Abstract
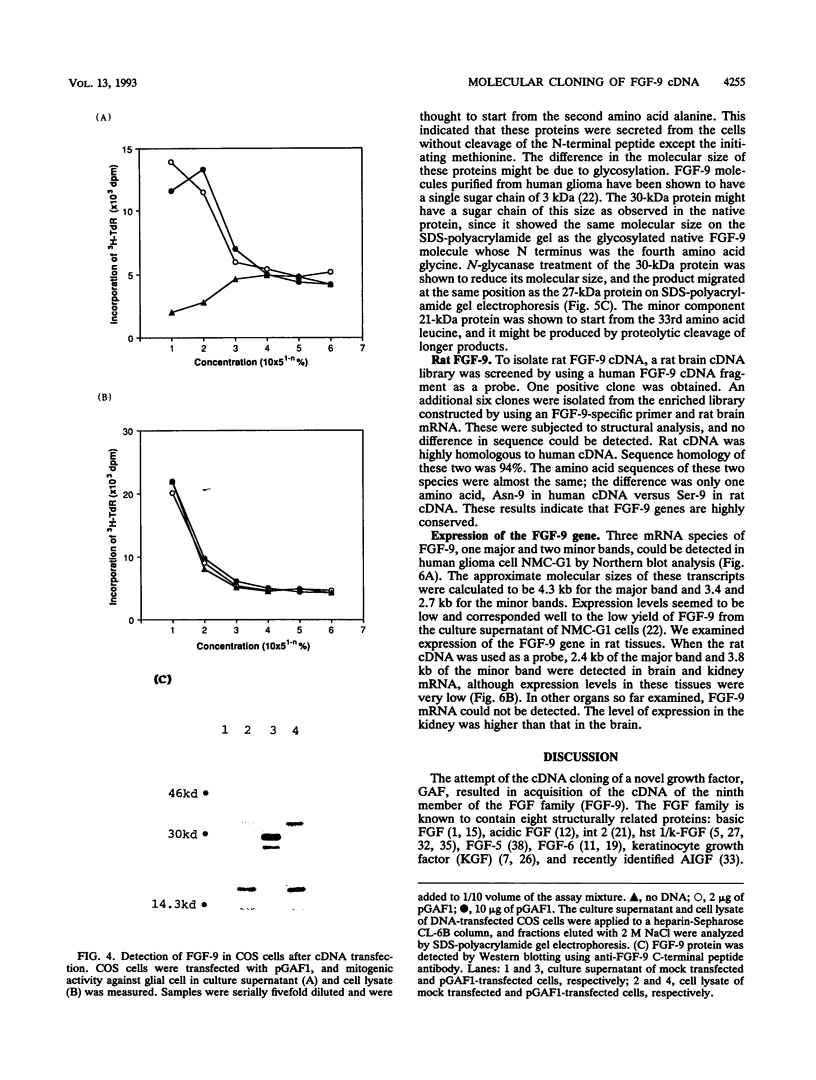
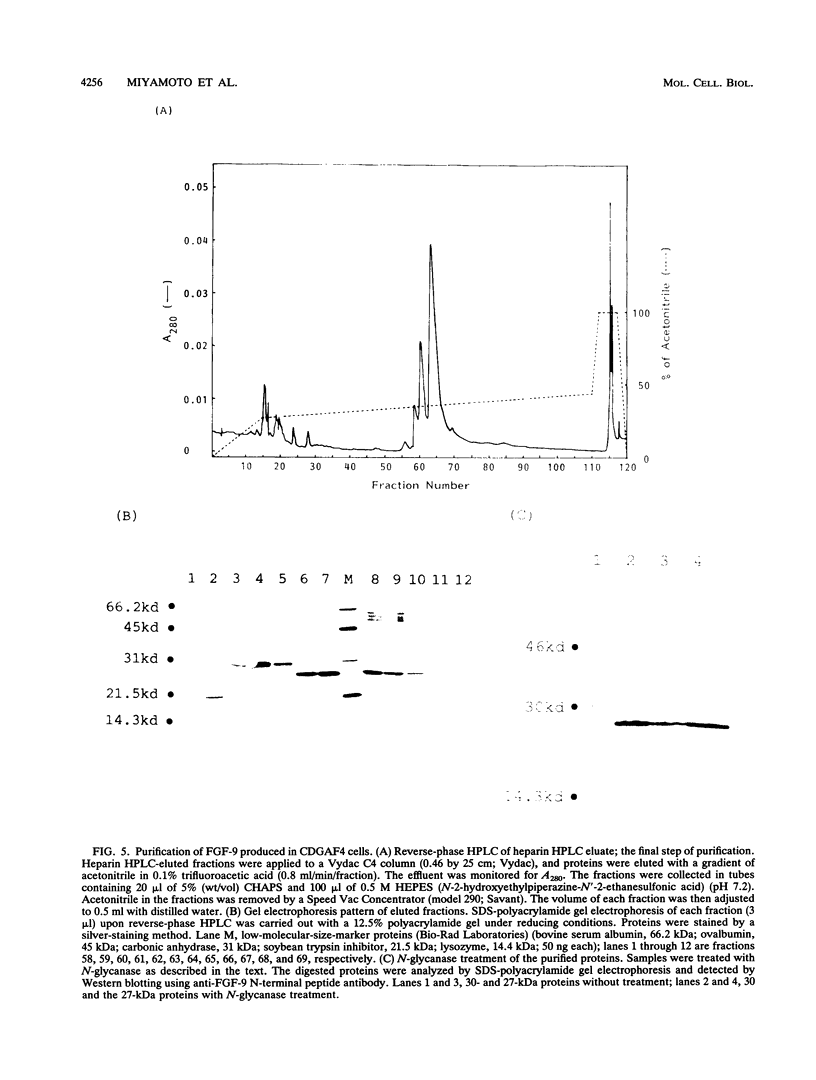
Glia-activating factor (GAF) is a novel heparin-binding growth factor purified from the culture supernatant of a human glioma cell line. It shows a spectrum of activity slightly different from those of other known growth factors. We have isolated the cDNA which encodes human GAF. A homology search revealed that GAF would be the ninth member of the FGF family, and we therefore call it FGF-9. The human FGF-9 cDNA cloned by using oligonucleotide probes encoded a polypeptide consisting of 208 amino acids. Sequence similarity to other members of the FGF family was estimated to be around 30%. Two cysteine residues and other consensus sequences in family members were also well conserved in the FGF-9 sequence. FGF-9 was found to have no typical signal sequence in its N terminus like those in acidic FGF and basic FGF. Acidic FGF and basic FGF are known not to be secreted from cells in a conventional manner. However, FGF-9 was found to be secreted from cells after synthesis despite its lack of a typical signal sequence. It could be detected exclusively in the culture medium of cDNA-transfected COS cells. The amino acid sequence of proteins purified from culture supernatant of the CHO cell line, which was cDNA transfected and selected as a high producer of FGF-9, showed that no peptides were cleaved from the N terminus except the initiation methionine. The rat FGF-9 cDNA was also cloned, and the structural analysis indicated that the PGF-9 gene is highly conserved. Expression of the FGF-9 gene could be detected in the brain and kidney of the adult rat. Restricted gene expression in organs and the unique secretion nature of the protein suggest that FGF-9 plays a physiological role which differs from those of well-characterized acidic FGF and basic FGF.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Whang J. L., Tumolo A., Mergia A., Friedman J., Gospodarowicz D., Fiddes J. C. Human basic fibroblast growth factor: nucleotide sequence and genomic organization. EMBO J. 1986 Oct;5(10):2523–2528. doi: 10.1002/j.1460-2075.1986.tb04530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland P., Dixon M., Peters G., Dickson C. Subcellular fate of the int-2 oncoprotein is determined by choice of initiation codon. Nature. 1990 Feb 15;343(6259):662–665. doi: 10.1038/343662a0. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Dionne C. A., Crumley G., Bellot F., Kaplow J. M., Searfoss G., Ruta M., Burgess W. H., Jaye M., Schlessinger J. Cloning and expression of two distinct high-affinity receptors cross-reacting with acidic and basic fibroblast growth factors. EMBO J. 1990 Sep;9(9):2685–2692. doi: 10.1002/j.1460-2075.1990.tb07454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Florkiewicz R. Z., Sommer A. Human basic fibroblast growth factor gene encodes four polypeptides: three initiate translation from non-AUG codons. Proc Natl Acad Sci U S A. 1989 Jun;86(11):3978–3981. doi: 10.1073/pnas.86.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb M., Deed R., MacAllan D., Walther W., Dickson C., Peters G. Cell transformation by Int-2--a member of the fibroblast growth factor family. Oncogene. 1991 Jan;6(1):65–71. [PubMed] [Google Scholar]
- Igarashi M., Nagata A., Jinno S., Suto K., Okayama H. Wee1(+)-like gene in human cells. Nature. 1991 Sep 5;353(6339):80–83. doi: 10.1038/353080a0. [DOI] [PubMed] [Google Scholar]
- Iida S., Yoshida T., Naito K., Sakamoto H., Katoh O., Hirohashi S., Sato T., Onda M., Sugimura T., Terada M. Human hst-2 (FGF-6) oncogene: cDNA cloning and characterization. Oncogene. 1992 Feb;7(2):303–309. [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Keegan K., Johnson D. E., Williams L. T., Hayman M. J. Isolation of an additional member of the fibroblast growth factor receptor family, FGFR-3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1095–1099. doi: 10.1073/pnas.88.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Kurokawa T., Sasada R., Iwane M., Igarashi K. Cloning and expression of cDNA encoding human basic fibroblast growth factor. FEBS Lett. 1987 Mar 9;213(1):189–194. doi: 10.1016/0014-5793(87)81489-8. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Marics I., Adelaide J., Raybaud F., Mattei M. G., Coulier F., Planche J., de Lapeyriere O., Birnbaum D. Characterization of the HST-related FGF.6 gene, a new member of the fibroblast growth factor gene family. Oncogene. 1989 Mar;4(3):335–340. [PubMed] [Google Scholar]
- Miyagawa K., Sakamoto H., Yoshida T., Yamashita Y., Mitsui Y., Furusawa M., Maeda S., Takaku F., Sugimura T., Terada M. hst-1 transforming protein: expression in silkworm cells and characterization as a novel heparin-binding growth factor. Oncogene. 1988 Oct;3(4):383–389. [PubMed] [Google Scholar]
- Moore R., Casey G., Brookes S., Dixon M., Peters G., Dickson C. Sequence, topography and protein coding potential of mouse int-2: a putative oncogene activated by mouse mammary tumour virus. EMBO J. 1986 May;5(5):919–924. doi: 10.1002/j.1460-2075.1986.tb04304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
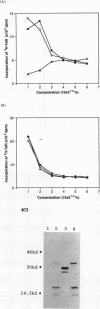
- Naruo K., Seko C., Kuroshima K., Matsutani E., Sasada R., Kondo T., Kurokawa T. Novel secretory heparin-binding factors from human glioma cells (glia-activating factors) involved in glial cell growth. Purification and biological properties. J Biol Chem. 1993 Feb 5;268(4):2857–2864. [PubMed] [Google Scholar]
- Ono Y., Kurokawa T., Fujii T., Kawahara K., Igarashi K., Kikkawa U., Ogita K., Nishizuka Y. Two types of complementary DNAs of rat brain protein kinase C. Heterogeneity determined by alternative splicing. FEBS Lett. 1986 Oct 6;206(2):347–352. doi: 10.1016/0014-5793(86)81010-9. [DOI] [PubMed] [Google Scholar]
- Partanen J., Mäkelä T. P., Eerola E., Korhonen J., Hirvonen H., Claesson-Welsh L., Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991 Jun;10(6):1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats H., Kaghad M., Prats A. C., Klagsbrun M., Lélias J. M., Liauzun P., Chalon P., Tauber J. P., Amalric F., Smith J. A. High molecular mass forms of basic fibroblast growth factor are initiated by alternative CUG codons. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1836–1840. doi: 10.1073/pnas.86.6.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto H., Mori M., Taira M., Yoshida T., Matsukawa S., Shimizu K., Sekiguchi M., Terada M., Sugimura T. Transforming gene from human stomach cancers and a noncancerous portion of stomach mucosa. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3997–4001. doi: 10.1073/pnas.83.11.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasada R., Onda H., Igarashi K. The establishment of IL-2 producing cells by genetic engineering. Cell Struct Funct. 1987 Apr;12(2):205–217. doi: 10.1247/csf.12.205. [DOI] [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Smith R., Peters G., Dickson C. Multiple RNAs expressed from the int-2 gene in mouse embryonal carcinoma cell lines encode a protein with homology to fibroblast growth factors. EMBO J. 1988 Apr;7(4):1013–1022. doi: 10.1002/j.1460-2075.1988.tb02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira M., Yoshida T., Miyagawa K., Sakamoto H., Terada M., Sugimura T. cDNA sequence of human transforming gene hst and identification of the coding sequence required for transforming activity. Proc Natl Acad Sci U S A. 1987 May;84(9):2980–2984. doi: 10.1073/pnas.84.9.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Miyamoto K., Minamino N., Takeda M., Sato B., Matsuo H., Matsumoto K. Cloning and characterization of an androgen-induced growth factor essential for the androgen-dependent growth of mouse mammary carcinoma cells. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8928–8932. doi: 10.1073/pnas.89.19.8928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The fibroblast growth factor family. Nomenclature meeting report and recommendations. January 17, 1991. Ann N Y Acad Sci. 1991;638:xiii–xvi. doi: 10.1111/j.1749-6632.1991.tb49011.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Miyagawa K., Odagiri H., Sakamoto H., Little P. F., Terada M., Sugimura T. Genomic sequence of hst, a transforming gene encoding a protein homologous to fibroblast growth factors and the int-2-encoded protein. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7305–7309. doi: 10.1073/pnas.84.20.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Muramatsu H., Muramatsu T., Sakamoto H., Katoh O., Sugimura T., Terada M. Differential expression of two homologous and clustered oncogenes, Hst1 and Int-2, during differentiation of F9 cells. Biochem Biophys Res Commun. 1988 Dec 15;157(2):618–625. doi: 10.1016/s0006-291x(88)80295-x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Tsutsumi M., Sakamoto H., Miyagawa K., Teshima S., Sugimura T., Terada M. Expression of the HST1 oncogene in human germ cell tumors. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1324–1329. doi: 10.1016/s0006-291x(88)81286-5. [DOI] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]