Abstract
Regulatory changes in cytokine permeation across the blood–brain barrier (BBB) may have crucial roles in central nervous system (CNS) autoimmune disease. Accordingly, we examined the interactions of interleukin (IL)-15 with the cerebral vasculature after induction of experimental autoimmune encephalomyelitis (EAE). In contrast to the influx of 125I-IL15 from blood to the CNS in normal mice and the persistence of IL15 influx in the spinal cord of EAE mice, influx was reduced in the EAE brain. Analyses of disappearance kinetics, FITC (fluorescein isothiocyanate)-albumin space, and delivery of IL15 by in situ perfusion, all indicate that the changes were not caused by BBB disruption but by the rapid availability (high volume of distribution) of IL15 and albumin. Although there was no significant change in the BBB permeation of IL15 in either direction in EAE mice, there was an upregulation of its specific receptor, IL15Rα, and an increased in situ production of IL15 mRNA that showed regional variation in both basal and EAE states. Overall, for IL15, its increased cerebral vascular space in the brain was equally as important as its persistent influx across the blood–spinal cord barrier, indicating that it is fully capable of activating the upregulated IL15Rα in the brain along with the intrinsic CNS source of IL15 in EAE mice.
Keywords: autoimmunity, blood–brain barrier, blood-spinal cord barrier, EAE, IL15
Introduction
Interleukin (IL)-15 is a 14-kD pleiotropic cytokine in the 4-α-helix bundle cytokine family, which also includes IL2, IL3, IL4, IL6, IL21, granulocyte-macrophage colony-stimulating factor, granulocyte colony-stimulating factor, erythropoietin, growth hormone, and prolactin (Budagian et al, 2006). Interleukin-15 has both proinflammatory and antiinflammatory effects and is a potent antiapoptotic cytokine and T-cell chemoattractant (McInnes et al, 1996; Wilkinson and Liew, 1995). The roles of IL15 and its receptors in vascular function and neuroinflammation are supported by their presence in the endothelia (Nilsen et al, 1998), microglia, and brain in general (Hanisch et al, 1997; Kurowska et al, 2002). The constitutive expression of IL15 in neurons and the induced expression in glial cells (Maslinska, 2001) suggest its crucial role in normal brain function and in central nervous system (CNS) development. Results from studies with IL2 knockout mice suggest that IL15 might be involved in hippocampal neurogenesis (Beck et al, 2005). Interleukin-15 induces dose-dependent increases of non-REM (rapid eye movement) sleep in rabbits after intracerebroventricular injection (Kubota et al, 2001), but the mechanism is not clear.
Interleukin-15 is a crucial target in the treatment of inflammatory and autoimmune diseases (Baslund et al, 2005; McInnes and Gracie, 2004; Waldmann, 2004). In autoimmune diseases, IL15 seems to be detrimental as it inhibits self-tolerance mediated by activation-induced cell death and induces other proinflammatory cytokines, including tumor necrosis factor-α (TNF-α) and IL1β (McInnes et al, 1997; McInnes and Schett, 2007). The concentration of IL15 is increased in both the serum and cerebrospinal fluid (CSF) of patients with multiple sclerosis, and the high serum/CSF ratio in these patients is believed to suggest a predominant role of the peripheral production of IL15 in immunopathology (Rentzos et al, 2006). Both IL15 and IL2 seem to worsen rheumatoid arthritis (Baslund et al, 2005). Daclizumab (Zenapax, Hoffmann-La Roche Inc., Nutley, NJ, USA), an antibody against IL2, has shown success in a recently completed phase II clinical trial of relapsing and remitting multiple sclerosis after monthly intravenous injections (Rose et al, 2007). This provides a strong basis for determining the interactions of IL15 with the blood–CNS barriers in the established animal model for multiple sclerosis—experimental autoimmune encephalomyelitis (EAE).
The blood–brain barrier (BBB) and blood-spinal cord barrier constitute a regulatory interface that allows the selective entry of certain peptides and cytokines, yet bars many others. Experimental autoimmune encephalomyelitis is associated with a dynamic pattern of BBB disruption that shows specificity in different brain and spinal cord regions during the course of the disease. The peak of neurologic deficits is associated with the maximal increase of BBB permeability to vascular markers, and there is an enhancement of the transport system for TNF (Pan et al, 1996). Tumor necrosis factor is a strong activator of the IL15 system in cerebral endothelia. It increases IL15 by reducing its protein turnover initially and upregulating mRNA expression afterwards, and it also increases the production of IL15Rα at both mRNA and protein levels (Yu C et al, unpublished observations). Although TNF crosses the BBB by a saturable influx system (Gutierrez et al, 1993; Pan et al, 1997; Pan and Kastin, 2001), the influx of IL15 cross saturably (Pan et al, 2008c). It is not known whether EAE changes the BBB permeation of IL15 as does TNF. A comparison of the patterns of interactions of TNF and IL15 with the BBB should also facilitate understanding of the cytokine cascades in EAE.
The transport of peptides and cytokines across the BBB can occur in either direction, involving an influx from blood to the CNS and an efflux from the CNS to blood. For the latter, there are known efflux transporters present in cerebral endothelia and perivascular astrocytes. The well-studied efflux transporter, P-glycoprotein (Pgp), is a 12-transmembrane protein located at the apical/luminal surface of endothelial cells and belongs to the ABC (adenosine triphosphate-binding cassette) family of energy-dependent transporters, which has a broad spectrum of substrates. Regulation of Pgp expression and activity occurs in inflammation (Yu et al, 2007, 2008). T-helper-1 and monocytes/macrophage cytokines induce robust Pgp upregulation in mixed glial culture (Lisak et al, 2009). Neither IL15 itself (Pan et al, 2008c) nor the related cytokine IL2 (Waguespack et al, 1994) enters the CNS by a saturable transport mechanism in the basal state, although neuroinflammation induced by lipopolysaccharide increases the permeation of IL15 from blood to the CNS (Pan et al, 2008c). Interleukin-2 leaves the brain through a brain-to-blood transport system (Banks et al, 2004), yet the fate of IL15 in CSF has not been studied. Therefore, we determined the regulatory changes in IL15 permeation from blood to the CNS (a summation or balance of influx and possible efflux if IL15 is a substrate for ABC transporters), and also measured the brain-to-blood transport in EAE mice.
Materials and methods
Experimental Autoimmune Encephalomyelitis Induction
The animal protocol was approved by the Institutional Animal Care and Use Committee. Eight-week-old female SJL/J mice (Jackson Laboratories, Bar Harbor, ME, USA) were injected subcutaneously with an emulsion of proteolipid protein fragment, PLP139–151 (AnaSpec, San Jose, CA, USA), along with adjuvant. The dose of PLP139–151 was 80 μg per mouse, delivered subcutaneously to the lower flank area, evenly divided into three spots. The initial experiments used RIBI adjuvant (RIBI Immunochem Research, Hamilton, MT, USA); as it is no longer available commercially, the later experiment used the Sigma Adjuvant System (Sigma, St Louis, MO, USA), which is an incomplete Freund’s adjuvant similar to RIBI, and eventually complete Freund’s adjuvant (CFA) (DIFCO Laboratories, Detroit, MI, USA) with two pertussis toxin (List Biological Laboratories, Campbell, CA, USA) boosters (200 ng in 200 μL PBS (phosphate-buffered saline) per mouse, one on the day of immunization and another 48 h later). PLP+RIBI produced a uniform and monophasic EAE with an onset of weakness on days 9 to 10, a peak of disease on day 14 with EAE scores of 2 to 3, and complete behavioral recovery by day 23 (Pan et al, 1996). PLP+CFA with two pertussis toxin boosters induced a biphasic distribution of the EAE score, with the first peak occurring on day 14 and the second peak about day 20. On the basis of preliminary data of the behavioral development of EAE, the mice were studied 2 days before the emergence of the classical symptoms of weakness of tail and hind limbs. Different batches of mice were used for the different studies described below.
Blood-to-Central Nervous System Influx of Interleukin-15 After Intravenous Injection
Carrier-free recombinant mouse IL15 (R&D Systems, Minneapolis, MN, USA) was labeled with 125I (Perkin Elmer, Waltham, MA, USA) using the chloramine-T (Sigma) method. Bovine serum albumin (BSA; Sigma) was labeled with 131I, also using the chloramine-T method. The iodinated proteins were separated from free iodine by elution on Sephadex G-10 columns (Sigma). The specific activity of 125I-IL15 was ~120 Ci/g, and that for 131I-albumin was ~60 Ci/g.
Multiple-time regression analysis (Kastin et al, 2001) was carried out to quantify the influx of IL15 from blood to the CNS. Two groups of mice were studied: EAE mice and adjuvant-only controls (RIBI adjuvant), both at 7 days after induction (n=8 per group). The mice were anesthetized by an intraperitoneal injection of urethane. The jugular veins were exposed and the right common carotid artery was isolated after a midline anterior cervical section. Each mouse received a bolus of 125I-IL15 and 131I-albumin (30,000 c.p.m./μL), delivered to the left jugular vein in a bolus of 100 μL of lactated Ringer’s solution containing 1% BSA (LR/BSA). At 1, 2, 3, 5, 7, 10, 15, and 20 mins, one mouse in each group was decapitated immediately after blood was collected from the right carotid artery. The brain was dissected into the hippocampus, striatum, hypothalamus, cerebellum, cerebral cortex, and subcortical regions. Cervical, thoracic, and lumbar spinal cord segments were also separated. The radioactivity of the weighed CNS tissue and 50 μL of serum were measured in a dual-channel (for 125I and 131I) γ-counter. Tissue/serum ratios of radioactivity were calculated ((c.p.m./g)/(c.p.m./μL)=μL/g) and plotted against exposure time, representing the theoretical steady-state value of circulation time if the blood concentration of 125I-IL15 was constant. Exposure time was calculated as the integral of serum radioactivity over time divided by experimental time (Kastin et al, 2001). The slope (Ki) of the linear regression between the CNS tissue/serum ratio and exposure time reflects the unidirectional influx rate, whereas the y intercept (Vi) represents the initial volume of distribution. The difference in the regression lines was compared using Prism GraphPad statistical software (GraphPad, San Diego, CA, USA).
Brain Distribution of Fluorescein Isothiocyanate-Albumin After Intravenous Delivery
Fluorescein isothiocyanate (FITC)-albumin (Sigma) solution (5 mg/mL) was freshly prepared in oxygenated modified Zlokovic’s perfusion buffer as follows (g/L): NaCl (7.19), KCl (0.3), CaCl2 (0.28), NaHCO3 (2.1), KH2PO4 (0.16), MgCl2 6H2O (0.37), D-glucose (0.99) (pH 7.4). The distribution of FITC-albumin was determined by an established procedure that was previously described (Zhao et al, 2007) with minor modifications. In brief, heparin (100 IU/kg in 0.2 mL PBS, Sigma) was injected intracardially to urethane-anesthetized control and EAE mice induced by PLP+CFA, followed immediately by perfusion of 5 mL FITC-albumin solution delivered through the left ventricle using a perfusion pump at a rate of 1.5 mL/min. Outflow was generated by severing the right atrium. The descending aorta was clamped during perfusion. Immediately afterwards, the brain was removed and immersed in 4% paraformaldehyde for 3 days, cryoprotected in 15% sucrose in PBS over night, and thereafter in 30% sucrose until the brain sank to the bottom. Coronal sections of 20 μm thickness were obtained with a cryostat. The distribution of FITC-albumin in microvessels and its potential extravasation into brain parenchyma were visualized after image capture on an FV1000 Olympus confocal microscope (Olympus America Inc., Center Valley, PA).
Degradation Assays
Our previous study using size-exclusion chromatography showed that 125I-IL15 remains stable in the blood and brain 10 mins after intravenous injection (Pan et al, 2008c). Thus, the stability of 125I-IL15 at a longer duration was determined here. Thirty minutes after intravenous injection of 125I-IL15 and 131I-albumin as described above, arterial blood was collected into a tube containing protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL, USA) on ice, and the mice were immediately decapitated. Whole brain was homogenized on ice in PBS in the presence of protease inhibitor cocktail, and supernatant was collected. The processing control involved the collection of blood and brain to which radioisotopes were added ex vivo to indicate the extent of degradation during tissue processing. Serum and brain homogenate samples were fractionated on Sephadex G-25 columns, and 30 fractions (20 secs per fraction) were collected. The eluant profiles of the samples were compared with those of 125I-IL15 and 131I-albumin stock solutions and free isotopes. The percentage of intact 125I-IL15 and 131I-albumin and degraded proteins in the total radioactivity was calculated.
In situ Brain Perfusion
To determine whether the IL15-specific receptor, IL15Rα, and the common γ-chain receptor, IL2Rγ, have a role in the permeation of IL15 from blood to the CNS in EAE mice induced by PLP with the Sigma Adjuvant System, in situ brain perfusion was performed over a 5-min interval in three groups of urethane-anesthetized mice. There were five to six EAE mice and five control mice in each group. At the time of study, 8 out of the 16 PLP-induced mice showed symptoms of EAE. These mice were evenly distributed into each group on the basis of their EAE score. One group of mice received a soluble IL15Rα receptor (sIL15Rα-Fc, R&D Systems), another group received a soluble IL2Rγ receptor (sIL2Rγ-Fc, Sigma), and the control group received an Fc antibody (R&D Systems) in addition to 125I-IL15 and 131I-albumin (1,000 c.p.m./μL for each). The molar ratio of the soluble receptor or Fc peptide to 125I-IL15 was 10:1. The reagents were dissolved in modified Zlokovic’s perfusion buffer. Albumin (1%) was added after oxygenation of the solution for 1 h to reduce the potential adsorption of IL15 to the plastic tubing. The solution was delivered by a perfusion pump at a constant rate of 2 mL/min. Pre-perfusion with only buffer for 2 mins removed blood components from the cerebral vasculature. After 5 mins of perfusion of the radioactive solution, postperfusion for 1 min was carried out to remove the residual radioactive solution. The brain was dissected into different regions as described above, weighed, and radioactivity measured along with 50 μL of perfusate. The tissue/perfusate ratios of 125I-IL15 and 131I-albumin were calculated. One-way ANOVA (analysis of variance) and Tukey’s post hoc tests were carried out to determine the difference between the control and the experimental groups.
Efflux Transport of Interleukin-15 After Intracerebroventricular Injection
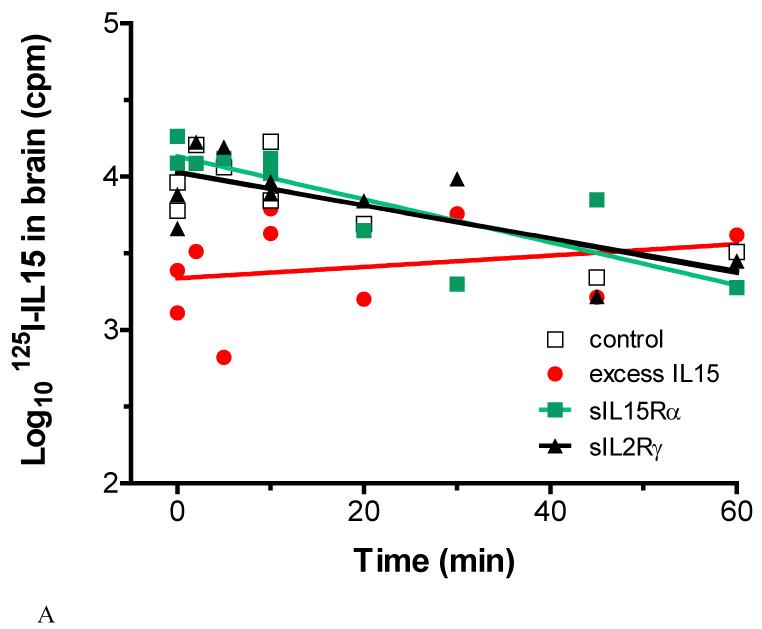
In the first study to determine whether the efflux of IL15 occurred by a regulatory process in normal mice, four groups of urethane-anesthetized 2-month-old male B6 mice (Jackson Laboratories) were studied (n=10 per group): (1) control group receiving 125I-IL15 and 131I-albumin and a control Fc antibody (6,000 c.p.m. in 2 μL); (2) with an additional 200-fold excess of unlabeled IL15 to test the self-inhibition characteristic of a saturable transport system; (3) with a 200-fold molar excess of sIL15Rα; and (4) with a 200-fold molar excess of sIL2Rγ. The bregma was exposed by a small incision on the scalp. The coordinates for intracerebroventricular injection to the left lateral ventricle were 1 mm lateral, 0.58 mm posterior, and 2 mm below the skull. Radioactive tracers were delivered by a 10-μL Hamilton syringe (Hamilton Company, Reno, NV, USA) at a rate of 1 μL/min. Individual mice in each group were killed at 0, 2, 5, 10, 10, 20, 30, 45, and 60 mins after the intracerebroventricular injection. Radioactivity in the brain was measured in a dual-channel γ-counter. Using the Prism GraphPad statistical software, the linear regression between the logarithm of brain radioactivity and time was measured, and significantly different changes among the groups were determined. The half-time disappearance (t1/2) from the brain for 125I-IL15 and 131I-albumin was derived from the slope of the regression line (b), in which the logarithm of the level of brain radioactivity is plotted against time (t) in minutes: Log10 (M)=b(t)+Log10 (A), where M is the residual count in the brain at time t, A (the intercept of the regression line) represents the amount available for transport at time 0, and t1/2=0.301/b (Banks et al, 1997; Pan et al, 1998).
In the second study to determine whether EAE mice show an altered brain-to-blood transport of IL15, three groups of mice were studied (n=8 per group). Experimental autoimmune encephalomyelitis was induced with PLP139–151 and CFA, and the mice were studied on day 13 when 18 of the 24 mice began to show EAE. These mice were assigned to different time points of intracerebroventricular injection evenly based on the score, and studied along with a group of control mice that received only CFA. A third group of mice received a 200-fold excess of unlabeled IL15 along with radioactive isotopes. The mice were anesthetized with urethane injected intraperitoneally, and received 125I-IL15 and 131I-albumin as described above. The half-time disappearance of 125I-IL15 and 131I-albumin was determined from the linear regression of the remaining brain radioactivity over time. The difference in regression lines was compared using the Prism GraphPad statistical software.
Real-time Reverse Transcriptase-PCR
Two groups of mice were compared: control and EAE mice on day 7 after induction by PLP+RIBI (n=4 per group). Brain and spinal cord tissues were collected after the mice were decapitated. The tissue was homogenized in RNA lysis buffer as previously described (Pan et al, 2008a, 2008b). Total RNA was obtained with an Absolutely RNA Miniprep Kit (Stratagene, La Jolla, CA, USA). A one-step core PCR kit (Applied Biosystems, Foster City, CA, USA) was used for reverse transcription and mRNA amplification by use of Taqman real-time PCR primers and fluorescent probes. The primers for IL15 were as follows: forward 5′-ATGTTCATCAACACGTCCTGACT, reverse 5′-GCAGCAGGTGGAGGTACCTTAA. The fluorescent probe was 5′-6-FAM-CATGCGAGCCTCTTCCGTGTTTCT-TAMRA. The primers for IL15Rα were forward 5′-CCAGGCCATTCCTGTGTTG, reverse 5′-GATGTCAGCATGCTCAATAGATACG. The fluorescent probe was 5′-6-FAM-CCGGGCACCACGTGTCCACC-TAMRA. The housekeeping gene, GAPDH (glyceraldehyde-3-phosphate dehydrogenase), was measured simultaneously as previously described (Pan et al, 2008a, 2008b). Standard curves showed an efficient amplification of the genes. The relative amount of mRNA was calculated from the cycle threshold by the established δδ-cycle threshold method, in which the mRNAs for IL15 and IL15Rα were normalized to the GAPDH mRNA. The difference between the control and EAE groups in each region was compared by Student’s t-test.
Results
Abolished Influx of Interleukin-15 and Albumin from Blood to Brain in EAE Mice
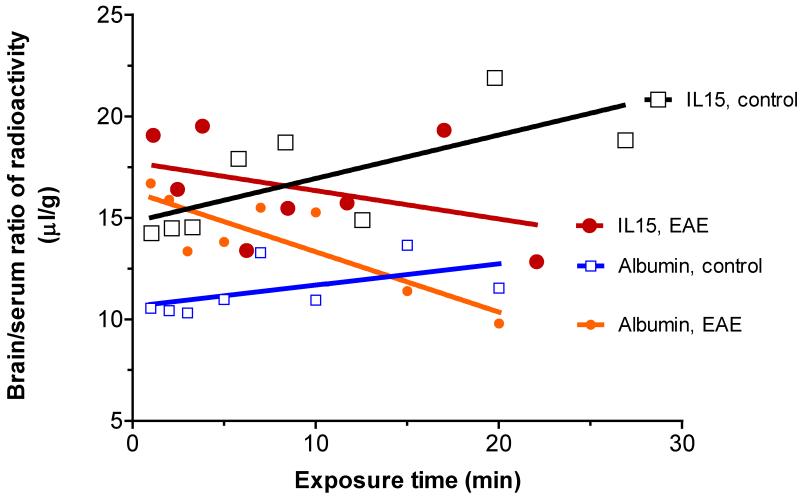
In control SJL/J mice, 125I-IL15 had a significant influx from blood to brain, with a Ki of 0.214±0.089 μL/g min and a Vi of 14.79±1.17 μL/g. In EAE mice (induced by PLP+RIBI and studied before the emergence of symptoms), the Ki was −0.140±0.134 μL/g min and Vi was 17.74±1.53 μL/g. The significant (P<0.05) difference between the two regression lines was reflected in the difference in the slope. In the same groups, coadministered 131I-albumin also showed a significant (P<0.005) decrease in Ki from 0.105±0.066 μL/g min in the control to −0.297±0.078 μL/g min in EAE mice, with an increase in Vi from 10.63±0.67 μL/g in the control to 16.30±0.79 μL/g in EAE mice (Figure 1). Owing to the major difference between Kis, it was not possible to determine whether Vis were also significantly different. Nevertheless, the initial volume of distribution for albumin in EAE mice was 1.5 times higher than that in controls, indicating an increase in vascular space. This is consistent with greater BBB permeability to vascular markers in EAE mice, shown by the use of radioactive tracers in our previous studies (Pan et al, 1996) and FITC-albumin indicated below.
Fig.1.
Influx of 125I-IL15 from blood to brain was determined in groups of control and EAE mice receiving PLP139-151 and RIBI adjuvant (n = 8/group). In contrast to the substantial uptake over time in the controls, there was a decrease of the brain/serum ratio of 125I-IL15 over time. The co-administered vascular marker 131I-albumin followed a similar pattern. The difference in Ki between the control and EAE groups was significant for both tracers.
Decreased Influx of Albumin But Not IL15 From Blood to Spinal Cord in EAE Mice
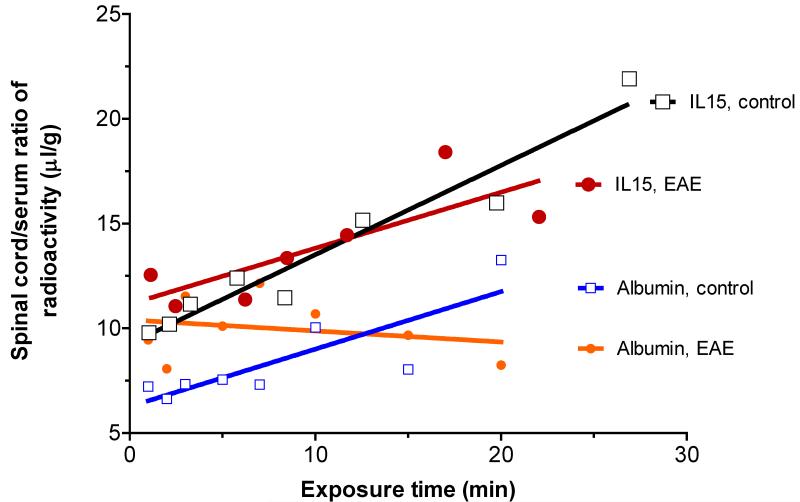
In the control spinal cord of the SJL/J mice, the influx rate Ki of 125I-IL15 was 0.426±0.045 μL/g min, and the initial volume of distribution Vi was 9.26±0.59 μL/g. In EAE mice, Ki was 0.267±0.090 μL/g min, whereas Vi was 11.15±1.10 μL/g. Although both groups had significant regression lines, the difference between them was not significant. The spinal cord influx of IL15 differed from the pattern in the brain, but the influx of 131I-albumin was similar to that observed in the brain. The control group had a Ki of 0.274±0.073 μL/g min and a Vi of 6.27±0.74 μL/g. The EAE group had a Ki of −0.053±0.085 μL/g min and a Vi of 10.40±0.86 μL/g (Figure 2). The difference in albumin influx in the spinal cord between the control and EAE mice was significant (P<0.05). The initial volume of distribution for 131I-albumin was 1.6-fold higher in EAE mice than in controls, again indicating an increased vascular space resulting from the greater permeability of the blood-spinal cord barrier in EAE mice.
Fig.2.
The influx of 125I-IL15 from blood to spinal cord in EAE mice (PLP + RIBI) did not differ significantly from that in the control group. The influx rate of 131I-albumin, however, was significantly reduced in the EAE mice.
Regional Differences in IL15 Permeation and the Level of IL15 Expression In Situ
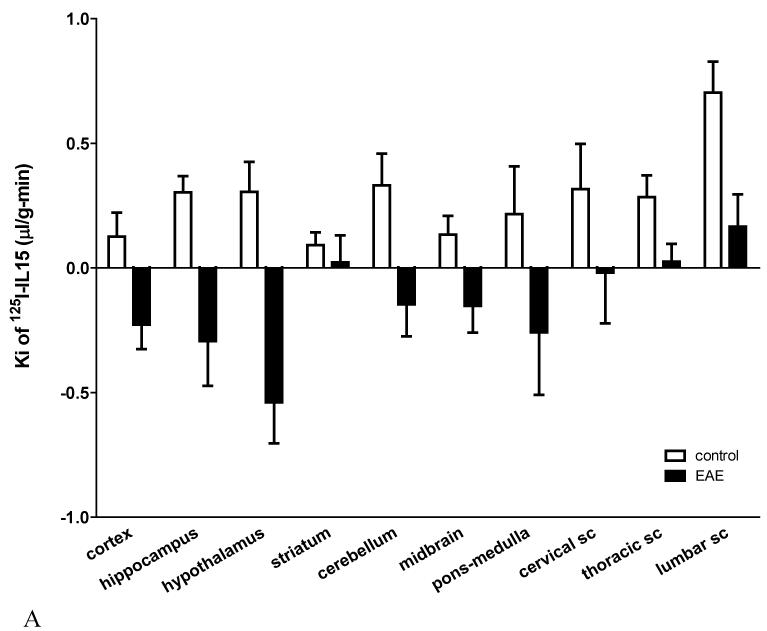
Major regional differences in the BBB permeation of 125I-IL15 were observed in control mice. The highest Ki was observed in the lumbar spinal cord (0.703±0.125 μL/g min). There were several areas with a moderate Ki of ~0.3 μL/g min, including the hippocampus, hypothalamus, cerebellum, and cervical and thoracic spinal cord. A low permeation of IL15 in descending order was observed in the pons-medulla, midbrain, cerebral cortex, and striatum. Interestingly, the striatum, which had the lowest influx rate (0.092±0.052 μL/g min) in controls, maintained a positive but minimal influx in EAE mice, whereas all other brain areas showed negative Kis attributed to rapid efflux. In the thoracic and lumbar spinal cord, where BBB disruption was maximal in EAE mice, there was a significant reduction in the influx of 125I-IL15 that still maintained positive values (Figure 3A).
Fig.3.
Regional variation in BBB permeability to 125I-IL15 and levels of IL15 and IL15Rα mRNA.
(A) In control mice, the lumbar spinal cord had the highest Ki whereas the striatum had the lowest. EAE induction was associated with a decreased tissue/serum ratio of 125I-IL15 (thus a negative Ki) in most regions except the striatum, and thoracic and lumbar spinal cord (sc).
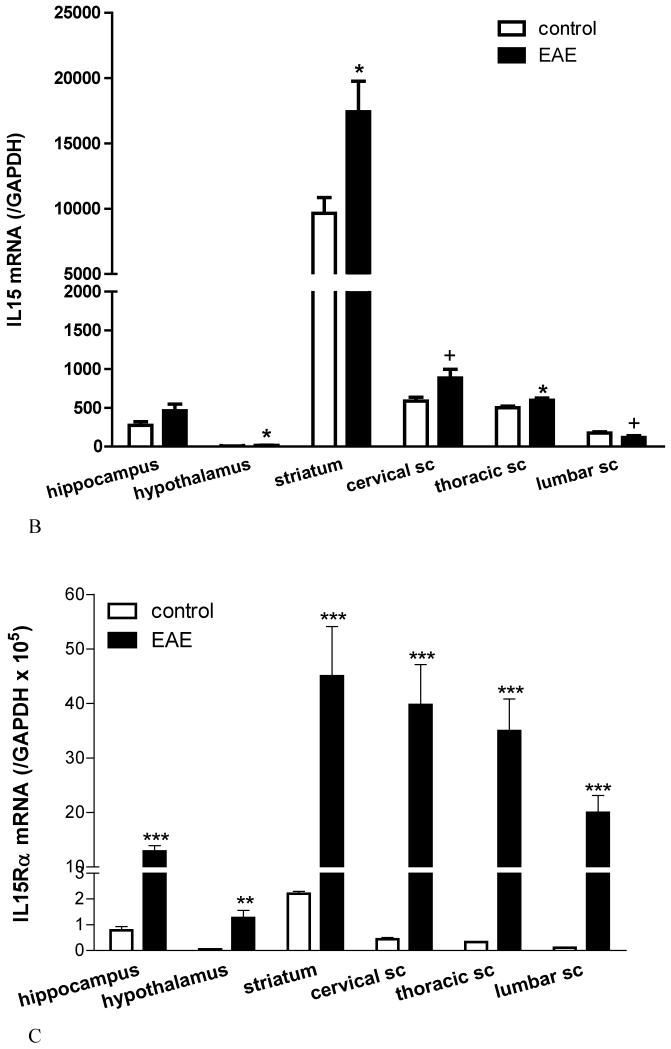
(B) IL15 mRNA was highest in the striatum and lowest in the hypothalamus in normal mice. EAE induction by PLP + RIBI caused a significant increase of IL15 mRNA in the hypothalamus, striatum, and thoracic spinal cord (sc) (*: p < 0.05). There was also a trend (+: p < 0.06) toward an increase in the cervical and lumbar sc.
(C) The basal level of IL15Rα mRNA was low in most regions; striatum had the highest. EAE (induced by PLP + RIBI) was associated with a significant increase of the receptor mRNA in all regions. **: p < 0.01; ***: p < 0.005.
In normal mice, the relationship between BBB permeability and IL15 mRNA was not linear, but showed a trend toward an inverse correlation. The hypothalamus (which had a high Ki) showed the lowest basal mRNA. The striatum had an extremely high basal IL15 mRNA in contrast to the low Ki. The highest Ki of the lumbar spinal cord was reflected in a relatively low level of IL15 mRNA. In all regions, with the exception of the hippocampus, there was an increase in IL15 mRNA after the induction of EAE (P<0.05 for the hypothalamus, striatum, and thoracic spinal cord and P<0.06 for cervical and lumbar spinal cord) (Figure 3B). By contrast, IL15Rα mRNA showed significant upregulation in all the tested regions (Figure 3C).
Vascular Space in the Experimental Autoimmune Encephalomyelitis Mice
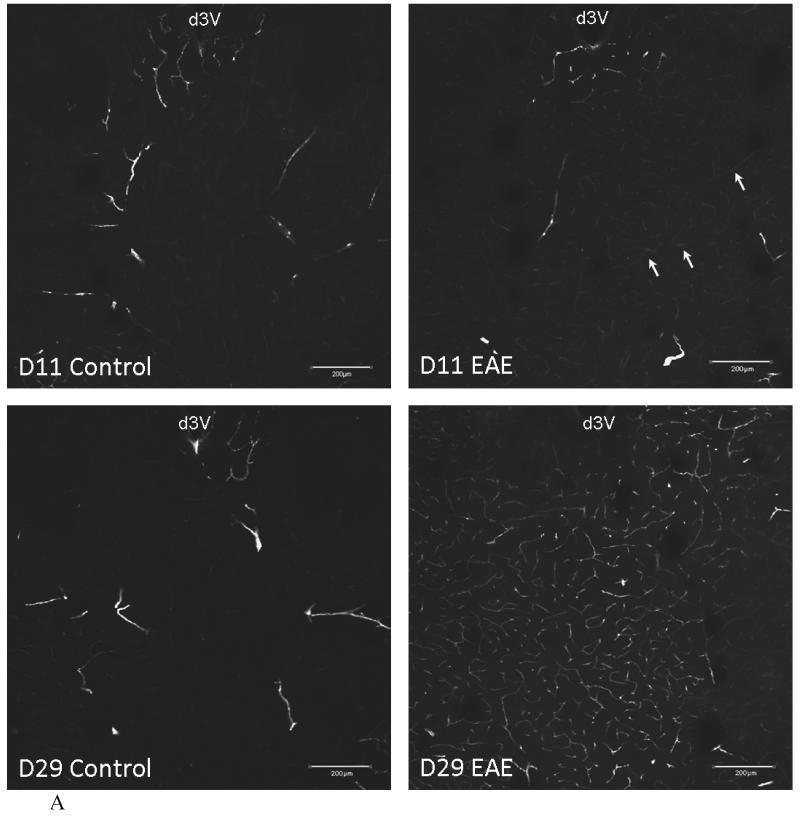
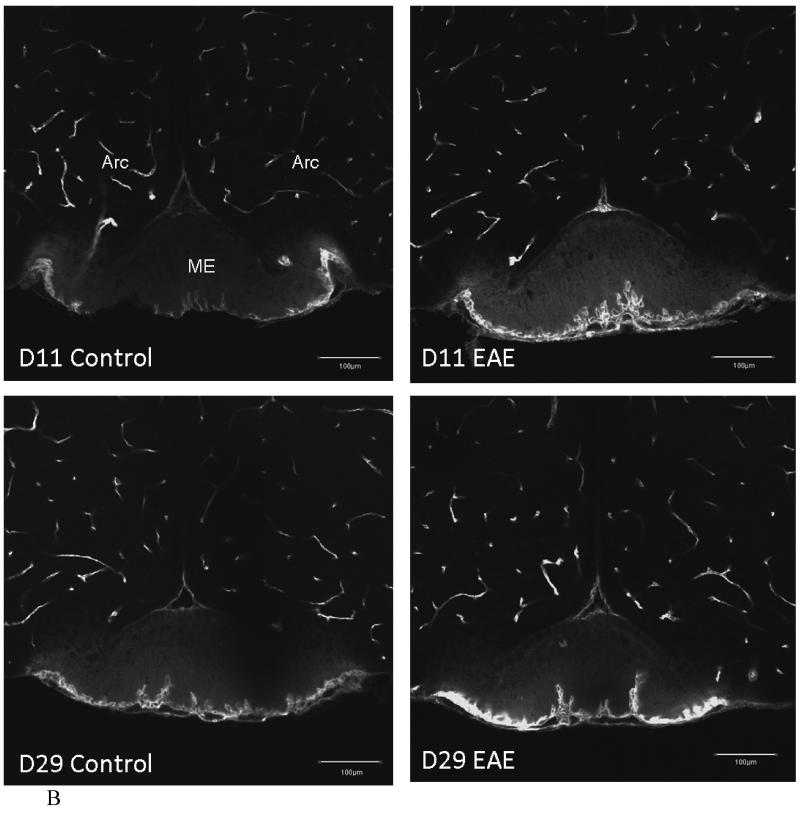
In EAE mice induced by PLP+CFA, there was an increase in the density of distribution of FITC-albumin in cerebral microvessels. More microvessels were delineated by FITC-albumin after intravenous delivery. In the striatum of control mice that received only adjuvant either 11 or 29 days earlier, there was a marked regional variation in the filling of microvessels. The EAE mouse 11 days after induction (asymptomatic) showed a mild increase in FITC-albumin fluorescence intensity (arrow). The EAE mouse 29 days after induction (with a score of 0, having just recovered from EAE) showed a major increase of delineated microvessels and a greater fluorescence intensity of FITC-albumin (Figure 4A). This suggests hyperemia and an increase in vascular space. In the hypothalamus, there was a moderate increase in the capillary density of postcapillary venules at the external rim of the median eminence on both days 11 and 29 after EAE induction, without apparent changes in the microvessels in the arcuate nucleus (Figure 4B). In none of these mice was there an extravasation of FITC-albumin. This indicates that the BBB remained relatively intact in most regions of the brain.
Fig.4.
Vascular space of control and EAE mice after induction with PLP and CFA.
(A) In the striatum and thalamus, the vascular space was slightly increased in the EAE mouse 11 days after PLP treatment (d11) in the area under the dorsal 3rd ventricle (d3V) (arrows), and was significantly increased at d29, compared with the controls.
(B) The blood vessels beneath the median eminence (ME) also showed a similar pattern in EAE mice. There is no significant difference between control and EAE mice in the vascular space in most of the brain regions, as shown in the arcuate nucleus (Arc). There was no BBB leakage seen in EAE mice within this perfusion time frame but the diffusion of FITC-albumin can be seen in CVOs, such as the ME (scale bar = 200 μm).
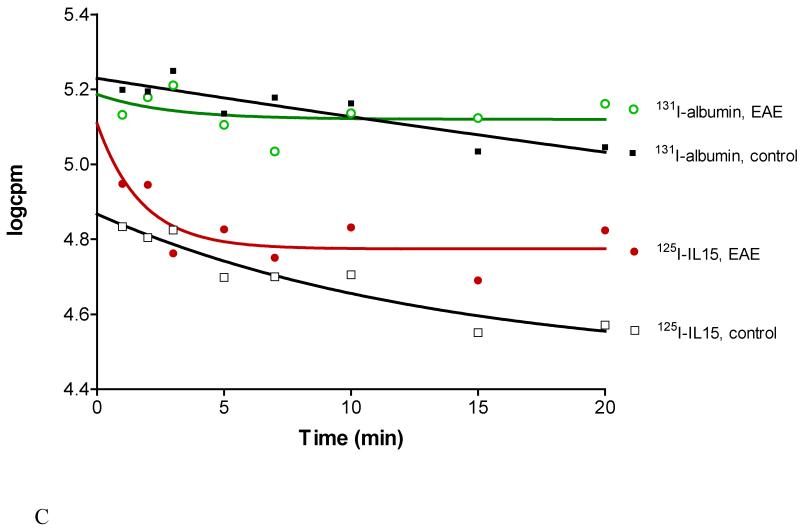
Fig.4C. Disappearance of 125I-IL15 and 131I-albumin from blood after iv bolus injection. EAE was associated with altered kinetics and a shortened half-life for both substances.
The disappearance half-life of 131I-albumin after intravenous injection differed significantly in EAE and control mice. The F-test showed the best fit of the concentration of 131I-albumin (log c.p.m.) with the following equation for one-phase exponential decay: Y=Span × exp(−K × X)+plateau, with K being the rate constant, and X being the time (min). The half-life of 131I-albumin was 83.96 mins in the control, but 1.97 mins in the EAE group (n=8 per group) (Figure 4C). This contrasts with 125I-IL15 in the same study, which had a half-life of 9.33 mins in the control and 1.22 mins in EAE mice.
Interleukin-15 and Albumin Remained Intact 30 mins After Intravenous Delivery
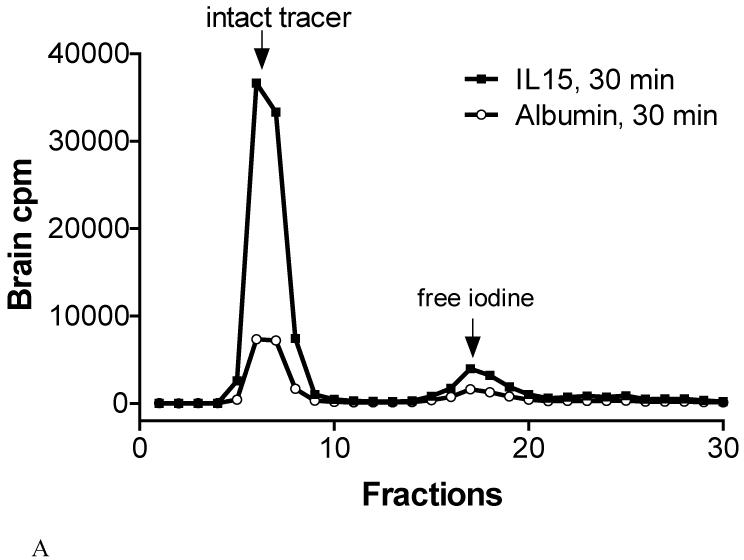
125I-IL15 coeluted with 131I-albumin in brain homogenates (Figure 5A) and serum (Figure 5B) 30 mins after intravenous injection. As shown in Table 1, at least 80% of the total radioactivity in the brain eluant 30 mins after intravenous injection represents intact 125I-IL15. Some of the degradation occurred during tissue processing, as shown by the result of 90% intact 125I-IL15 in the brain after ex vivo processing of control. In the serum, more than 92% of radioactivity eluted in the same position as 125I-IL15, 30 mins after intravenous injection. 131I-albumin showed even less dissociation of free iodine. Its co-migration with 125I-IL15 in size-exclusion chromatography suggests that IL15 binds to albumin in both blood and brain homogenates.
Fig.5.
Degradation assay of 125I-IL15 and 131I-albumin. In both brain (A) and serum (B), most of the radioactivity remained as intact IL15 or albumin.
Table 1.
Degradation assays showed that most of the radioactivity in blood and brain represents intact 125I-IL15
| Brain, 30 min | Brain, control | Serum, 30 min | Serum, control | |
|---|---|---|---|---|
| 125I-IL15 | 80.3 % | 90.8 % | 92.6 % | 98.6 % |
| Free 125I | 11.7 % | 6.0 % | 6.0 % | 4.5 % |
In Situ Brain Perfusion Overrides the Decrease of IL15 Permeation Across the Blood–Brain Barrier
The brain/perfusate ratio of 131I-albumin was higher in EAE mice induced by PLP with the Sigma Adjuvant System as they began to show signs of weakness. This was observed in all brain regions and in the whole brain, but the increase in the adjuvant-only controls was not significant. The brain/perfusate ratio of 125I-IL15 did not show significant changes, despite a tendency toward a reduced uptake in the hypothalamus and striatum (Figure 6). In neither the control nor EAE mice did the coadministration of sIL15Rα or sIL2Rγ (after 1 h of incubation with the radioactively labeled tracers) have a significant effect on the brain uptake of 125I-IL15 or 131I-albumin. The lack of decrease of IL15 uptake in the brain of EAE mice argues against the presence of an efflux transport system. Rather, it supports the possibility that the negative Ki in the multiple-time regression analysis after intravenous injection was caused by the rapid disappearance of IL15 and albumin in blood to reach the cerebral vasculature during the first few minutes, followed by redistribution with backflux.
Fig.6.
In-situ brain perfusion was performed in control and EAE mice for 5 min. EAE tended to increase the brain uptake of 131I-albumin in the hypothalamus (p = 0.07) but not in other brain regions. The uptake of 125I-IL15 did not differ significantly between the control and EAE groups, although there was a non-significant decrease in the striatum (n = 5/group).
The Brain-to-Blood Transport of Interleukin-15 in Normal and EAE Mice
In normal mice, the rate of 125I-IL15 exiting the brain could be predicted by the following equation: log10(M)=−0.011(t)+4.033, r=0.78. The t1/2 was 27.4 mins. Mice receiving sIL15Rα or sIL2Rγ did not show significant changes in the t1/2 of 125I-IL15. The presence of excess unlabeled IL15, however, greatly slowed the disappearance of 125I-IL15 from the brain: log10(M)=−0.004(t)+3.337, r=0.24, t1/2=81.2 mins (Figure 7A). This represents a significant change from the control and from the groups coadministered with soluble receptors. Surprisingly, the efflux of 131I-albumin followed the same pattern and was also reduced significantly (P<0.05) in the presence of excess unlabeled IL15 (data not shown). This may be indicative of IL15-albumin binding or suppression of CSF reabsorption by excess IL15.
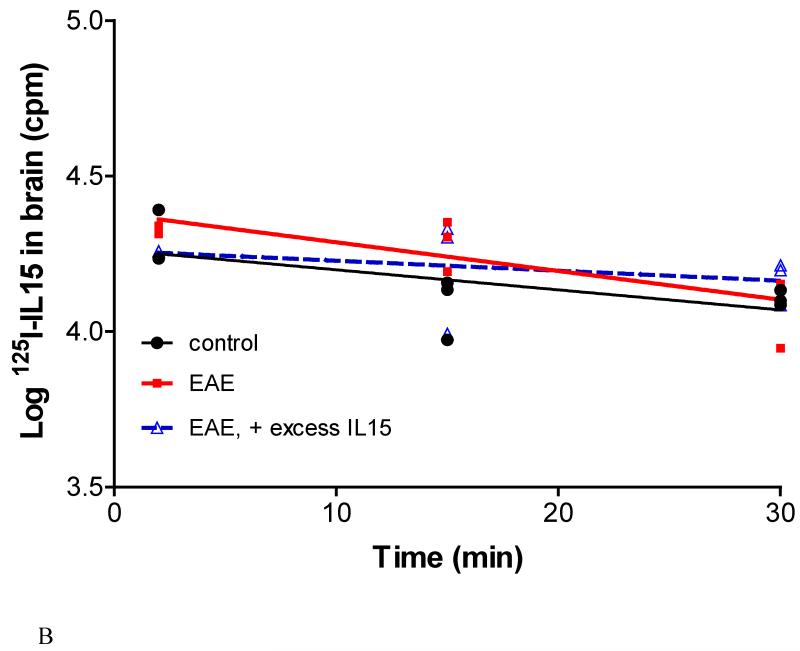
Fig.7.
Brain-to-blood transport of IL15, shown by the logarithm of residual radioactivity in the brain was plotted against time after icv injection.
(A) In normal mice, the half-time disappearance of 125I-IL15 was delayed significantly by excess unlabeled IL15 but was not affected by sIL15Rα or sIL2Rγ.
(B) In EAE mice, the half-time disappearance of 125I-IL15 was not significantly different from that in the control mice, and it was not affected by excess unlabeled IL15.
In EAE mice induced by PLP+CFA and showing early signs of weakness, there was no significant change in the disappearance of 125I-IL15 from the brain in comparison with that in control mice (n=9 per group). Excess IL15 had no effect on the brain-to-blood transport of 125I-IL15 (Figure 7B). This is reminiscent of the results seen in the study of blood-to-spinal cord influx of IL15 in EAE mice as shown in Figure 2. Coadministered 131I-albumin showed a similar pattern (data not shown).
Discussion
In this study, we show that the development of EAE was associated with a decreased brain/serum ratio of IL15 and vascular marker albumin over time. The influx rate of IL15 into the spinal cord remained high, despite a decrease in the spinal cord/serum ratio of albumin over time. Multiple-time regression analysis is typically applied to situations in which the blood concentration of a tracer is relatively stable and permeation across the BBB is low, as observed for most nonvasoactive peptides and proteins (Kastin et al, 2001). In situations with a rapid extraction of a blood-borne substance by the CNS, an oscillation of efflux and influx, or extremely unstable blood concentrations, the method may no longer be valid. In the case of EAE mice, the negative Ki seemed to be caused by a rapid filling of the cerebral vascular space within the first 5 mins and by a later redistribution of serum proteins (including albumin and IL15) over time. Although not perfect for this situation, the multiple-time regression analysis reflects dynamic interactions of IL15 and albumin with cerebral vasculature. Therefore, the increase in Vi in the brain was equally as important as the persistent Ki in the spinal cord. This is further supported by results from in situ perfusion influx studies in which EAE was not associated with significant changes in the brain uptake of IL15, and from brain-to-blood transport studies in which the disappearance of IL15 from CSF was not accelerated. Nevertheless, there was an increase in cerebral vascular space in EAE mice, most apparent at the peak of the disease in the striatum. The blood-borne IL15 probably acts in concert with the in situ production of IL15 and cerebral IL15Rα, both of which showed significant transcriptional upregulation.
Our previous findings of IL15 permeation across the BBB after lipopolysaccharide challenge by intraperitoneal injection showed relatively smaller changes than would be expected from the increase of vascular permeability shown by albumin (Pan et al, 2008c). The results of this study with EAE mice showed an entirely different pattern of altered BBB permeation. As a negative influx rate of 131I-albumin may be caused by a reduction in CNS vascular space resulting from either vasoconstriction or hypoperfusion, we determined the regional distribution of FITC-albumin after intravenous injection. There was no vasoconstriction; rather, EAE mice showed an increase in vascular space, apparent in the striatum. Neither was there extravasation of FITC-albumin indicative of focal BBB disruption. An increased vascular space was probably mainly responsible for this rapid turnover of exogenous albumin. This is also supported by findings that albumin nanoparticles show increased distribution in meningeal and perivascular areas in the lumbar spinal cord (Merodio et al, 2000). In addition, perivascular extravasation of serum albumin, immunoglobulins, and exogenous horseradish peroxidase, are all prominent in the brainstem and cervical spinal cord in EAE (Rabchevsky et al, 1999). Concurrently, laser flow Doppler measurement at the cortical surface did not show a significant change in cerebral perfusion (Pan W, unpublished observations). The Ki for IL15 in the spinal cord remained high, suggesting an increase in the spinal cord uptake of IL15. Therefore, EAE is associated with an increased cerebral vascular space and an accelerated turnover of serum proteins. The altered kinetics of CNS extraction of IL15 from blood have never been observed in other disease models in mice, including neuroinflammation by lipopolysaccharide (Pan et al, 2008d), spinal cord injury (Pan et al, 2003a), brain trauma (Pan et al, 2003b), or stroke (Pan et al, 2006).
As EAE is a heterogenous disease with regional variation in CNS pathology, we determined whether the regional difference of IL15 permeation was related to the level of expression of IL15 and its receptors within the CNS. Owing to technical limitations in protein analyses by western blotting or immunohistochemistry (issues of sensitivity and specificity), mRNA was measured. The changes in IL15 permeation and in situ production in the spinal cord, striatum, and cortex may be directly responsible for the motor deficits observed in EAE mice, whereas that in the hippocampus could provide a biochemical basis for altered feeding behavior and weight loss. Surprisingly, there seemed to be an inverse correlation between endogenous IL15 expression and permeation of 125I-IL15 from blood to the CNS. It is not known whether there is a reciprocal inhibition between parenchymal IL15 and blood-borne IL15. Interleukin-15Rα, the specific receptor for IL15 that initiates signaling through Janus kinases/Signal Transducer and Activator of Transcription, was increased significantly in all regions measured, and did not show a correlation with the Ki of blood-borne IL15. Nevertheless, the increase in IL15Rα in EAE mice enables its enhanced signaling in response to blood-borne as well as intrathecally produced IL15.
An efflux system could be present at the cerebral endothelia, such as the Pgp system at the luminal surface of microvessels (apical surface of the endothelial cells), or on the brain side of the barrier structures. Cytokines, such as TNF, have been shown to increase Pgp activity and facilitate the efflux of its substrates across the BBB (Bauer et al, 2007; Yu et al, 2007). Thus, we further determined the influx of IL15 by intracardial (in situ) brain perfusion. The uptake of IL15 after 5 mins of continuous perfusion did not differ between the EAE and control groups, although there was a nonsignificant increase in albumin uptake. Thus, the altered IL15 influx from blood did not occur at the level of the luminal surface of the BBB. Interleukin-15 is apparently not a substrate for a Pgp-like efflux transporter. The lack of modification of IL15 uptake by soluble receptors suggests that the permeation of IL15 was not mediated by receptor-mediated transport in either control or EAE mice. This is consistent with what we have shown in mice after lipopolysaccharide challenge (Pan et al, 2008c).
Once the blood-borne IL15 reaches the CSF through either the BBB or blood-cerebrospinal fluid barrier, there is an increase in the CSF/blood ratio. However, in the human disorder of multiple sclerosis, which EAE models, there is an increased serum/CSF ratio of IL15 (Rentzos et al, 2006). This decrease in the CSF/serum ratio might suggest brain-to-blood transport. Thus, we determined the fate of IL15 after permeation across the BBB or intrathecal production by intracerebroventricular injection. In normal mice, IL15 exits the brain by CSF reabsorption, as shown by the similar disappearance half-life of IL15 and albumin. Albumin enters the CNS through a variety of routes collectively termed ‘extracellular pathways,’ and the CSF levels of albumin are ~0.5% of serum levels (Banks, 2004). Albumin exits CSF by reabsorption at the granular process in the subarachnoid space, making it a reliable marker of CSF turnover. However, excess IL15 seemed to inhibit the process, with a concurrent reduction of both IL15 and albumin disappearance from the CSF. Results from the use of soluble receptors indicated that brain-to-blood transport was unlikely to be receptor-mediated. Overall, the prolonged half-life of 125I-IL15 and 131I-albumin in the CSF in the presence of excess IL15 suggests altered CSF dynamics. This was changed in EAE mice, in which excess IL15 was no longer effective.
Thus, EAE is associated with drastic changes in the vascular space, with lack of saturation of the blood-to-CNS influx and brain-to-blood transport, and with the transcriptional upregulation of IL15 and its specific receptor IL15Rα in the brain. The results indicate a complex role of the BBB in mediating communication between blood-borne IL15 and the CNS during the development of EAE.
Acknowledgments
This work was supported by NIH (NS62291, NS45751, and DK54880). We thank Dr Kyha D Williams (Duke University), Dr Hong Tu (Shanghai Cancer Institute and PBRC), and Dr Jiming Feng (Lousiana State University School of Veterinary Medicine) for their helpful suggestions on the use of adjuvants to induce EAE.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Banks WA. Are the extracellular pathways a conduit for the delivery of therapeutics to the brain? Curr Pharm Des. 2004;10:1365–1370. doi: 10.2174/1381612043384862. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 2.Banks WA, Fasold MB, Kastin AJ. Measurement of efflux rates from brain to blood. In: Irvine GB, Williams CH, editors. Methods in molecular biology, neuropeptide protocols. Humana Press Inc.; Totowa, NJ: 1997. pp. 353–360. [DOI] [PubMed] [Google Scholar]
- 3.Banks WA, Niehoff ML, Zalcman SS. Permeability of the mouse blood-brain barrier to murine interleukin-2: predominance of a saturable efflux system. Brain Behav Immun. 2004;18:434–442. doi: 10.1016/j.bbi.2003.09.013. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 4.Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen L, Beurskens F, Schuurman J, van de Winkel J, Parren P, Gracie J, Jongbloed S, Liew F, McInnes I. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52:2686–2692. doi: 10.1002/art.21249. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 5.Bauer B, Hartz AM, Miller DS. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 6.Beck RD, Jr, Wasserfall C, Ha GK, Cushman JD, Huang Z, Atkinson MA, Petitto JM. Changes in hippocampal IL-15, related cytokines, and neurogenesis in IL-2 deficient mice. Brain Res. 2005;1041:223–230. doi: 10.1016/j.brainres.2005.02.010. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 7.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez EG, Banks WA, Kastin AJ. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 9.Hanisch UK, Lyons SA, Prinz M, Nolte C, Weber JR, Kettenmann H, Kirchhoff F. Mouse brain microglia express interleukin-15 and its multimeric receptor complex functionally coupled to Janus kinase activity. J Biol Chem. 1997;272:28853–28860. doi: 10.1074/jbc.272.46.28853. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 10.Kastin AJ, Akerstrom V, Pan W. Validity of multiple-time regression analysis in measurement of tritiated and iodinated leptin crossing the blood-brain barrier: meaningful controls. Peptides. 2001;22:2127–2136. doi: 10.1016/s0196-9781(01)00569-1. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 11.Kubota T, Brown RA, Fang J, Krueger JM. Interleukin-15 and interleukin-2 enhance non-REM sleep in rabbits. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1004–R1012. doi: 10.1152/ajpregu.2001.281.3.R1004. | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 12.Kurowska M, Rudnicka W, Maslinska D, Maslinski W. Expression of IL-15 and IL-15 receptor isoforms in select structures of human fetal brain. Ann NY Acad Sci. 2002;966:441–445. doi: 10.1111/j.1749-6632.2002.tb04245.x. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 13.Lisak RP, Benjamins JA, Bealmear B, Nedelkoska L, Studzinski D, Retland E, Yao B, Land S. Differential effects of Th1, monocyte/macrophage and Th2 cytokine mixtures on early gene expression for molecules associated with metabolism, signaling and regulation in central nervous system mixed glial cell cultures. J Neuroinflammation. 2009;6:4. doi: 10.1186/1742-2094-6-4. | Article | PubMed | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maslinska D. The cytokine network and interleukin-15 (IL-15) in brain development. Folia Neuropathol. 2001;39:43–47. | PubMed | ChemPort |. [PubMed] [Google Scholar]
- 15.McInnes IB, al-Mughales J, Field M, Leung BP, Huang FP, Dixon R, Sturrock RD, Wilkinson PC, Liew FY. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–182. doi: 10.1038/nm0296-175. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 16.McInnes IB, Gracie JA. Interleukin-15: a new cytokine target for the treatment of inflammatory diseases. Curr Opin Pharmacol. 2004;4:392–397. doi: 10.1016/j.coph.2004.04.003. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 17.McInnes I, Leung B, Sturrock R, Field M, Liew F. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 18.McInnes I, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 19.Merodio M, Irache JM, Eclancher F, Mirshahi M, Villarroya H. Distribution of albumin nanoparticles in animals induced with the experimental allergic encephalomyelitis. J Drug Target. 2000;8:289–303. doi: 10.3109/10611860008997907. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 20.Nilsen E, Johansen F, Jahnsen F, Lundin K, Scholz T, Brandtzaeg P, Haraldsen G. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut. 1998;42:635–642. doi: 10.1136/gut.42.5.635. | PubMed | ISI | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmcol. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. | Article | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 22.Pan W, Banks WA, Kastin AJ. Permeability of the blood-brain and blood-spinal cord barriers to interferons. J Neuroimmunol. 1997;76:105–111. doi: 10.1016/s0165-5728(97)00034-9. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 23.Pan W, Banks WA, Kennedy MK, Gutierrez EG, Kastin AJ. Differential permeability of the BBB in acute EAE: enhanced transport of TNF-α. Am J Physiol. 1996;271:E636–E642. doi: 10.1152/ajpendo.1996.271.4.E636. | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 24.Pan W, Csernus B, Kastin AJ. Upregulation of p55 and p75 receptors mediating TNF alpha transport across the injured blood-spinal cord barrier. J Mol Neurosci. 2003a;21:173–184. doi: 10.1385/JMN:21:2:173. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 25.Pan W, Ding Y, Yu Y, Ohtaki H, Nakamachi T, Kastin AJ. Stroke upregulates TNF alpha transport across the blood-brain barrier. Exp Neurol. 2006;198:222–233. doi: 10.1016/j.expneurol.2005.11.020. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 26.Pan W, Hsuchou H, He Y, Sakharkar A, Cain C, Yu C, Kastin AJ. Astrocyte leptin receptor (ObR) and leptin transport in adult-onset obese mice. Endocrinology. 2008a;149:2798–2806. doi: 10.1210/en.2007-1673. | Article | PubMed | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan W, Hsuchou H, Tu H, Kastin AJ. Developmental changes of leptin receptors in cerebral microvessels: unexpected relation to leptin transport. Endocrinology. 2008b;149:877–885. doi: 10.1210/en.2007-0893. | Article | PubMed | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan W, Hsuchou H, Yu C, Kastin AJ. Permeation of blood-borne IL15 across the blood-brain barrier and the effect of LPS. J Neurochem. 2008c;106:313–319. doi: 10.1111/j.1471-4159.2008.05390.x. | Article | PubMed | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan W, Kastin AJ. Increase in TNF transport after SCI is specific for time, region, and type of lesion. Exp Neurol. 2001;170:357–363. doi: 10.1006/exnr.2001.7702. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 30.Pan W, Kastin AJ, McLay RN, Rigai T, Pick CG. Increased hippocampal uptake of TNF and behavioral changes in mice. Exp Brain Res. 2003b;149:195–199. doi: 10.1007/s00221-002-1355-7. | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 31.Pan W, Yu C, Hsuchou H, Zhang Y, Kastin AJ. Neuroinflammation facilitates LIF entry into brain: role of TNF. Am J Physiol Cell Physiol. 2008d;294:C1436–C1442. doi: 10.1152/ajpcell.00489.2007. | Article | PubMed | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rabchevsky AG, Degos JD, Dreyfus PA. Peripheral injections of Freund’s adjuvant in mice provoke leakage of serum proteins through the blood-brain barrier without inducing reactive gliosis. Brain Res. 1999;832:84–96. doi: 10.1016/s0006-8993(99)01479-1. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 33.Rentzos M, Cambouri C, Rombos A, Nikolaou C, Anagnostouli M, Tsoutsou A, Dimitrakopoulos A, Triantafyllou N, Vassilopoulos D. IL-15 is elevated in serum and cerebrospinal fluid of patients with multiple sclerosis. J Neurol Sci. 2006;241:25–29. doi: 10.1016/j.jns.2005.10.003. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 34.Rose JW, Burns JB, Bjorklund J, Klein J, Watt HE, Carlson NG. Daclizumab phase II trial in relapsing and remitting multiple sclerosis: MRI and clinical results. Neurology. 2007;69:785–789. doi: 10.1212/01.wnl.0000267662.41734.1f. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 35.Waguespack PL, Banks WA, Kastin AJ. Interleukin-2 does not cross the blood-brain barrier by a saturable transport system. Brain Res Bull. 1994;34:103–109. doi: 10.1016/0361-9230(94)90005-1. | Article | PubMed | ISI | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 36.Waldmann TA. Targeting the interleukin-15/interleukin-15 receptor system in inflammatory autoimmune diseases. Arthritis Res Ther. 2004;6:174–177. doi: 10.1186/ar1202. | Article | PubMed | ISI | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–1259. doi: 10.1084/jem.181.3.1255. | Article | PubMed | ISI | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu C, Argyropoulos G, Zhang Y, Kastin AJ, Hsuchou H, Pan W. Neuroinflammation activates Mdr1b efflux transport through NFkappaB: promoter analysis in BBB endothelia. Cell Physiol Biochem. 2008;22:745–756. doi: 10.1159/000185558. | Article | PubMed | ChemPort |. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu C, Pan W, Tu H, Waters S, Kastin AJ. TNF activates MDR1 (P-glycoprotein) in cerebral microvascular endothelial cells. Cell Physiol Biochem. 2007;20:853–858. doi: 10.1159/000110445. | Article | PubMed | ChemPort |. [DOI] [PubMed] [Google Scholar]
- 40.Zhao C, Ling Z, Newman MB, Bhatia A, Carvey PM. TNF-alpha knockout and minocycline treatment attenuates blood-brain barrier leakage in MPTP-treated mice. Neurobiol Dis. 2007;26:36–46. doi: 10.1016/j.nbd.2006.11.012. | Article | PubMed | ChemPort. [DOI] [PMC free article] [PubMed] [Google Scholar]