Abstract
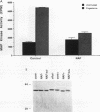
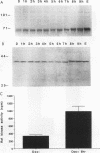
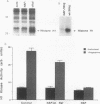
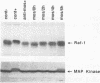
In somatic cells, the Raf-1 serine/threonine protein kinase is activated by several polypeptide growth factors. We investigated the role of Raf-1 in progesterone-induced meiotic maturation of Xenopus laevis oocytes. Raf-1 enzymatic activity and phosphorylation (reflected by a mobility shift on sodium dodecyl sulfate gels) were increased in oocytes following progesterone stimulation. The increase in Raf-1 activity was concurrent with an elevation in the activity of mitogen-activated protein (MAP) kinase. When RNA encoding an oncogenic form of Raf-1 (v-Raf) was injected into immature oocytes, MAP kinase mobility shift, germinal vesicle breakdown, and histone H1 phosphorylation increased markedly. When RNA encoding a dominant-negative version of Raf-1 was injected, progesterone-induced oocyte maturation was blocked. When RNA encoding Xenopus mos (mosxe) was injected into oocytes, Raf-1 and MAP kinase mobility shifts were observed after several hours. Also, when antisense mosxe oligonucleotides were injected into oocytes, progesterone-induced Raf-1 and MAP kinase mobility shifts were blocked. Finally, when antisense mosxe oligonucleotides were coinjected with v-Raf RNA into oocytes, histone H1 kinase activation, germinal vesicle breakdown, and MAP kinase mobility shift occurred. These findings suggest that Raf-1 activity is required for progesterone-induced oocyte maturation and that Raf-1 is downstream of mosxe activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Wu M., Gerhart J. C., Martin G. S. Cell cycle tyrosine phosphorylation of p34cdc2 and a microtubule-associated protein kinase homolog in Xenopus oocytes and eggs. Mol Cell Biol. 1991 Apr;11(4):1965–1971. doi: 10.1128/mcb.11.4.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. S., Meyer A. N., Li J., Donoghue D. J. Phosphorylation of conserved serine residues does not regulate the ability of mosxe protein kinase to induce oocyte maturation or function as cytostatic factor. J Cell Biol. 1992 Feb;116(3):725–735. doi: 10.1083/jcb.116.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman R. S., Pickham K. M., Kanki J. P., Lee B. A., Pena S. V., Donoghue D. J. Xenopus homolog of the mos protooncogene transforms mammalian fibroblasts and induces maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5805–5809. doi: 10.1073/pnas.86.15.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Kanki J. P., Donoghue D. J. Progression from meiosis I to meiosis II in Xenopus oocytes requires de novo translation of the mosxe protooncogene. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5794–5798. doi: 10.1073/pnas.88.13.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizaka-Kondoh S., Sato K., Tamura K., Nojima H., Okayama H. Raf-1 protein kinase is an integral component of the oncogenic signal cascade shared by epidermal growth factor and platelet-derived growth factor. Mol Cell Biol. 1992 Nov;12(11):5078–5086. doi: 10.1128/mcb.12.11.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Lloyd P., Rapp U. R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991 Jan 31;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Kovacina K. S., Yonezawa K., Brautigan D. L., Tonks N. K., Rapp U. R., Roth R. A. Insulin activates the kinase activity of the Raf-1 proto-oncogene by increasing its serine phosphorylation. J Biol Chem. 1990 Jul 25;265(21):12115–12118. [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Le Guellec R., Couturier A., Le Guellec K., Paris J., Le Fur N., Philippe M. Xenopus c-raf proto-oncogene: cloning and expression during oogenesis and early development. Biol Cell. 1991;72(1-2):39–45. doi: 10.1016/0248-4900(91)90076-y. [DOI] [PubMed] [Google Scholar]
- MacArthur H., Walter G. Monoclonal antibodies specific for the carboxy terminus of simian virus 40 large T antigen. J Virol. 1984 Nov;52(2):483–491. doi: 10.1128/jvi.52.2.483-491.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNicol A. M., Muslin A. J., Williams L. T. Raf-1 kinase is essential for early Xenopus development and mediates the induction of mesoderm by FGF. Cell. 1993 May 7;73(3):571–583. doi: 10.1016/0092-8674(93)90143-e. [DOI] [PubMed] [Google Scholar]
- Mark G. E., MacIntyre R. J., Digan M. E., Ambrosio L., Perrimon N. Drosophila melanogaster homologs of the raf oncogene. Mol Cell Biol. 1987 Jun;7(6):2134–2140. doi: 10.1128/mcb.7.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Escobedo J. A., Rapp U. R., Roberts T. M., Williams L. T. Direct activation of the serine/threonine kinase activity of Raf-1 through tyrosine phosphorylation by the PDGF beta-receptor. Cell. 1989 Aug 25;58(4):649–657. doi: 10.1016/0092-8674(89)90100-1. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rapp U., Roberts T. M. Signal transduction from membrane to cytoplasm: growth factors and membrane-bound oncogene products increase Raf-1 phosphorylation and associated protein kinase activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8855–8859. doi: 10.1073/pnas.85.23.8855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada J., Cooper J. A. Requirements for phosphorylation of MAP kinase during meiosis in Xenopus oocytes. Science. 1992 Jan 10;255(5041):212–215. doi: 10.1126/science.1313186. [DOI] [PubMed] [Google Scholar]
- Rapp U. R., Goldsborough M. D., Mark G. E., Bonner T. I., Groffen J., Reynolds F. H., Jr, Stephenson J. R. Structure and biological activity of v-raf, a unique oncogene transduced by a retrovirus. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4218–4222. doi: 10.1073/pnas.80.14.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp U. R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991 Apr;6(4):495–500. [PubMed] [Google Scholar]
- Sagata N., Daar I., Oskarsson M., Showalter S. D., Vande Woude G. F. The product of the mos proto-oncogene as a candidate "initiator" for oocyte maturation. Science. 1989 Aug 11;245(4918):643–646. doi: 10.1126/science.2474853. [DOI] [PubMed] [Google Scholar]
- Sagata N., Oskarsson M., Copeland T., Brumbaugh J., Vande Woude G. F. Function of c-mos proto-oncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988 Oct 6;335(6190):519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- Smith L. D. The induction of oocyte maturation: transmembrane signaling events and regulation of the cell cycle. Development. 1989 Dec;107(4):685–699. doi: 10.1242/dev.107.4.685. [DOI] [PubMed] [Google Scholar]
- Stanton V. P., Jr, Nichols D. W., Laudano A. P., Cooper G. M. Definition of the human raf amino-terminal regulatory region by deletion mutagenesis. Mol Cell Biol. 1989 Feb;9(2):639–647. doi: 10.1128/mcb.9.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Gunn M., Dell K., Tseng A., Jr, Williams L. A truncated form of fibroblast growth factor receptor 1 inhibits signal transduction by multiple types of fibroblast growth factor receptor. J Biol Chem. 1992 Jan 25;267(3):1470–1476. [PubMed] [Google Scholar]
- Watanabe N., Vande Woude G. F., Ikawa Y., Sagata N. Specific proteolysis of the c-mos proto-oncogene product by calpain on fertilization of Xenopus eggs. Nature. 1989 Nov 30;342(6249):505–511. doi: 10.1038/342505a0. [DOI] [PubMed] [Google Scholar]