Abstract
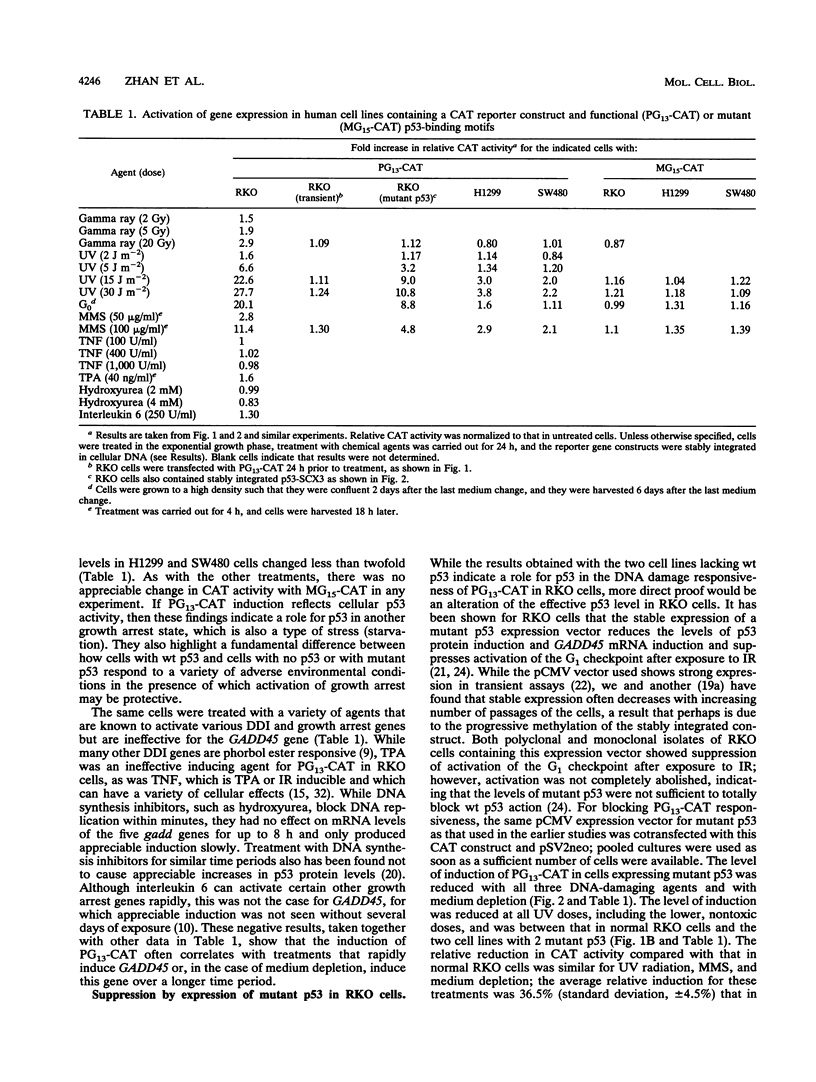
The tumor suppressor p53 can function as a sequence-specific transcription factor and is required for activation by ionizing radiation (IR) of one or more downstream effector genes, such as the human GADD45 gene. One important consequence of IR that is probably mediated by these downstream effector genes is activation of the p53-mediated G1 cell cycle checkpoint. While the induction of reporter constructs containing p53-binding sites has already been demonstrated with p53 expression vectors, we have now demonstrated the direct activation of such a construct after treatment of the human RKO line, which has a normal p53 phenotype, with various types of DNA-damaging agents and also after growth arrest produced by medium depletion (starvation). IR, UV radiation, and methylmethane sulfonate were found to induce p53 activity when a stably integrated reporter construct containing functional p53-binding sites was used and also in mobility shift assays with a p53-binding site from the GADD45 gene, and IR-inducible gene previously associated with growth arrest. The same cell treatments that induced this p53 activity also caused an increase in cellular p53 protein levels. The response in cells lacking normal p53 or in RKO cells expressing a dominant negative mutant p53 was markedly reduced. Interestingly, the spectrum of effective inducing agents for the above-described experiments was similar to that which induces GADD45 either in cells with a normal p53 status or, with the exception of IR, in cells lacking normal p53. These results indicate a role for p53 in the IR pathway, which is completely p53 dependent, and in other genotoxic stress responses, in which p53 has a cooperative effect but is not required.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agoff S. N., Hou J., Linzer D. I., Wu B. Regulation of the human hsp70 promoter by p53. Science. 1993 Jan 1;259(5091):84–87. doi: 10.1126/science.8418500. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Markowitz S., Fearon E. R., Willson J. K., Vogelstein B. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 1990 Aug 24;249(4971):912–915. doi: 10.1126/science.2144057. [DOI] [PubMed] [Google Scholar]
- Bohr V. A., Evans M. K., Fornace A. J., Jr DNA repair and its pathogenetic implications. Lab Invest. 1989 Aug;61(2):143–161. [PubMed] [Google Scholar]
- Carrier F., Owens R. A., Nebert D. W., Puga A. Dioxin-dependent activation of murine Cyp1a-1 gene transcription requires protein kinase C-dependent phosphorylation. Mol Cell Biol. 1992 Apr;12(4):1856–1863. doi: 10.1128/mcb.12.4.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devary Y., Gottlieb R. A., Smeal T., Karin M. The mammalian ultraviolet response is triggered by activation of Src tyrosine kinases. Cell. 1992 Dec 24;71(7):1081–1091. doi: 10.1016/s0092-8674(05)80058-3. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Jackman J., Hollander M. C., Hoffman-Liebermann B., Liebermann D. A. Genotoxic-stress-response genes and growth-arrest genes. gadd, MyD, and other genes induced by treatments eliciting growth arrest. Ann N Y Acad Sci. 1992 Nov 21;663:139–153. doi: 10.1111/j.1749-6632.1992.tb38657.x. [DOI] [PubMed] [Google Scholar]
- Fornace A. J., Jr, Nebert D. W., Hollander M. C., Luethy J. D., Papathanasiou M., Fargnoli J., Holbrook N. J. Mammalian genes coordinately regulated by growth arrest signals and DNA-damaging agents. Mol Cell Biol. 1989 Oct;9(10):4196–4203. doi: 10.1128/mcb.9.10.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk W. D., Pak D. T., Karas R. H., Wright W. E., Shay J. W. A transcriptionally active DNA-binding site for human p53 protein complexes. Mol Cell Biol. 1992 Jun;12(6):2866–2871. doi: 10.1128/mcb.12.6.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacca M., Gutierrez M. I., Menzo S., d'Adda di Fagagna F., Falaschi A. A human binding site for transcription factor USF/MLTF mimics the negative regulatory element of human immunodeficiency virus type 1. Virology. 1992 Jan;186(1):133–147. doi: 10.1016/0042-6822(92)90067-y. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan D. E., Spriggs D. R., Beckett M. A., Kufe D. W., Weichselbaum R. R. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch C. L., Bonner W. M. The human histone H2A.Z gene. Sequence and regulation. J Biol Chem. 1990 Sep 5;265(25):15211–15218. [PubMed] [Google Scholar]
- Holbrook N. J., Fornace A. J., Jr Response to adversity: molecular control of gene activation following genotoxic stress. New Biol. 1991 Sep;3(9):825–833. [PubMed] [Google Scholar]
- Holbrook N. J., Gulino A., Ruscetti F. Cis-acting transcriptional regulatory sequences in the gibbon ape leukemia virus (GALV) long terminal repeat. Virology. 1987 Mar;157(1):211–219. doi: 10.1016/0042-6822(87)90330-8. [DOI] [PubMed] [Google Scholar]
- Hollander M. C., Fornace A. J., Jr Induction of fos RNA by DNA-damaging agents. Cancer Res. 1989 Apr 1;49(7):1687–1692. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kastan M. B., Zhan Q., el-Deiry W. S., Carrier F., Jacks T., Walsh W. V., Plunkett B. S., Vogelstein B., Fornace A. J., Jr A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992 Nov 13;71(4):587–597. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Kessis T. D., Slebos R. J., Nelson W. G., Kastan M. B., Plunkett B. S., Han S. M., Lorincz A. T., Hedrick L., Cho K. R. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3988–3992. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltzman W., Czyzyk L. UV irradiation stimulates levels of p53 cellular tumor antigen in nontransformed mouse cells. Mol Cell Biol. 1984 Sep;4(9):1689–1694. doi: 10.1128/mcb.4.9.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papathanasiou M. A., Kerr N. C., Robbins J. H., McBride O. W., Alamo I., Jr, Barrett S. F., Hickson I. D., Fornace A. J., Jr Induction by ionizing radiation of the gadd45 gene in cultured human cells: lack of mediation by protein kinase C. Mol Cell Biol. 1991 Feb;11(2):1009–1016. doi: 10.1128/mcb.11.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitschke W. W., Lin Z. Y., DePonti-Zilli L., Paterson B. M. The beta actin promoter. High levels of transcription depend upon a CCAAT binding factor. J Biol Chem. 1989 Jun 5;264(16):9539–9546. [PubMed] [Google Scholar]
- Seto E., Usheva A., Zambetti G. P., Momand J., Horikoshi N., Weinmann R., Levine A. J., Shenk T. Wild-type p53 binds to the TATA-binding protein and represses transcription. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12028–12032. doi: 10.1073/pnas.89.24.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subler M. A., Martin D. W., Deb S. Inhibition of viral and cellular promoters by human wild-type p53. J Virol. 1992 Aug;66(8):4757–4762. doi: 10.1128/jvi.66.8.4757-4762.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerie K., Rosenberg M. Chromatin structure implicated in activation of HIV-1 gene expression by ultraviolet light. New Biol. 1990 Aug;2(8):712–718. [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Weichselbaum R. R., Hallahan D. E., Sukhatme V., Dritschilo A., Sherman M. L., Kufe D. W. Biological consequences of gene regulation after ionizing radiation exposure. J Natl Cancer Inst. 1991 Apr 3;83(7):480–484. doi: 10.1093/jnci/83.7.480. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Kern S. E., Pietenpol J. A., Kinzler K. W., Vogelstein B. Definition of a consensus binding site for p53. Nat Genet. 1992 Apr;1(1):45–49. doi: 10.1038/ng0492-45. [DOI] [PubMed] [Google Scholar]





