Abstract
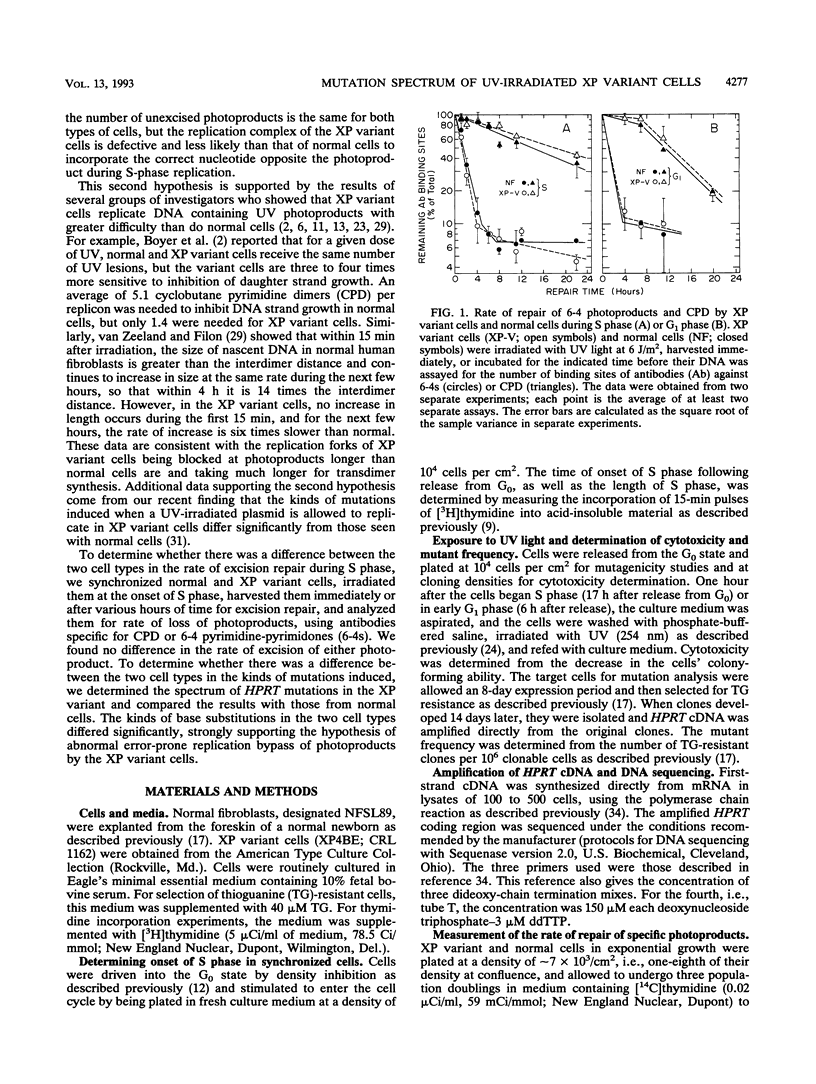
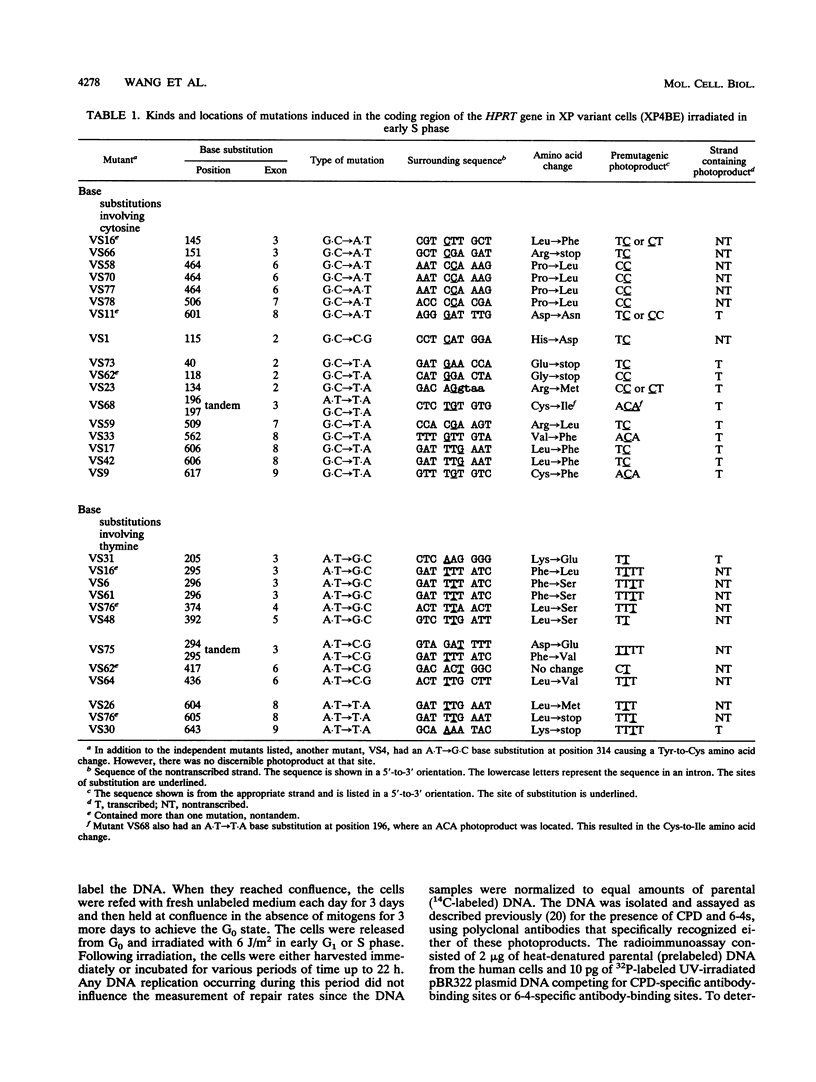
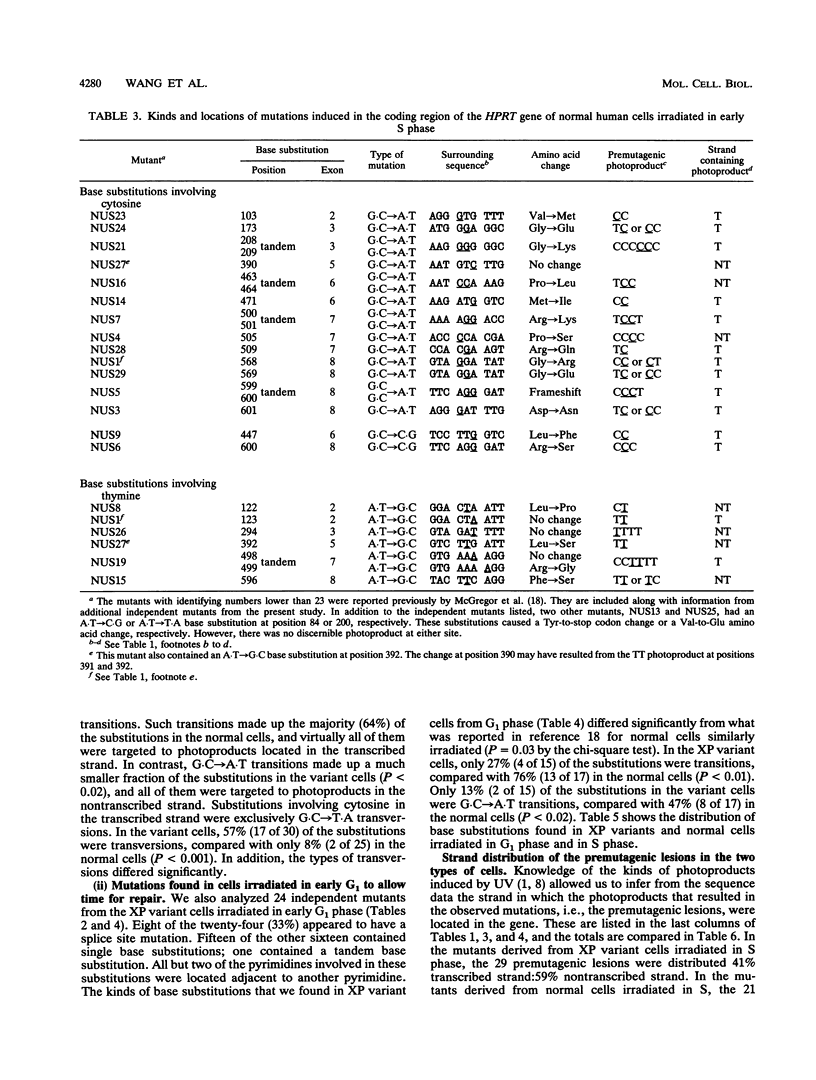
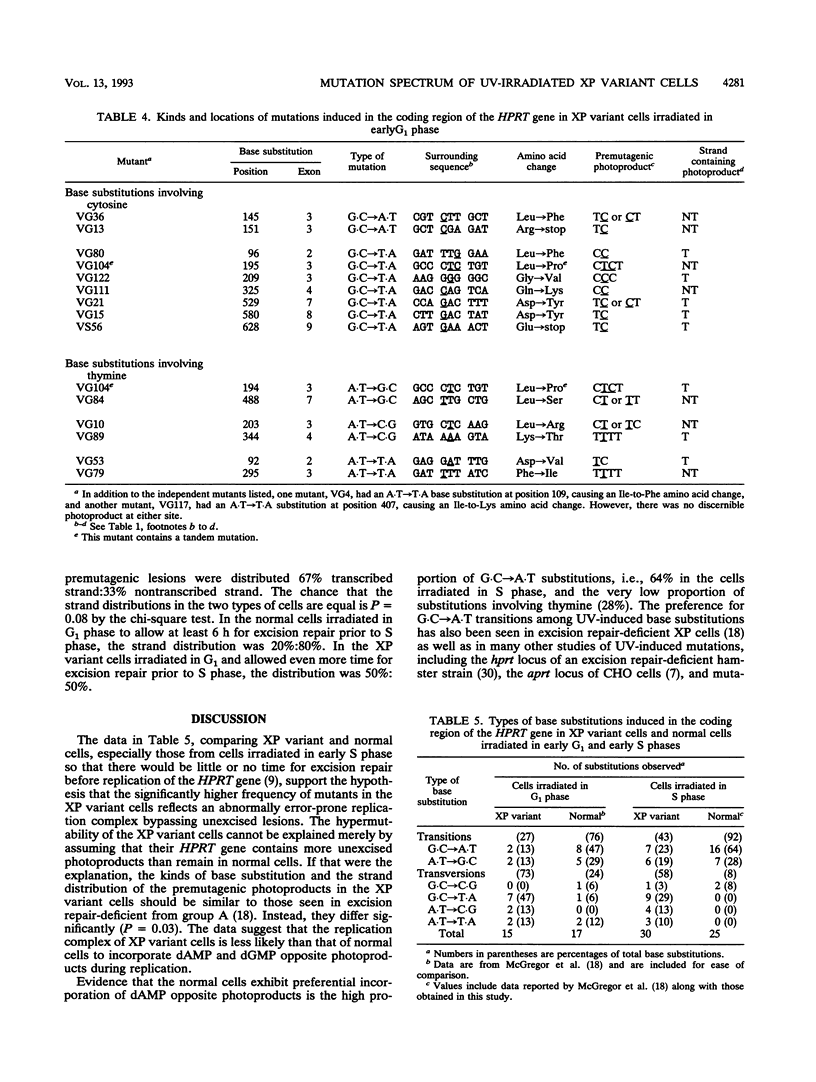
Xeroderma pigmentosum (XP) variant patients are genetically predisposed to sunlight-induced skin cancer. Fibroblasts derived from these patients are extremely sensitive to the mutagenic effect of UV radiation and are abnormally slow in replicating DNA containing UV-induced photoproducts. However, unlike cells from the majority of XP patients, XP variant cells have a normal or nearly normal rate of nucleotide excision repair of such damage. To determine whether their UV hypermutability reflected a slower rate of excision of photoproducts specifically during early S phase when the target gene for mutations, i.e., the hypoxanthine (guanine) phosphoribosyltransferase gene (HPRT), is replicated, we synchronized diploid populations of normal and XP variant fibroblasts, irradiated them in early S phase, and compared the rate of loss of cyclobutane pyrimidine dimers and 6-4 pyrimidine-pyrimidones from DNA during S phase. There was no difference. Both removed 94% of the 6-4 pyrimidine-pyrimidones within 8 h and 40% of the dimers within 11 h. There was also no difference between the two cell lines in the rate of repair during G1 phase. To determine whether the hypermutability resulted from abnormal error-prone replication of DNA containing photoproducts, we determined the spectra of mutations induced in the coding region of the HPRT gene of XP variant cells irradiated in early S and G1 phases and compared with those found in normal cells. The majority of the mutations in both types of cells were base substitutions, but the two types of cells differed significantly from each other in the kinds of substitutions, but the two types differed significantly from each other in the kinds of substitutions observed either in mutants from S phase (P < 0.01) or from G1 phase (P = 0.03). In the variant cells, the substitutions were mainly transversions (58% in S, 73% in G1). In the normal cells irradiated in S, the majority of the substitutions were G.C --> A.T, and most involved CC photoproducts in the transcribed strand. In the variant cells irradiated in S, substitutions involving cytosine in the transcribed strand were G.C --> T.A transversions exclusively. G.C --> A.T transitions made up a much smaller fraction of the substitutions than in normal cells (P < 0.02), and all of them involved photoproducts located in the nontranscribed strand. The data strongly suggest that XP variant cells are much less likely than normal cells to incorporate either dAMP or dGMP opposite the pyrimidines involved in photoproducts. This would account for their significantly higher frequency of mutants and might explain their abnormal delay in replicating a UV-damaged template.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourre F., Renault G., Sarasin A. Sequence effect on alkali-sensitive sites in UV-irradiated SV40 DNA. Nucleic Acids Res. 1987 Nov 11;15(21):8861–8875. doi: 10.1093/nar/15.21.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. C., Kaufmann W. K., Brylawski B. P., Cordeiro-Stone M. Defective postreplication repair in xeroderma pigmentosum variant fibroblasts. Cancer Res. 1990 May 1;50(9):2593–2598. [PubMed] [Google Scholar]
- Bredberg A., Kraemer K. H., Seidman M. M. Restricted ultraviolet mutational spectrum in a shuttle vector propagated in xeroderma pigmentosum cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8273–8277. doi: 10.1073/pnas.83.21.8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M. P., Hauser J., Levine A. S., Dixon K. Replication and mutagenesis of UV-damaged DNA templates in human and monkey cell extracts. Mol Cell Biol. 1993 Jan;13(1):533–542. doi: 10.1128/mcb.13.1.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Thomas G. H., Park S. D. Xeroderma pigmentosum variants have a slow recovery of DNA synthesis after irradiation with ultraviolet light. Biochim Biophys Acta. 1979 Aug 29;564(1):122–131. doi: 10.1016/0005-2787(79)90193-x. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Xeroderma pigmentosum: variants with normal DNA repair and normal sensitivity to ultraviolet light. J Invest Dermatol. 1972 Mar;58(3):124–128. doi: 10.1111/1523-1747.ep12538913. [DOI] [PubMed] [Google Scholar]
- Drobetsky E. A., Grosovsky A. J., Glickman B. W. The specificity of UV-induced mutations at an endogenous locus in mammalian cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9103–9107. doi: 10.1073/pnas.84.24.9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossmann A., Maher V. M., McCormick J. J. The frequency of mutants in human fibroblasts UV-irradiated at various times during S-phase suggests that genes for thioguanine- and diphtheria toxin-resistance are replicated early. Mutat Res. 1985 Oct;152(1):67–76. doi: 10.1016/0027-5107(85)90047-8. [DOI] [PubMed] [Google Scholar]
- HOWARD B. D., TESSMAN I. IDENTIFICATION OF THE ALTERED BASES IN MUTATED SINGLE-STRANDED DNA. 3. MUTAGENESIS BY ULTRAVIOLET LIGHT. J Mol Biol. 1964 Aug;9:372–375. doi: 10.1016/s0022-2836(64)80214-x. [DOI] [PubMed] [Google Scholar]
- Kaufmann W. K., Cleaver J. E. Mechanisms of inhibition of DNA replication by ultraviolet light in normal human and xeroderma pigmentosum fibroblasts. J Mol Biol. 1981 Jun 25;149(2):171–187. doi: 10.1016/0022-2836(81)90297-7. [DOI] [PubMed] [Google Scholar]
- Konze-Thomas B., Hazard R. M., Maher V. M., McCormick J. J. Extent of excision repair before DNA synthesis determines the mutagenic but not the lethal effect of UV radiation. Mutat Res. 1982 Jun;94(2):421–434. doi: 10.1016/0027-5107(82)90305-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Paterson M. C., Lohman P. H., de Weerd-Kastelein E. A., Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A. 1975 Jan;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb L. A., Preston B. D. Mutagenesis by apurinic/apyrimidinic sites. Annu Rev Genet. 1986;20:201–230. doi: 10.1146/annurev.ge.20.120186.001221. [DOI] [PubMed] [Google Scholar]
- Maher V. M., Ouellette L. M., Curren R. D., McCormick J. J. Frequency of ultraviolet light-induced mutations is higher in xeroderma pigmentosum variant cells than in normal human cells. Nature. 1976 Jun 17;261(5561):593–595. doi: 10.1038/261593a0. [DOI] [PubMed] [Google Scholar]
- Mayne L. V., Lehmann A. R. Failure of RNA synthesis to recover after UV irradiation: an early defect in cells from individuals with Cockayne's syndrome and xeroderma pigmentosum. Cancer Res. 1982 Apr;42(4):1473–1478. [PubMed] [Google Scholar]
- McGregor W. G., Chen R. H., Lukash L., Maher V. M., McCormick J. J. Cell cycle-dependent strand bias for UV-induced mutations in the transcribed strand of excision repair-proficient human fibroblasts but not in repair-deficient cells. Mol Cell Biol. 1991 Apr;11(4):1927–1934. doi: 10.1128/mcb.11.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. (6-4)Photoproducts are removed from the DNA of UV-irradiated mammalian cells more efficiently than cyclobutane pyrimidine dimers. Mutat Res. 1985 Jul;143(3):109–112. doi: 10.1016/s0165-7992(85)80018-x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Haipek C. A., Clarkson J. M. Xeroderma pigmentosum variant cells are not defective in the repair of (6-4) photoproducts. Int J Radiat Biol Relat Stud Phys Chem Med. 1987 Aug;52(2):201–205. doi: 10.1080/09553008714551661. [DOI] [PubMed] [Google Scholar]
- Myhr B. C., Turnbull D., DiPaolo J. A. Ultraviolet mutagenesis of normal and xeroderma pigmentosum variant human fibroblasts. Mutat Res. 1979 Sep;62(2):341–353. doi: 10.1016/0027-5107(79)90089-7. [DOI] [PubMed] [Google Scholar]
- Park S. D., Cleaver J. E. Postreplication repair: questions of its definition and possible alteration in xeroderma pigmentosum cell strains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3927–3931. doi: 10.1073/pnas.76.8.3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. D., Rowan L. A., Mendrala A. L., Howell J. N., Maher V. M., McCormick J. J. Xeroderma pigmentosum fibroblasts including cells from XP variants are abnormally sensitive to the mutagenic and cytotoxic action of broad spectrum simulated sunlight. Photochem Photobiol. 1984 Jan;39(1):37–42. doi: 10.1111/j.1751-1097.1984.tb03401.x. [DOI] [PubMed] [Google Scholar]
- Rabkin S. D., Moore P. D., Strauss B. S. In vitro bypass of UV-induced lesions by Escherichia coli DNA polymerase I: specificity of nucleotide incorporation. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1541–1545. doi: 10.1073/pnas.80.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Roberts J. D., Kunkel T. A. Fidelity of a human cell DNA replication complex. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7064–7068. doi: 10.1073/pnas.85.19.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharam S., Seidman M. M. Modulation of an ultraviolet mutational hotspot in a shuttle vector Xeroderma cells. Nucleic Acids Res. 1991 Apr 11;19(7):1601–1604. doi: 10.1093/nar/19.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling H., Van Rooijen M. L., Groen N. A., Zdzienicka M. Z., Simons J. W., Lohman P. H., van Zeeland A. A. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. C., Maher V. M., McCormick J. J. Xeroderma pigmentosum variant cells are less likely than normal cells to incorporate dAMP opposite photoproducts during replication of UV-irradiated plasmids. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7810–7814. doi: 10.1073/pnas.88.17.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Maher V. M., McCormick J. J. Excision repair of UV- or benzo[a]pyrene diol epoxide-induced lesions in xeroderma pigmentosum variant cells is 'error free'. Mutat Res. 1985 Nov;146(3):285–294. doi: 10.1016/0167-8817(85)90070-7. [DOI] [PubMed] [Google Scholar]
- Yagi T., Tatsumi-Miyajima J., Sato M., Kraemer K. H., Takebe H. Analysis of point mutations in an ultraviolet-irradiated shuttle vector plasmid propagated in cells from Japanese xeroderma pigmentosum patients in complementation groups A and F. Cancer Res. 1991 Jun 15;51(12):3177–3182. [PubMed] [Google Scholar]
- Yang J. L., Maher V. M., McCormick J. J. Amplification and direct nucleotide sequencing of cDNA from the lysate of low numbers of diploid human cells. Gene. 1989 Nov 30;83(2):347–354. doi: 10.1016/0378-1119(89)90121-2. [DOI] [PubMed] [Google Scholar]
- Zelle B., Lohman P. H. Repair of UV-endonuclease-susceptible sites in the 7 complementation groups of xeroderma pigmentosum A through G. Mutat Res. 1979 Sep;62(2):363–368. doi: 10.1016/0027-5107(79)90091-5. [DOI] [PubMed] [Google Scholar]


