Abstract
Generation of meiotic crossovers in many eukaryotes requires the elimination of anti-crossover activities by utilizing the Msh4–Msh5 heterodimer to block helicases. Msh4 and Msh5 have been lost from the flies Drosophila and Glossina but we identified a complex of mini-chromosome maintenance (MCM) proteins that functionally replace Msh4–Msh5. REC, an ortholog of MCM8 that evolved under strong positive selection in flies, interacts with MEI-217 and MEI-218, which arose from a previously undescribed metazoan-specific MCM protein. Meiotic crossovers are reduced in Drosophila rec, mei-217, and mei-218 mutants; however, removal of the Bloom syndrome helicase ortholog restores crossovers. Thus, MCMs were co-opted into a novel complex that replaces the meiotic pro-crossover function of Msh4–Msh5 in flies.
Crossovers (COs) between homologous chromosomes can be beneficial or detrimental, depending on their context (1). Meiotic COs increase genetic diversity and promote accurate chromosome segregation, whereas mitotic COs can lead to loss of heterozygosity, potentially triggering tumorigenesis. Mitotic COs are prevented by “anti-CO” proteins. A key anti-CO protein is the Bloom syndrome helicase BLM, which generates non-crossover products by unwinding recombination intermediates that might otherwise be processed into COs (2). In meiosis, CO formation is encouraged through inhibition of anti-CO proteins. The budding yeast Msh4–Msh5 heterodimer antagonizes the BLM ortholog Sgs1 (3). Msh4 and Msh5 are found in all metazoans for which sequence is available except Drosophila species and their fellow Schizophoran the tsetse fly Glossina morsitans (Figs. S1 and S2). The lack of recognizable orthologs of these proteins suggests that these species evolved another protein or complex to block the anti-CO activity of BLM.
Like S. cerevisiae Msh4 and Msh5 mutants, the only defects in Drosophila rec, mei-217 and mei-218 mutants are in meiotic recombination (4–9). REC is orthologous to MCM8 (6); MCMs have properties reminiscent of Msh4–Msh5. MCM2-7, which are essential for replication in eukaryotes, form a heterohexamer that encircles DNA (10). Similarly, Msh4–Msh5 is thought to encircle recombination intermediates (11). In both cases, this activity is regulated by ATP binding and hydrolysis (10, 11).
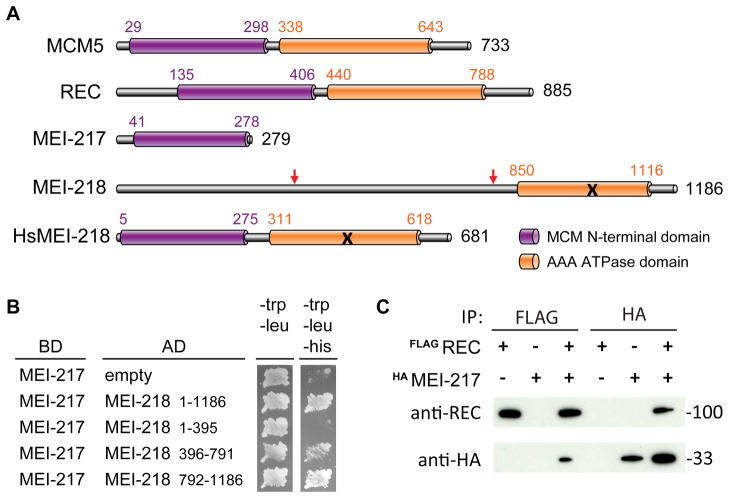
MEI-217 initially appeared to be novel, as BLAST searches fail to identify homologs outside Dipterans and searches of the Conserved Domain Database (CDD) (12) do not detect any domains. BLAST searches with MEI-218 identify a single putative ortholog in metazoans (Figs. S1 and S2). A CDD search returns a hit to the MCM domain in the C-terminus of MEI-218, but the score is low and the match covers only a third of the domain (Fig. S3). To verify the presence of this domain we conducted structure-based searches with PHYRE (Fig. 1A, (13)). This analysis reveals that the C-terminus of MEI-218 has a similar structure to the AAA ATPase domain of MCMs (Fig. S3b). Canonical MCMs have both an N-terminal MCM domain and a C-terminal ATPase domain. The N-terminal domain is present in vertebrate MEI-218 but not in Drosophila MEI-218. However, PHYRE with MEI-217 shows that its predicted structure is similar to the MCM N-terminal domain. Since MEI-217 and MEI-218 are encoded by overlapping open reading frames on the same transcript (7), we infer they evolved from an MCM-like protein represented by a single polypeptide in other metazoans.
Fig. 1.
(A) Structural domains identified through PHYRE. “MCM N-terminal domain” corresponds to Protein Data Bank fold 3f9v and “AAA ATPase domain” to fold ID 3f8t. The “x” on MEI-218 symbolizes changes in the ATP binding and hydrolysis motifs predicted to abolish ATPase activity (Fig. S3B). Red arrows on MEI-218 indicate segments used in yeast two-hybrid analysis. (B) Yeast two-hybrid interactions between MEI-217 and MEI-218. Cells expressing the indicated fusions to the GAL4 DNA binding domain (BD) or activating domain (AD) were streaked onto selective media. Growth on – trp – leu – his indicates an interaction. (C) Co-immunoprecipitation of REC and MEI-217. Epitope-tagged mei-MCMs were co-expressed in insect cells, immunoprecipitated with anti-tag antibodies, blotted, and probed with antibodies to REC and to the HA tag (19).
The shared phenotypes and MCM domains suggest that REC, MEI-217, and MEI-218 function together in meiotic recombination. To distinguish them from the replicative MCMs, we hereafter call REC, MEI-217, and MEI-218 “mei-MCMs”. Since MCM2-7 function as a heterohexamer, we investigated whether the mei-MCMs form a complex. MEI-217 interacts strongly with both the C-terminal third of MEI-218 and REC (Figs. 1B and 1C), suggesting that the mei-MCMs form a complex. This complex likely also contains one or more replicative MCMs. A meiosis-specific mutation in Mcm5 causes the same phenotypes as mei-MCM mutants (14), making MCM5 a strong candidate to be a component of the complex.
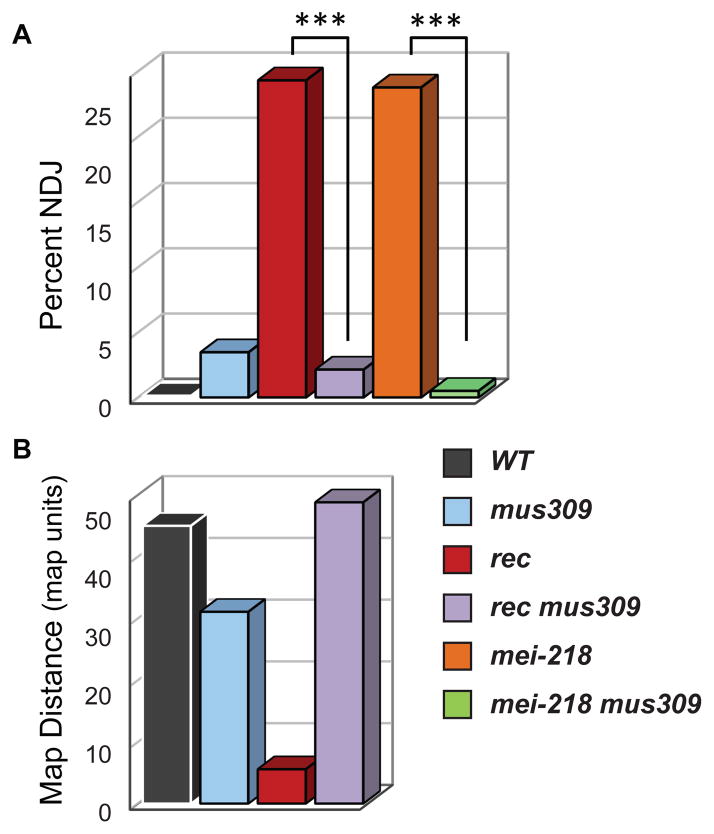
Noting the genetic and biochemical similarities between mei-MCMs and Msh4–Msh5, we hypothesized that the mei-MCMs antagonize DmBLM in lieu of Msh4–Msh5. This hypothesis predicts that removing DmBLM should compensate for mei-MCM mutations; in budding yeast the CO defect in msh4 mutants is suppressed by removing Sgs1 (3). Few COs are made in rec and mei-218 single mutants, resulting in high nondisjunction (NDJ) of meiotic chromosomes (Fig. 2A). In contrast, mutations in mus309, which encodes DmBLM, cause only a mild reduction in COs and correspondingly low levels of NDJ. Strikingly, mus309 mutations suppress the high NDJ phenotype of rec and mei-218 mutants (Fig. 2A). Furthermore, the low CO rate in rec mutants returns to an approximately wild-type rate in mus309 rec double mutants (Figs. 2B and S4, tables S1–S4), indicating that mei-MCMs are not essential for generating meiotic COs if DmBLM is absent, and supporting our hypothesis that mei-MCMs oppose the known anti-CO activities of DmBLM (15, 16).
Fig. 2.
(A) X chromosome non-disjunction (NDJ) across more than 1500 individuals for each genotype except mei-218; mus309 (n=383). ***, P < 0.0001. (B) This graph shows the summed map distance in map units (m. u., equivalent to centiMorgans) across five intervals spanning ~20% of the genome for over 1000 individuals for each genotype (19). P < 0.0001 for all comparisons except WT versus mus309 rec, P = 0.0674.
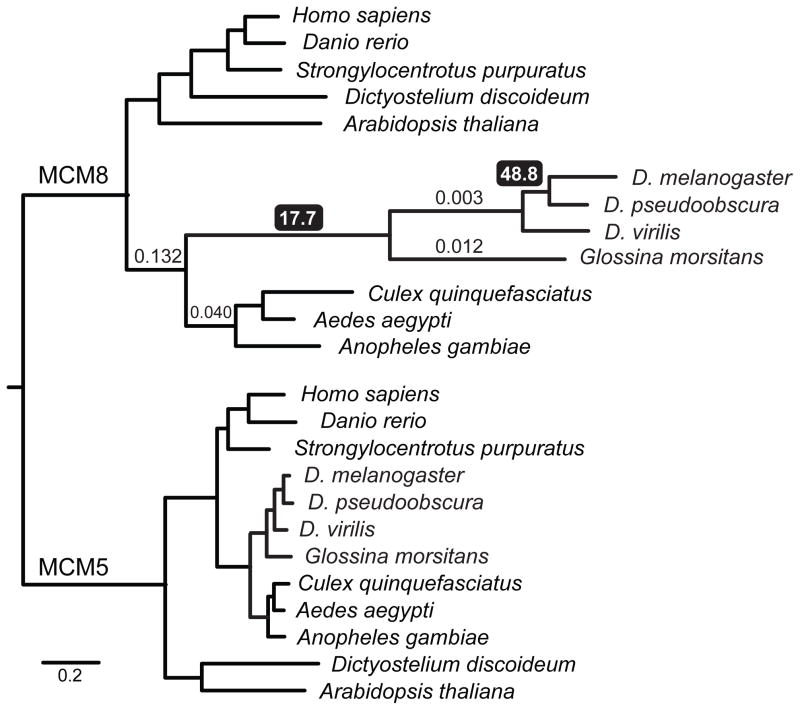
mei-MCMs appear to functionally replace Msh4–Msh5 in Schizophora, and presumably evolved to do so in response to natural selection. Several evolutionary scenarios could lead to this result (Fig. S5), but most predict that there would be evidence of adaptive divergence of mei-MCM genes in Schizophora. REC was previously noted to be highly diverged in Drosophila (6, 17); we found that Glossina MCM8/REC is similarly divergent (Fig. 3). The presence or absence of MCM8 correlates with that of its functional partner MCM9 throughout eukaryotes, except in Drosophila and Glossina, which retained MCM8/REC while losing MCM9 (Fig. S1 and S2). The loss of MCM9 suggests that MCM8 evolved a novel function in an ancestor to Schizophora.
Fig. 3.
Maximum-likelihood tree from an alignment of the conserved MCM domains of MCM8/REC and MCM5 from diverse taxa. Branch lengths indicate the number of substitutions per site (see scale). Numbers above branches show dN/dS estimates for selected branches; those with black background highlight branches with dN/dS estimates greater than one, suggesting positive selection. See fig. S6 for more description and additional dN/dS estimates.
Divergence in rec and loss of MCM9 occurred after the split between mosquitoes and higher flies 200–250 million years ago (MYA), but prior to the emergence of the Schizophora 65 MYA. To test whether patterns of sequence evolution were consistent with positive selection leading to the divergence of rec, we estimated the ratio between the rate of base pair substitutions at non-synonymous sites (dN) and the rate at synonymous sites (dS) among Dipterans in MCM8/rec. We compared 15 evolutionary models, ranging from conservation of dN/dS ratios across all taxa surveyed to allowing free evolution of dN/dS ratios along all branches, and including models testing specific hypotheses about the evolution of rec along different branches of the insect phylogeny. The best fitting model (P = 0.0002 vs. next best model) supports the hypothesis that rapid protein coding divergence was driven by positive selection prior to the split of tsetse flies from fruit flies (Figs. 3 and S6, table S5). Thus we infer that natural selection likely drove the repurposing of REC into its new role as an antagonist of DmBLM. Recent evolution of rec shows much lower levels of non-synonymous changes, suggesting subsequent functional constraint (Fig. S6). MEI-217 and MEI-218 have also diverged substantially from the ancestral MCM structure: they split into two polypeptides and MEI-218 acquired an N-terminal extension (Figs. S7 and S8).
Our data show that flies evolved a novel MCM complex to antagonize anti-CO functions of BLM during meiosis, a role held by Msh4–Msh5 in other organisms. Although we do not know what evolutionary forces ultimately drove the loss of Msh4–Msh5 and the repurposing of mei-MCMs, it is tempting to speculate that these forces also led to another fundamental meiotic difference in Drosophila and Glossina compared to mosquitoes – the absence of recombination in males, which was first noted in Drosophila by Morgan 100 years ago (Fig. S5, (18)). Resolving the conundrum of why the mei-MCMs supplanted Msh4–Msh5 will require a deeper understanding of both the evolutionary origins of the mei-MCMs and the functional differences between mei-MCMs and Msh4–Msh5.
Supplementary Material
Acknowledgments
We thank K. McKim for helpful discussions and for sharing unpublished results and N. Crown and other members of the Sekelsky laboratory for helpful comments on the manuscript. This work was supported by a grant from the NIH to JS (GM061252) and from the NSF to JS, CDJ, and G.C. Copenhaver (MCB-0618691). KPK was supported in part by NIH grant 5T32GM007092. Alignments and trees were submitted to TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S13435).
Footnotes
References and Notes
- 1.Andersen SL, Sekelsky J. Meiotic versus mitotic recombination: two different routes for double-strand break repair: the different functions of meiotic versus mitotic DSB repair are reflected in different pathway usage and different outcomes. Bioessays. 2010;32:1058. doi: 10.1002/bies.201000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu WK, Hickson ID. RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer. 2009;9:644. doi: 10.1038/nrc2682. [DOI] [PubMed] [Google Scholar]
- 3.Jessop L, Rockmill B, Roeder GS, Lichten M. Meiotic chromosome synapsis-promoting proteins antagonize the anti-crossover activity of sgs1. PLoS Genet. 2006;2:e155. doi: 10.1371/journal.pgen.0020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross-Macdonald P, Roeder GS. Mutation of a meiosis-specific MutS homolog decreases crossing over but not mismatch correction. Cell. 1994;79:1069. doi: 10.1016/0092-8674(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 5.Hollingsworth NM, Ponte L, Halsey C. MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes Dev. 1995;9:1728. doi: 10.1101/gad.9.14.1728. [DOI] [PubMed] [Google Scholar]
- 6.Blanton HL, et al. REC, Drosophila MCM8, drives formation of meiotic crossovers. PLoS Genet. 2005;1:343. doi: 10.1371/journal.pgen.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Jang JK, Graham J, Nycz K, McKim KS. Two genes required for meiotic recombination in Drosophila are expressed from a dicistronic message. Genetics. 2000;154:1735. doi: 10.1093/genetics/154.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker BS, Carpenter ATC. Genetic analysis of sex chromosomal meiotic mutants in Drosophila melanogaster. Genetics. 1972;71:255. doi: 10.1093/genetics/71.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manheim EA, Jang JK, Dominic D, McKim KS. Cytoplasmic localization and evolutionary conservation of MEI-218, a protein required for meiotic crossing-over in Drosophila. Mol Biol Cell. 2002;13:84. doi: 10.1091/mbc.01-06-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remus D, et al. Concerted loading of Mcm2-7 double hexamers around DNA during DNA replication origin licensing. Cell. 2009;139:719. doi: 10.1016/j.cell.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snowden T, Acharya S, Butz C, Berardini M, Fishel R. hMSH4-hMSH5 recognizes Holliday Junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell. 2004;15:437. doi: 10.1016/j.molcel.2004.06.040. [DOI] [PubMed] [Google Scholar]
- 12.Marchler-Bauer A, et al. CDD: a Conserved Domain Database for the functional annotation of proteins. Nucleic Acids Res. 2011;39:D225. doi: 10.1093/nar/gkq1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protocols. 2009;4:363. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 14.Lake CM, Teeter K, Page SL, Nielsen R, Hawley RS. A genetic analysis of the Drosophila mcm5 gene defines a domain specifically required for meiotic recombination. Genetics. 2007;176:2151. doi: 10.1534/genetics.107.073551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams MD, McVey M, Sekelsky J. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science. 2003;299:265. doi: 10.1126/science.1077198. [DOI] [PubMed] [Google Scholar]
- 16.McVey M, Andersen S, Broze Y, Sekelsky J. Multiple functions of DmBlm helicase in maintenance of genome stability. Genetics. 2007;176:1979. doi: 10.1534/genetics.106.070052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Richards TA, Aves SJ. Ancient diversification of eukaryotic MCM DNA replication proteins. BMC Evol Biol. 2009;9:60. doi: 10.1186/1471-2148-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan TH. Complete linkage in the second chromosome of the male of Drosophila. Science. 1912;36:719. [Google Scholar]
- 19.Materials and methods are available as supplementary material on Science Online.
- 20.Boyd JB, Golino MD, Shaw KES, Osgood CJ, Green MM. Third-chromosome mutagen-sensitive mutants of Drosophila melanogaster. Genetics. 1981;97:607. doi: 10.1093/genetics/97.3-4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKim KS, Dahmus JB, Hawley RS. Cloning of the Drosophila melanogaster meiotic recombination gene mei-218: a genetic and molecular analysis of interval 15E. Genetics. 1996;144:215. doi: 10.1093/genetics/144.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rørth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 23.McGaugh SE, Noor MA. Genomic impacts of chromosomal inversions in parapatric Drosophila species. Philos Trans R Soc Lond B Biol Sci. 2012;367:422. doi: 10.1098/rstb.2011.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol. 2001;18:691. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- 27.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 28.Grimaldi DA, Engel MS. Evolution of the Insects. Cambridge University Press; 2005. [Google Scholar]
- 29.Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci. 1997 Oct;13:555. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 30.Goldman N, Yang Z. A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol Biol Evol. 1994;11:725. doi: 10.1093/oxfordjournals.molbev.a040153. [DOI] [PubMed] [Google Scholar]
- 31.James P, Halladay J, Craig EA. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics. 1996;144:1425. doi: 10.1093/genetics/144.4.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Muyt A, et al. BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol Cell. 2012;46:43. doi: 10.1016/j.molcel.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zakharyevich K, Tang S, Ma Y, Hunter N. Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell. 2012;149:334. doi: 10.1016/j.cell.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutzmann M, et al. MCM8- and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol Cell. 2012;47:523. doi: 10.1016/j.molcel.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 35.Nishimura K, et al. Mcm8 and Mcm9 form a complex that functions in homologous recombination repair induced by DNA interstrand crosslinks. Mol Cell. 2012;47:511. doi: 10.1016/j.molcel.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 36.Collin R, Cipriani R. Dollo’s law and the re-evolution of shell coiling. Proc Biol Sci. 2003;270:2551. doi: 10.1098/rspb.2003.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Collin R, Miglietta MP. Reversing opinions on Dollo’s Law. Trends Ecol Evol. 2008;23:602. doi: 10.1016/j.tree.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 38.Adams MD, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 39.Wiegmann BM, et al. Episodic radiations in the fly tree of life. Proc Natl Acad Sci USA. 2011 Apr 5;108:5690. doi: 10.1073/pnas.1012675108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gooding RH, Rolseth BM. Genetics of Glossina palpalis palpalis: designation of linkage groups and the mapping of eight biochemical and visible marker genes. Genome. 1995;38:833. doi: 10.1139/g95-109. [DOI] [PubMed] [Google Scholar]
- 41.Gilchrist BM, Haldane JBS. Sex linkage and sex determination in a mosquito, Culex molestus. Hereditas. 1947;33:175. [Google Scholar]
- 42.McClelland GA. Sex-linkage at two loci affecting eye pigment in the mosquito Aedes aegypti (diptera: culicidae) Canadian J Genet Cytol. 1966;8:192. doi: 10.1139/g66-024. [DOI] [PubMed] [Google Scholar]
- 43.Zheng L, Benedict MQ, Cornel AJ, Collins FH, Kafatos FC. An integrated genetic map of the African human malaria vector mosquito, Anopheles gambiae. Genetics. 1996;143:941. doi: 10.1093/genetics/143.2.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.