Activation of transcription by two factors that bind promoter and enhancer sequences of the human metallothionein gene and SV40. Nature. 1987 Jan 22;325(6102):368–372. doi: 10.1038/325368a0. [DOI] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B., Mitchell P. J., Williams T., Tjian R. Regulation of transcription factor AP-2 by the morphogen retinoic acid and by second messengers. Genes Dev. 1989 Oct;3(10):1507–1517. doi: 10.1101/gad.3.10.1507. [DOI] [PubMed] [Google Scholar]
- Martin G. R. Teratocarcinomas and mammalian embryogenesis. Science. 1980 Aug 15;209(4458):768–776. doi: 10.1126/science.6250214. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Timmons P. M., Hébert J. M., Rigby P. W., Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991 Jan;5(1):105–119. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Wang C., Tjian R. Positive and negative regulation of transcription in vitro: enhancer-binding protein AP-2 is inhibited by SV40 T antigen. Cell. 1987 Sep 11;50(6):847–861. doi: 10.1016/0092-8674(87)90512-5. [DOI] [PubMed] [Google Scholar]
- Mumberg D., Lucibello F. C., Schuermann M., Müller R. Alternative splicing of fosB transcripts results in differentially expressed mRNAs encoding functionally antagonistic proteins. Genes Dev. 1991 Jul;5(7):1212–1223. doi: 10.1101/gad.5.7.1212. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y., Nathans D. A naturally occurring truncated form of FosB that inhibits Fos/Jun transcriptional activity. Cell. 1991 Feb 22;64(4):751–759. doi: 10.1016/0092-8674(91)90504-r. [DOI] [PubMed] [Google Scholar]
- Nyborg J. K., Dynan W. S. Interaction of cellular proteins with the human T-cell leukemia virus type I transcriptional control region. Purification of cellular proteins that bind the 21-base pair repeat elements. J Biol Chem. 1990 May 15;265(14):8230–8236. [PubMed] [Google Scholar]
- Perkins K. K., Dailey G. M., Tjian R. Novel Jun- and Fos-related proteins in Drosophila are functionally homologous to enhancer factor AP-1. EMBO J. 1988 Dec 20;7(13):4265–4273. doi: 10.1002/j.1460-2075.1988.tb03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Zhu H. In vitro squelching of activated transcription by serum response factor: evidence for a common coactivator used by multiple transcriptional activators. Nucleic Acids Res. 1992 Feb 11;20(3):513–520. doi: 10.1093/nar/20.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman C., Cohn L., Calame K. A dominant negative form of transcription activator mTFE3 created by differential splicing. Science. 1991 Oct 4;254(5028):94–97. doi: 10.1126/science.1840705. [DOI] [PubMed] [Google Scholar]
- Ruben S. M., Narayanan R., Klement J. F., Chen C. H., Rosen C. A. Functional characterization of the NF-kappa B p65 transcriptional activator and an alternatively spliced derivative. Mol Cell Biol. 1992 Feb;12(2):444–454. doi: 10.1128/mcb.12.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Acampora D., Arcioni L., Andrews P. W., Boncinelli E., Mavilio F. Sequential activation of HOX2 homeobox genes by retinoic acid in human embryonal carcinoma cells. Nature. 1990 Aug 23;346(6286):763–766. doi: 10.1038/346763a0. [DOI] [PubMed] [Google Scholar]
- Skerra A., Plückthun A. Assembly of a functional immunoglobulin Fv fragment in Escherichia coli. Science. 1988 May 20;240(4855):1038–1041. doi: 10.1126/science.3285470. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Snape A. M., Winning R. S., Sargent T. D. Transcription factor AP-2 is tissue-specific in Xenopus and is closely related or identical to keratin transcription factor 1 (KTF-1). Development. 1991 Sep;113(1):283–293. doi: 10.1242/dev.113.1.283. [DOI] [PubMed] [Google Scholar]
- Stellmach V., Leask A., Fuchs E. Retinoid-mediated transcriptional regulation of keratin genes in human epidermal and squamous cell carcinoma cells. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4582–4586. doi: 10.1073/pnas.88.11.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tainsky M. A., Cooper C. S., Giovanella B. C., Vande Woude G. F. An activated rasN gene: detected in late but not early passage human PA1 teratocarcinoma cells. Science. 1984 Aug 10;225(4662):643–645. doi: 10.1126/science.6740333. [DOI] [PubMed] [Google Scholar]
- Tainsky M. A., Krizman D. B., Chiao P. J., Yim S. O., Giovanella B. C. PA-1, a human cell model for multistage carcinogenesis: oncogenes and other factors. Anticancer Res. 1988 Sep-Oct;8(5A):899–913. [PubMed] [Google Scholar]
- Tainsky M. A., Shamanski F., Blair D., Giovanella B. C. Causal role for an activated N-ras oncogene in the induction of tumorigenicity acquired by a human cell line. Cancer Res. 1987 Jun 15;47(12):3235–3238. [PubMed] [Google Scholar]
- Tainsky M. A., Yim S. O., Krizman D. B., Kannan P., Chiao P. J., Mukhopadhyay T., Buettner R. Modulation of differentiation in PA-1 human teratocarcinoma cells after N-ras oncogene-induced tumorigenicity. Oncogene. 1991 Sep;6(9):1575–1582. [PubMed] [Google Scholar]
- Treacy M. N., He X., Rosenfeld M. G. I-POU: a POU-domain protein that inhibits neuron-specific gene activation. Nature. 1991 Apr 18;350(6319):577–584. doi: 10.1038/350577a0. [DOI] [PubMed] [Google Scholar]
- Van Dyke M. W., Sirito M., Sawadogo M. Single-step purification of bacterially expressed polypeptides containing an oligo-histidine domain. Gene. 1992 Feb 1;111(1):99–104. doi: 10.1016/0378-1119(92)90608-r. [DOI] [PubMed] [Google Scholar]
- Wagner M., Thaller C., Jessell T., Eichele G. Polarizing activity and retinoid synthesis in the floor plate of the neural tube. Nature. 1990 Jun 28;345(6278):819–822. doi: 10.1038/345819a0. [DOI] [PubMed] [Google Scholar]
- Williams T., Admon A., Lüscher B., Tjian R. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 1988 Dec;2(12A):1557–1569. doi: 10.1101/gad.2.12a.1557. [DOI] [PubMed] [Google Scholar]
- Williams T., Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991 Apr;5(4):670–682. doi: 10.1101/gad.5.4.670. [DOI] [PubMed] [Google Scholar]
- Williams T., Tjian R. Characterization of a dimerization motif in AP-2 and its function in heterologous DNA-binding proteins. Science. 1991 Mar 1;251(4997):1067–1071. doi: 10.1126/science.1998122. [DOI] [PubMed] [Google Scholar]
- Wirth T., Priess A., Annweiler A., Zwilling S., Oeler B. Multiple Oct2 isoforms are generated by alternative splicing. Nucleic Acids Res. 1991 Jan 11;19(1):43–51. doi: 10.1093/nar/19.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen J., Wisdom R. M., Tratner I., Verma I. M. An alternative spliced form of FosB is a negative regulator of transcriptional activation and transformation by Fos proteins. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5077–5081. doi: 10.1073/pnas.88.12.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeuthen J., Nørgaard J. O., Avner P., Fellous M., Wartiovaara J., Vaheri A., Rosén A., Giovanella B. C. Characterization of a human ovarian teratocarcinoma-derived cell line. Int J Cancer. 1980 Jan 15;25(1):19–32. doi: 10.1002/ijc.2910250104. [DOI] [PubMed] [Google Scholar]