Abstract
Synapses exhibit several forms of short-term plasticity that play a multitude of computational roles. Short-term depression suppresses neurotransmitter release for hundreds of milliseconds to tens of seconds; facilitation and post-tetanic potentiation lead to synaptic enhancement lasting hundreds of milliseconds to minutes. Recent advances have provided insight into the mechanisms underlying these forms of plasticity. Vesicle depletion and inactivation of release sites and calcium channels contribute to synaptic depression. Mechanisms of short-term enhancement include calcium channel facilitation, local depletion of calcium buffers, increases in the probability of release downstream of calcium influx, altered vesicle pool properties, and increases in quantal size. Moreover, there is a growing appreciation of the heterogeneity of vesicles and release sites and how they can contribute to use-dependent plasticity.
Introduction
A ubiquitous property of synapses is the ability to keep track of the history of activity. This history is encoded in various forms of activity-dependent plasticity that shape synaptic output and may form the basis of learning and memory. Short-term plasticity lasts from tens of milliseconds to several minutes and is thought to underlie information processing. It can lead to bidirectional changes in synaptic strength, which can be reduced for hundreds of milliseconds to seconds (depression), or it can be enhanced for hundreds of milliseconds to seconds (facilitation), to tens of seconds to minutes (augmentation and post-tetanic potentiation, PTP). Net plasticity at synapses reflects an interaction between multiple forms of plasticity. Here we will discuss recent advances in clarifying the mechanisms underlying these different forms of plasticity.
Synaptic depression
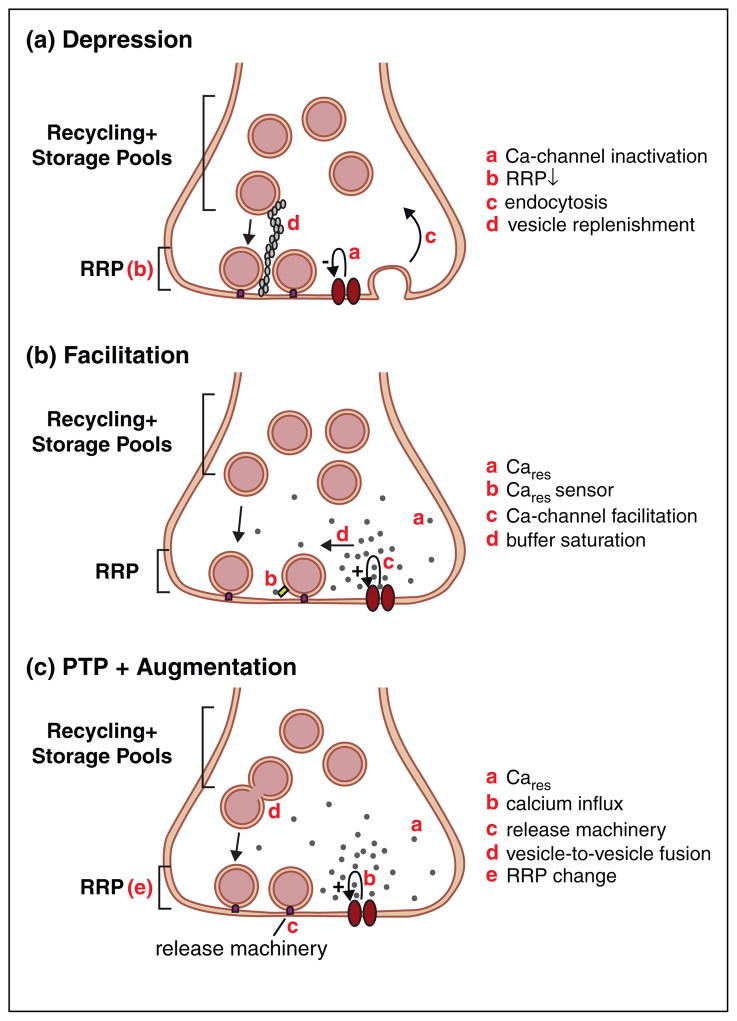
At many synapses, repeated stimuli delivered at short time intervals lead to a transient decrease in synaptic strength. Here, we will focus on presynaptic mechanisms that contribute to a decrease in neurotransmitter release [1]. Several factors can account for reduced release, including but not limited to vesicle depletion, inactivation of release sites, and decreased presynaptic calcium influx (Figure 1a).
Figure 1.
Presynaptic mechanisms of use-dependent short-term plasticity. Schematic diagrams illustrate proposed mechanisms for depression (a), facilitation (b), and post-tetanic potentiation (PTP) and augmentation (c). RRP: readily releasable pool of vesicles; Cares: residual calcium.
Depletion of the readily releasable pool
There are typically hundreds of vesicles associated with one active zone, but usually fewer than 5% of these vesicles are readily released with repeated stimulation [2]. The number of vesicles released by an action potential depends on the size of this readily releasable pool (RRP) of vesicles, and on the probability of release of these vesicles. Because the number of vesicles in the RRP is limiting, if an action potential releases a large fraction of the RRP, subsequent stimuli delivered before RRP replenishment will release fewer vesicles [1]. This model predicts that depression will increase when the initial release probability and the frequency of activation are increased. These predictions hold true for many synapses such as corticothalamic synapses and synapses in the auditory brainstem [1,3–5]. Recovery from depression occurs within several seconds as vesicles from a recycling pool of vesicles replenish the RRP. Recovery can be significantly accelerated by elevations of presynaptic calcium in a calmodulin-dependent manner [6–11].
Inactivation of release sites
According to a second model of synaptic depression, fusion of a vesicle at a release site can inhibit subsequent fusion events at that site even if the RRP is not depleted [12,13]. This proposed site inactivation lasts for seconds following exocytosis and could reflect the time it takes to clear vesicular membrane proteins, which get incorporated into the plasma membrane upon vesicle fusion, from the release site [12]. A recent study suggests a surprising role for endocytosis in limiting the extent of depression by allowing sites to recover from such inactivation. Blocking endocytosis presynaptically reduces the recruitment of readily releasable vesicles and leads to more pronounced depression during trains [14••]. These findings are consistent with endocytosis clearing vesicular membrane proteins from the plasma membrane where they interfere with release, thereby allowing sites to recover from inactivation more rapidly than if these proteins were removed by diffusion within the membrane.
Reduction in calcium influx
At many synapses including some neocortical synapses, axo-axonic synapses of the Mauthner neuron in the gold-fish, and vestibular afferent synapses, the properties of depression are inconsistent with RRP depletion [15–17]. Activity-dependent decreases in calcium influx could account for depression at these synapses. Because of the steep dependence of neurotransmitter release on calcium [12], even small activity-dependent changes in calcium entry can lead to significant presynaptic plasticity. At the calyx of Held, a synapse in the auditory brainstem, calcium-dependent decreases in calcium influx contribute to synaptic depression [18,19]. Calcium-sensing proteins (CaS), including calmodulin, calcium binding protein 1 (CaBP1), and neuronal calcium sensor 1 (NCS-1), interact with calcium channels and bidirectionally modulate their function [20•]. A recent study in cultures of superior cervical ganglion neurons provides compelling evidence that calcium-dependent inactivation of calcium channels can contribute to synaptic depression: Deleting the calmodulin-binding domain on P-type calcium channels to prevent their inactivation reduces synaptic depression [21••]. Among various synapses, the frequency dependencies of calcium channel inactivation and vesicle depletion are different [4,20•,22], and this could explain differences in the relative contributions of each mechanism for specific experimental conditions [21••].
Molecular determinants of depression and recovery from depression
Pharmacological or genetic manipulation of many proteins can influence depression [1,23–25]. This is not surprising considering that the initial probability of release, presynaptic calcium signaling, endocytosis, the size of vesicle pools, and replenishment of these pools can all influence depression and recovery from depression [4,13,26,27]. Consequently it is often difficult to interpret a change in the extent of depression. This is illustrated by considering the dramatic alleviation of depression when RIM proteins are eliminated [28]. This reduction in depression arises from a decrease in the probability of release [28,29], which is set by RIM via its functions in priming vesicles for release and localizing calcium channels to the active zone [30–32]. Some synapses have molecular specializations that limit the extent of depression. For example, at the cerebellar mossy fiber-to-granule cell synapse genetic deletion of Bassoon, a protein of the active zone, results in more pronounced synaptic depression. Additional results suggest that Bassoon reduces synaptic depression by aiding vesicle replenishment at release sites [33•].
Facilitation
For most synapses with a low initial probability of release, repeated stimulation at short time intervals leads to a transient increase in transmitter release probability [34]. This short-lived synaptic facilitation depends on presynaptic calcium. Several mechanisms have been proposed to account for facilitation (Figure 1b).
Residual calcium
One proposed mechanism for facilitation involves residual calcium (Cares) that persists in the presynaptic terminal following synaptic activation [1]. At the calyx of Held, linear summation of Cares (hundreds of nanomolar) with the high local calcium levels at a release site evoked by an action potential (Calocal of tens to hundreds of micromolar) will not lead to sufficient enhancement of synaptic transmission [35]. It has therefore been hypothesized that Cares increases the probability of release by binding to a sensor distinct from synaptotagmin, the sensor for synchronous release, and activating a site distinct from the low affinity sites on synaptotagmin that are responsible for vesicle fusion [36,37]. At present no such calcium sensor has been identified.
Saturation of endogenous calcium buffers
Another potential mechanism for facilitation involves calcium-binding proteins within presynaptic terminals that normally intercept calcium ions between calcium channels and release sites, thus reducing the initial probability of release [38,39]. If the first stimulus leads to calcium occupying some of these calcium-binding proteins, then more calcium will reach the release site in response to the second stimulus, and the probability of release will be elevated. This mechanism of facilitation has been demonstrated at some neocortical synapses that contain a high concentration of the calcium binding protein calbindin D-28k [40].
Facilitation of calcium currents
An increase in presynaptic calcium influx could increase probability of release and contribute to facilitation. It has been known for some time that calcium currents can be enhanced in a use-dependent manner [41,42]. Moreover, calcium-sensitive proteins such as calmodulin have previously been implicated in use-dependent increases in presynaptic calcium entry [20•]. A crucial link among these two sets of observations and facilitation was made when it was found that mutating P-type calcium channels to prevent calcium-dependent facilitation of calcium currents also suppressed synaptic facilitation [21• •].
Augmentation and post-tetanic potentiation
Augmentation and PTP are two closely related forms of enhancement that are observed following sustained, high-frequency synaptic activation [1]. PTP lasts for tens of seconds to minutes, and becomes longer lasting when the stimulus frequency and duration are increased. Augmentation is induced with less prolonged stimulation and lasts for 5–10 s. Different synapses exhibit considerable differences in the frequency and number of stimuli needed to induce augmentation and PTP, and the distinction between the two phenomena is not always clear [1].
Numerous mechanisms have been implicated in PTP (Figure 1c). PTP is accompanied by a decrease in paired-pulse plasticity, suggesting an increase in the probability of release. This increase may result from either an increase in presynaptic calcium entry or changes in the release machinery itself. At the calyx of Held synapse, tetanus-induced increases in action potential-evoked calcium entry could contribute to PTP [43,44], but at superior cervical ganglion cell synapses calcium-induced enhancement of calcium channels does not contribute significantly to PTP even though it accounts for approximately half of augmentation [21• •]. The probability of release can also be altered independently of changes in presynaptic calcium entry. For example, protein kinase C (PKC), which has been implicated in PTP [44–46], can decrease the calcium cooperativity such that the same calcium signal can evoke the release of more synaptic vesicles [47•]. Tetanus-induced alterations in the properties of the RRP can also contribute to PTP. At the calyx of Held synapse, it is thought that activation of myosin light chain kinase (MLCK) can produce alterations in the RRP that can account for about 20% of PTP [48]. Tetanic stimulation can also increase the size of miniature synaptic currents that can contribute to PTP [1]. At the calyx of Held synapse, tetanic stimulation can cause some of the vesicles to fuse with each other before fusion with the plasma membrane [49• •].
Calcium signals within the presynaptic bouton play a central role in all of the proposed mechanisms mediating PTP. At the calyceal synapse, tetanic stimulation elevates Cares to several hundred nanomolar, and this Cares decays with a time course similar to PTP [44,50], suggesting that the time course of Cares may dictate the time course of PTP. At the hippocampal Schaffer collateral-CA1 synapse, Cares decays more rapidly than PTP [45], suggesting that Cares activates biochemical cascades with slower kinetics that regulate the duration of PTP. In addition to PKC, possible targets of Cares include Munc13 [51], calmodulin/CaM kinase II [52] and its downstream effector protein synapsin [24], and the calcium-activated protease calpain [53]. Pharmacological studies support a role for PKC [44,45,54,55], but these studies have been called into question [48] and molecular genetic evidence is unavailable, in part because there are many PKC iso-forms [56]. Munc13 proteins, which are required for synaptic transmission, are essential for vesicle priming and can regulate short-term synaptic plasticity [57•,58]. There is still considerable uncertainty about the relative contributions of PKC, Munc13, and other calcium-sensitive proteins to PTP, and it has even been suggested that related forms of synaptic enhancement require both Munc13 and PKC, as well as its downstream target Munc18 [47•,59].
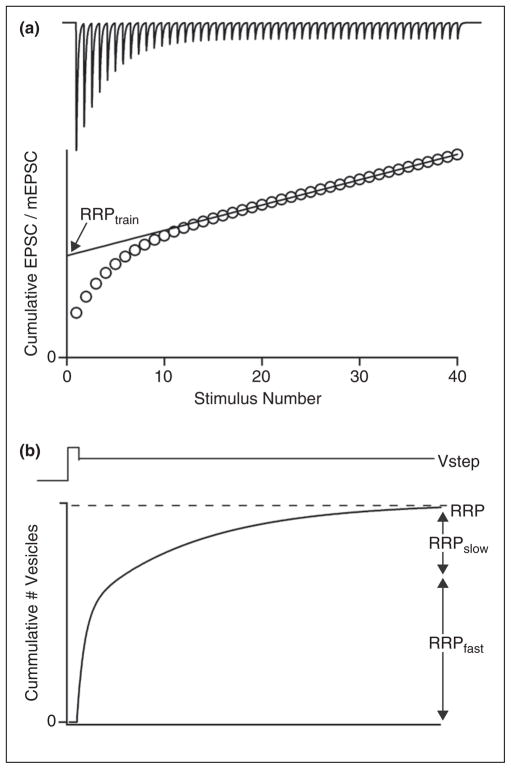
Studies of the contribution of vesicle pool size to PTP at the calyx of Held synapse highlight some of the challenges in interpreting data and drawing mechanistic conclusions. Tetanic stimulation can increase the size of the vesicle pool that is released by a high-frequency train (RRPtrain) [48], but paradoxically there is little change in the overall size of the RRP determined by large prolonged presynaptic voltage steps [60•] (Figure 2). Similarly, PKC activators can produce large increases in RRPtrain whereas they only produce small increases in the RRP assayed by voltage steps [47•]. The differences in the RRPtrain and RRP can be explained by nonuniformity in the vesicles that make up the RRP [61,62]. Vesicles that are readily released by action potentials are thought to be near voltage-gated calcium channels, whereas it is difficult for action potentials to liberate vesicles that are far from voltage-gated calcium channels [12]. In contrast, vesicles both near and far from voltage-gated calcium channels contribute to the RRP that is determined using protocols that lead to large and prolonged calcium increases [61,63]. Such non-uniformity in vesicles complicates the interpretation of short-term plasticity. For example, an increase in the calcium sensitivity of vesicles might increase RRPtrain by making some distant vesicles responsive to action potentials.
Figure 2.
Two common approaches of assessing vesicle pools relevant to understanding the mechanisms of short-term plasticity. One approach to assessing the properties of vesicles is to stimulate synapses at high frequencies under conditions where desensitization and saturation of postsynaptic receptors are blocked (a, top). (a, bottom) The amplitudes of the synaptic currents evoked by each stimulus are then measured, and a graph is made of the cumulative excitatory postsynaptic current (EPSC). When these values are divided by the amplitude of miniature EPSCs (mEPSCs) they represent the cumulative number of vesicles. The readily releasable pool liberated by the stimulus train (RRPtrain) is then determined by fitting over a linear region of this curve and extrapolating back to zero. (b) Another approach is to provide a prolonged voltage step that opens presynaptic calcium channels for a long time. The resulting postsynaptic currents then provide a measure of the readily releasable pool (again using the mEPSC size to convert from current to number of vesicles). The total number of vesicles liberated is the readily releasable pool (RRP), which consists of a fast component (RRPfast) and a slow component (RRPslow). As discussed in the text, synaptic plasticity can affect RRPtrain without influencing RRP. Understanding RRPtrain, RRPfast, RRPslow, and RRP has important implications for determining the mechanisms underlying short-term plasticity.
Conclusions
In the past decade significant advances have been made in clarifying the mechanisms responsible for short-term plasticity. Depletion of readily releasable vesicles, inactivation of release sites, and inactivation of presynaptic calcium channels can all contribute to synaptic depression. Local saturation of calcium buffers, facilitation of presynaptic calcium channels, and Cares-dependent processes can lead to synaptic facilitation. Increased quantal size, Cares-dependent increases in the probability of release, facilitation of calcium channels, and alterations in vesicles have all been implicated in PTP. But there are many unresolved questions. Why do some release sites inactivate whereas other do not? Although much is known about the molecular mechanism of calcium channel regulation, much less is known about other mechanisms. Are there specialized calcium sensors that can respond to Cares to produce facilitation, and if so what are they and how do they work? What are the molecular mechanisms that allow Cares to produce PTP? How does the heterogeneity of vesicles and release sites influence short-term plasticity? Our current view of synaptic transmission and short-term plasticity is based to a large extent on the calyx of Held, but to what extent can the properties of this synapse be generalized to others? Thus, despite recent progress in the field, many questions remain to be addressed.
Acknowledgments
We thank Miklos Antal, Aaron Best, John Crowley, Court Hull, Skyler Jackman, Michael Myoga, Todd Pressler, and Monica Thanawala for comments on a previous version of the manuscript. This work was supported by NIH grant R37 NS032405 to WGR.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 2.Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat Rev Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- 3.Ran I, Quastel DM, Mathers DA, Puil E. Fluctuation analysis of tetanic rundown (short-term depression) at a corticothalamic synapse. Biophys J. 2009;96:2505–2531. doi: 10.1016/j.bpj.2008.12.3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002;25:206–212. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Manis PB. Short-term synaptic depression and recovery at the mature mammalian endbulb of Held synapse in mice. J Neurophysiol. 2008;100:1255–1264. doi: 10.1152/jn.90715.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dittman JS, Regehr WG. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J Neurosci. 1998;18:6147–6162. doi: 10.1523/JNEUROSCI.18-16-06147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallermann S, Kittel RJ, Wichmann C, Weyhersmuller A, Fouquet W, Mertel S, Owald D, Eimer S, Depner H, Schwarzel M, et al. Naked dense bodies provoke depression. J Neurosci. 2010;30:14340–14345. doi: 10.1523/JNEUROSCI.2495-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hosoi N, Sakaba T, Neher E. Quantitative analysis of calcium-dependent vesicle recruitment and its functional role at the calyx of Held synapse. J Neurosci. 2007;27:14286–14298. doi: 10.1523/JNEUROSCI.4122-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaba T. Two Ca(2+)-dependent steps controlling synaptic vesicle fusion and replenishment at the cerebellar basket cell terminal. Neuron. 2008;57:406–419. doi: 10.1016/j.neuron.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Sakaba T, Neher E. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron. 2001;32:1119–1131. doi: 10.1016/s0896-6273(01)00543-8. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Xu-Friedman MA. Relative roles of different mechanisms of depression at the mouse endbulb of Held. J Neurophysiol. 2008;99:2510–2521. doi: 10.1152/jn.01293.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 13.von Gersdorff H, Borst JG. Short-term plasticity at the calyx of Held. Nat Rev Neurosci. 2002;3:53–64. doi: 10.1038/nrn705. [DOI] [PubMed] [Google Scholar]
- 14••.Hosoi N, Holt M, Sakaba T. Calcium dependence of exo- and endocytotic coupling at a glutamatergic synapse. Neuron. 2009;63:216–229. doi: 10.1016/j.neuron.2009.06.010. In a series of elegant experiments at the calyx of Held synapse, Hosoi et al. find that blocking slow endocytosis enhances synaptic depression and slows recovery from depression, which they attribute to delayed recruitment of vesicles back to the readily releasable pool. [DOI] [PubMed] [Google Scholar]
- 15.Bagnall MW, McElvain LE, Faulstich M, du Lac S. Frequency-independent synaptic transmission supports a linear vestibular behavior. Neuron. 2008;60:343–352. doi: 10.1016/j.neuron.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomson AM, Bannister AP. Release-independent depression at pyramidal inputs onto specific cell targets: dual recordings in slices of rat cortex. J Physiol. 1999;519(Pt 1):57–70. doi: 10.1111/j.1469-7793.1999.0057o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waldeck RF, Pereda A, Faber DS. Properties and plasticity of paired-pulse depression at a central synapse. J Neurosci. 2000;20:5312–5320. doi: 10.1523/JNEUROSCI.20-14-05312.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/s0896-6273(00)81017-x. [DOI] [PubMed] [Google Scholar]
- 19.Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 20•.Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. This comprehensive review discusses the cellular and molecular mechanisms through which presynaptic calcium channels are regulated as well as the role of calcium channel signaling complexes in synaptic transmission and plasticity. A brief discussion of the involvement of these channels in neurological disorders is also included. [DOI] [PubMed] [Google Scholar]
- 21••.Mochida S, Few AP, Scheuer T, Catterall WA. Regulation of presynaptic Ca(V)2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron. 2008;57:210–216. doi: 10.1016/j.neuron.2007.11.036. By manipulating different CaS-binding domains on P-type calcium channels, the authors generated recombinant calcium channels without CaS-dependent inactivation or CaS-dependent facilitation properties. Expression of these mutated channels in cultured superior cervical ganglion neurons provided compelling evidence for the role of CaS-mediated calcium current inactivation in synaptic depression, as well as for a role of CaS-mediated calcium current enhancement in facilitation and augmentation, but not PTP. [DOI] [PubMed] [Google Scholar]
- 22.Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P. Distinct pools of synaptic vesicles in neurotransmitter release. Nature. 1995;375:493–497. doi: 10.1038/375493a0. [DOI] [PubMed] [Google Scholar]
- 23.Powell CM. Gene targeting of presynaptic proteins in synaptic plasticity and memory: across the great divide. Neurobiol Learn Mem. 2006;85:2–15. doi: 10.1016/j.nlm.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cesca F, Baldelli P, Valtorta F, Benfenati F. The synapsins: key actors of synapse function and plasticity. Prog Neurobiol. 2010;91:313–348. doi: 10.1016/j.pneurobio.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Mittelstaedt T, Alvarez-Baron E, Schoch S. RIM proteins and their role in synapse function. Biol Chem. 2010;391:599–606. doi: 10.1515/BC.2010.064. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, He L, Wu LG. Role of Ca(2+) channels in short-term synaptic plasticity. Curr Opin Neurobiol. 2007;17:352–359. doi: 10.1016/j.conb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Wilkinson RS, Lin MY. Endocytosis and synaptic plasticity: might the tail wag the dog? Trends Neurosci. 2004;27:171–174. doi: 10.1016/j.tins.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron. 2004;42:889–896. doi: 10.1016/j.neuron.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 29.Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415:321–326. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 30.Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM determines Ca(2+) channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC. RIM proteins tether Ca(2+) channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deng L, Kaeser PS, Xu W, Sudhof TC. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Hallermann S, Fejtova A, Schmidt H, Weyhersmuller A, Silver RA, Gundelfinger ED, Eilers J. Bassoon speeds vesicle reloading at a central excitatory synapse. Neuron. 2010;68:710–723. doi: 10.1016/j.neuron.2010.10.026. Using Bassoon knockout mice, the authors investigated the function of this active zone component in synaptic transmission and plasticity at cerebellar mossy fiber-to-granule cell synapses. They reported enhancement of short-term depression in knockouts, with no effect on basal transmission. Using fluctuation and quantal analysis as well as computer simulations, the authors concluded that elevated depression is due to impaired vesicle reloading rates, and proposed a role for Bassoon in replenishing release sites with vesicles. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz B, Miledi R. The role of calcium in neuromuscular facilitation. J Physiol. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felmy F, Neher E, Schneggenburger R. Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron. 2003;37:801–811. doi: 10.1016/s0896-6273(03)00085-0. [DOI] [PubMed] [Google Scholar]
- 36.Tang Y, Schlumpberger T, Kim T, Lueker M, Zucker RS. Effects of mobile buffers on facilitation: experimental and computational studies. Biophys J. 2000;78:2735–2751. doi: 10.1016/s0006-3495(00)76819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matveev V, Zucker RS, Sherman A. Facilitation through buffer saturation: constraints on endogenous buffering properties. Biophys J. 2004;86:2691–2709. doi: 10.1016/S0006-3495(04)74324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 40.Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa T, Kaneko M, Shin HS, Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J Physiol. 2005;568:199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inchauspe CG, Martini FJ, Forsythe ID, Uchitel OD. Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of Held presynaptic terminal. J Neurosci. 2004;24:10379–10383. doi: 10.1523/JNEUROSCI.2104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Habets RL, Borst JG. An increase in calcium influx contributes to post-tetanic potentiation at the rat calyx of Held synapse. J Neurophysiol. 2006;96:2868–2876. doi: 10.1152/jn.00427.2006. [DOI] [PubMed] [Google Scholar]
- 44.Korogod N, Lou X, Schneggenburger R. Posttetanic potentiation critically depends on an enhanced Ca(2+) sensitivity of vesicle fusion mediated by presynaptic PKC. Proc Natl Acad Sci U S A. 2007;104:15923–15928. doi: 10.1073/pnas.0704603104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brager DH, Cai X, Thompson SM. Activity-dependent activation of presynaptic protein kinase C mediates post-tetanic potentiation. Nat Neurosci. 2003;6:551–552. doi: 10.1038/nn1067. [DOI] [PubMed] [Google Scholar]
- 46.Beierlein M, Fioravante D, Regehr WG. Differential expression of posttetanic potentiation and retrograde signaling mediate target-dependent short-term synaptic plasticity. Neuron. 2007;54:949–959. doi: 10.1016/j.neuron.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47•.Lou X, Korogod N, Brose N, Schneggenburger R. Phorbol esters modulate spontaneous and Ca2+-evoked transmitter release via acting on both Munc13 and protein kinase C. J Neurosci. 2008;28:8257–8267. doi: 10.1523/JNEUROSCI.0550-08.2008. The authors examined the effect of PKC/Munc13 activators on synaptic transmission. Using trains of action potentials and the deconvolution method (see Figure 2 of this review), they estimated the size of the RRPtrain and of the RRP before and after application of the activators. Although estimates of RRPtrain increased in the presence of the activator, RRP size did not change. Increasing extracellular calcium also increased RRPtrain. The authors concluded that the increase in RRPtrain probably does not reflect a true increase in the size of the readily releasable pool but an increase in release probability. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JS, Kim MH, Ho WK, Lee SH. Presynaptic release probability and readily releasable pool size are regulated by two independent mechanisms during posttetanic potentiation at the calyx of Held synapse. J Neurosci. 2008;28:7945–7953. doi: 10.1523/JNEUROSCI.2165-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49••.He L, Xue L, Xu J, McNeil BD, Bai L, Melicoff E, Adachi R, Wu LG. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature. 2009;459:93–97. doi: 10.1038/nature07860. He et al. performed cell-attached capacitance recordings from the release face of the calyx of Held and found that elevated presynaptic calcium lead to compound fusion (vesicles fusing with each other before fusing with the plasma membrane). They showed that this leads to an increase in the size of miniature excitatory postsynaptic currents, which contributes to PTP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habets RL, Borst JG. Post-tetanic potentiation in the rat calyx of Held synapse. J Physiol. 2005;564:173–187. doi: 10.1113/jphysiol.2004.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rodriguez-Castaneda F, Maestre-Martinez M, Coudevylle N, Dimova K, Junge H, Lipstein N, Lee D, Becker S, Brose N, Jahn O, et al. Modular architecture of Munc13/calmodulin complexes: dual regulation by Ca2+ and possible function in short-term synaptic plasticity. EMBO J. 2010;29:680–691. doi: 10.1038/emboj.2009.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fiumara F, Milanese C, Corradi A, Giovedi S, Leitinger G, Menegon A, Montarolo PG, Benfenati F, Ghirardi M. Phosphorylation of synapsin domain A is required for post-tetanic potentiation. J Cell Sci. 2007;120:3228–3237. doi: 10.1242/jcs.012005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khoutorsky A, Spira ME. Activity-dependent calpain activation plays a critical role in synaptic facilitation and post-tetanic potentiation. Learn Mem. 2009;16:129–141. doi: 10.1101/lm.1275709. [DOI] [PubMed] [Google Scholar]
- 54.Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber–interneuron synapse. Proc Natl Acad Sci U S A. 2001;98:14708–14713. doi: 10.1073/pnas.251610898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee D, Lee KH, Ho WK, Lee SH. Target cell-specific involvement of presynaptic mitochondria in post-tetanic potentiation at hippocampal mossy fiber synapses. J Neurosci. 2007;27:13603–13613. doi: 10.1523/JNEUROSCI.3985-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Newton A. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 57•.Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, Camacho-Perez M, Brose N, Machius M, Rizo J, et al. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol. 2010;17:280–288. doi: 10.1038/nsmb.1758. Munc13 is a presynaptic protein involved in vesicle priming. Here, the authors showed that Mun13 can bind phospholipids in a calcium-dependent manner. Mutations that inactivated this binding increased synaptic depression, whereas mutations that increased this binding enhanced basal release as well as augmentation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Junge HJ, Rhee JS, Jahn O, Varoqueaux F, Spiess J, Waxham MN, Rosenmund C, Brose N. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell. 2004;118:389–401. doi: 10.1016/j.cell.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 59.Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron. 2007;54:275–290. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 60•.Lee JS, Ho WK, Lee SH. Post-tetanic increase in the fast-releasing synaptic vesicle pool at the expense of the slowly releasing pool. J Gen Physiol. 2010;136:259–272. doi: 10.1085/jgp.201010437. Previous studies showed that tetanic stimulation increases RRPtrain (Figure 2a), suggesting that pool size increases contribute to PTP. Previously it had not been determined if RRP increases (as in Figure 2b) contribute to PTP, because PTP is not observed during whole cell recordings from presynaptic boutons. Lee et al. found that PTP and changes in RRPtrain were intact when calmodulin was included in the recording pipette. They found that tetanic stimulation did not increase overall RRP size but it did shift the balance between the two components of the RRP, so that vesicles from the slow pool are converted to fast-releasing vesicles. This mechanism, which depends on calmodulin and myosin light chain kinase, can account for 20% of PTP previously observed by this group under control conditions. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakaba T. Roles of the fast-releasing and the slowly releasing vesicles in synaptic transmission at the calyx of Held. J Neurosci. 2006;26:5863–5871. doi: 10.1523/JNEUROSCI.0182-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller M, Goutman JD, Kochubey O, Schneggenburger R. Interaction between facilitation and depression at a large CNS synapse reveals mechanisms of short-term plasticity. J Neurosci. 2010;30:2007–2016. doi: 10.1523/JNEUROSCI.4378-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sakaba T, Neher E. Quantitative relationship between transmitter release and calcium current at the calyx of Held synapse. J Neurosci. 2001;21:462–476. doi: 10.1523/JNEUROSCI.21-02-00462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]