Abstract
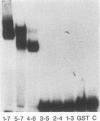
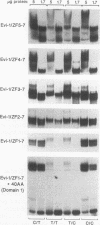
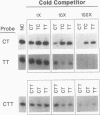
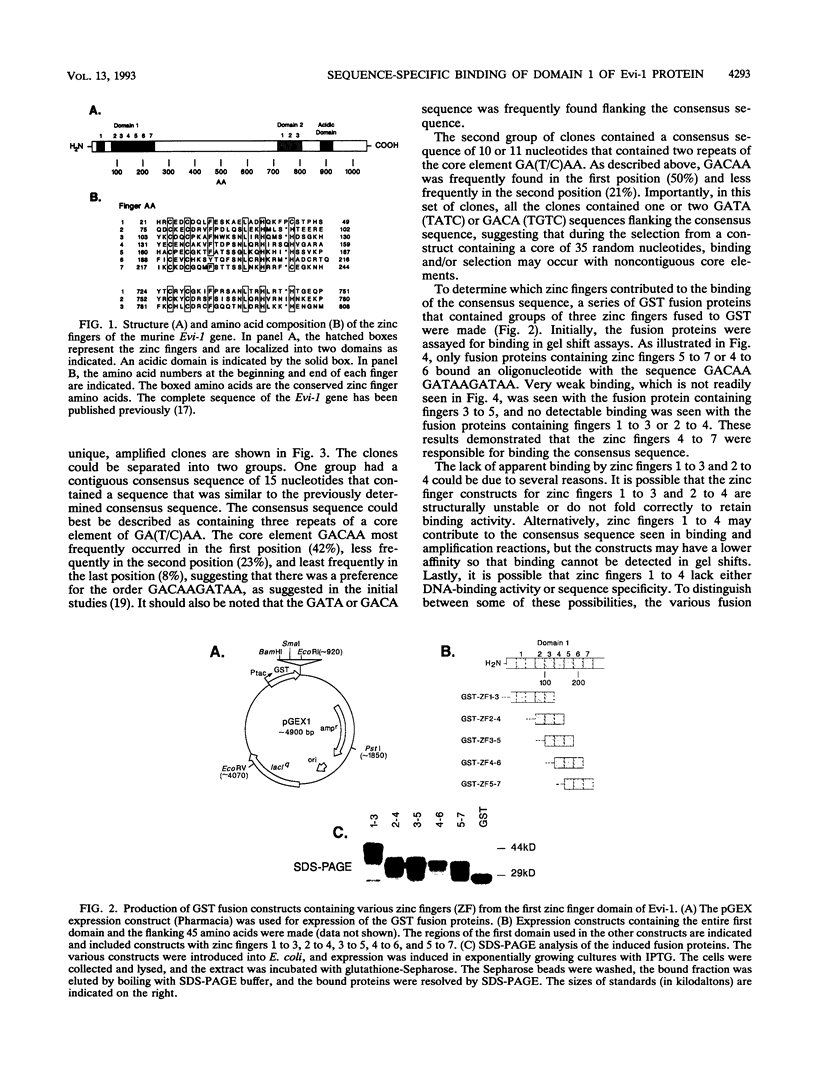
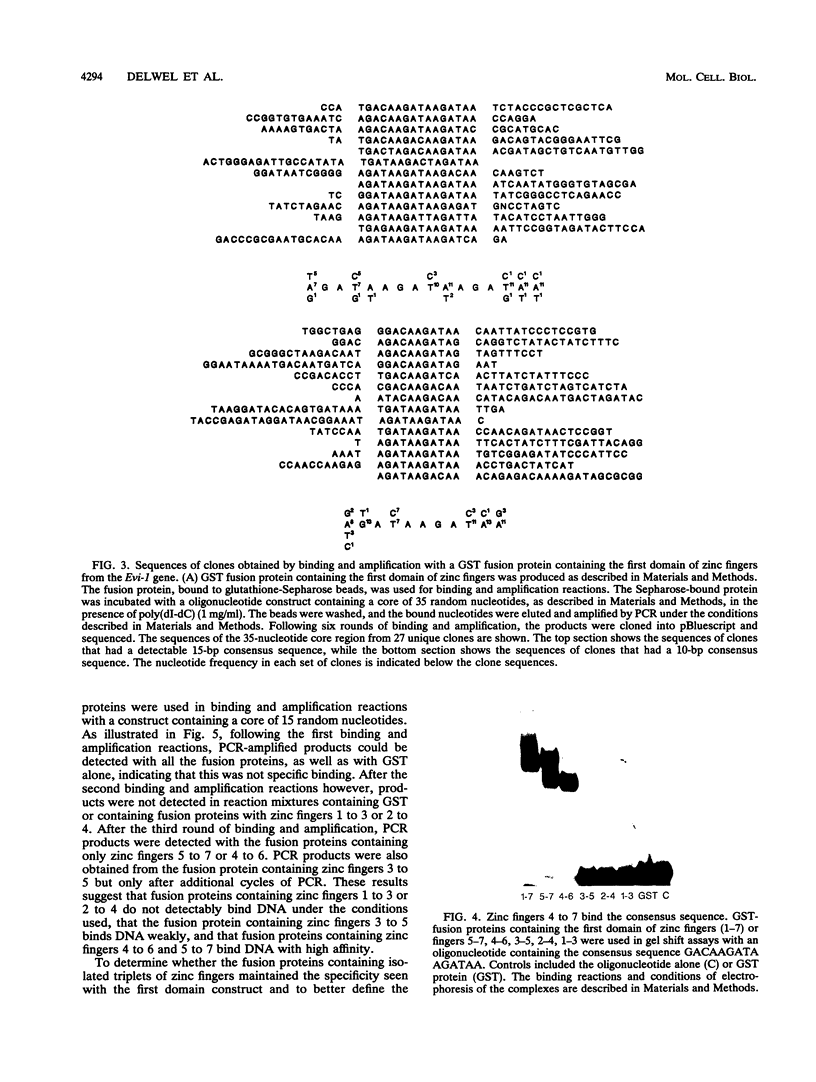
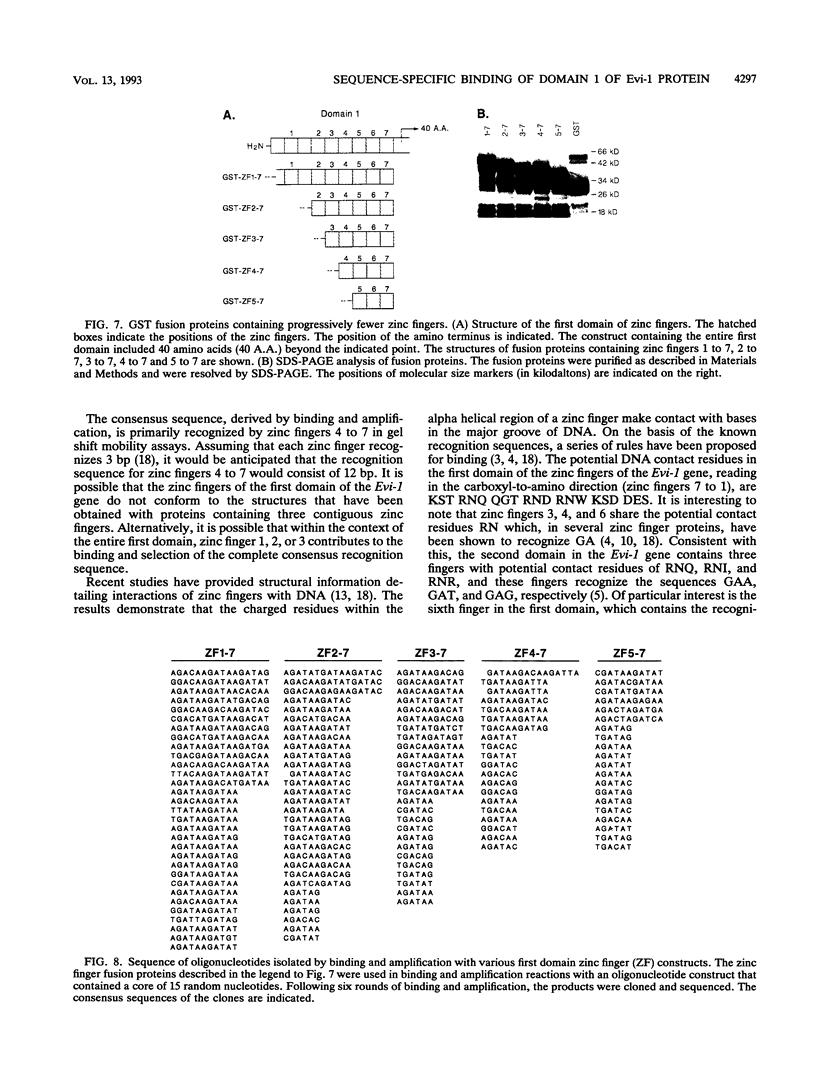
Expression of the Evi-1 gene is activated in murine myeloid leukemias by retroviral insertions and in human acute myelogenous leukemia by translocations and inversions involving chromosome band 3q26 where the gene resides. Aberrant expression of the Evi-1 gene has been shown to interfere with myeloid differentiation, which is proposed to be the basis for its role in leukemias. The Evi-1 gene encodes a 145-kDa DNA-binding protein containing two domains of seven and three Cys2-His2 zinc fingers. Previous studies identified a portion of the consensus DNA-binding sequence for the first domain of zinc fingers. The experiments presented here extend these studies and demonstrate that the first domain recognizes a consensus of 15 nucleotides consisting of GA(C/T)AAGA(T/C)AAGATAA. The first three fingers of the first domain do not detectably bind DNA but contribute to the binding by conferring a relative specificity for GACAA verses GATAA in the first position. The first three fingers also contribute to optimal binding of the 15-nucleotide consensus sequence.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartholomew C., Ihle J. N. Retroviral insertions 90 kilobases proximal to the Evi-1 myeloid transforming gene activate transcription from the normal promoter. Mol Cell Biol. 1991 Apr;11(4):1820–1828. doi: 10.1128/mcb.11.4.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew C., Morishita K., Askew D., Buchberg A., Jenkins N. A., Copeland N. G., Ihle J. N. Retroviral insertions in the CB-1/Fim-3 common site of integration activate expression of the Evi-1 gene. Oncogene. 1989 May;4(5):529–534. [PubMed] [Google Scholar]
- Desjarlais J. R., Berg J. M. Redesigning the DNA-binding specificity of a zinc finger protein: a data base-guided approach. Proteins. 1992 Feb;12(2):101–104. doi: 10.1002/prot.340120202. [DOI] [PubMed] [Google Scholar]
- Desjarlais J. R., Berg J. M. Toward rules relating zinc finger protein sequences and DNA binding site preferences. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7345–7349. doi: 10.1073/pnas.89.16.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho I. C., Vorhees P., Marin N., Oakley B. K., Tsai S. F., Orkin S. H., Leiden J. M. Human GATA-3: a lineage-restricted transcription factor that regulates the expression of the T cell receptor alpha gene. EMBO J. 1991 May;10(5):1187–1192. doi: 10.1002/j.1460-2075.1991.tb08059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Only two of the five zinc fingers of the eukaryotic transcriptional repressor PRDI-BF1 are required for sequence-specific DNA binding. Mol Cell Biol. 1992 May;12(5):1940–1949. doi: 10.1128/mcb.12.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevit R. E. Recognition of DNA by Cys2,His2 zinc fingers. Science. 1991 Sep 20;253(5026):1367–1393. doi: 10.1126/science.1896847. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Gippert G. P., Soman K. V., Case D. A., Wright P. E. Three-dimensional solution structure of a single zinc finger DNA-binding domain. Science. 1989 Aug 11;245(4918):635–637. doi: 10.1126/science.2503871. [DOI] [PubMed] [Google Scholar]
- Matsugi T., Morishita K., Ihle J. N. Identification, nuclear localization, and DNA-binding activity of the zinc finger protein encoded by the Evi-1 myeloid transforming gene. Mol Cell Biol. 1990 Mar;10(3):1259–1264. doi: 10.1128/mcb.10.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Parganas E., Matsugi T., Ihle J. N. Expression of the Evi-1 zinc finger gene in 32Dc13 myeloid cells blocks granulocytic differentiation in response to granulocyte colony-stimulating factor. Mol Cell Biol. 1992 Jan;12(1):183–189. doi: 10.1128/mcb.12.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Parganas E., Parham D. M., Matsugi T., Ihle J. N. The Evi-1 zinc finger myeloid transforming gene is normally expressed in the kidney and in developing oocytes. Oncogene. 1990 Sep;5(9):1419–1423. [PubMed] [Google Scholar]
- Morishita K., Parganas E., William C. L., Whittaker M. H., Drabkin H., Oval J., Taetle R., Valentine M. B., Ihle J. N. Activation of EVI1 gene expression in human acute myelogenous leukemias by translocations spanning 300-400 kilobases on chromosome band 3q26. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3937–3941. doi: 10.1073/pnas.89.9.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita K., Parker D. S., Mucenski M. L., Jenkins N. A., Copeland N. G., Ihle J. N. Retroviral activation of a novel gene encoding a zinc finger protein in IL-3-dependent myeloid leukemia cell lines. Cell. 1988 Sep 9;54(6):831–840. doi: 10.1016/s0092-8674(88)91175-0. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Pabo C. O. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991 May 10;252(5007):809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- Perkins A. S., Fishel R., Jenkins N. A., Copeland N. G. Evi-1, a murine zinc finger proto-oncogene, encodes a sequence-specific DNA-binding protein. Mol Cell Biol. 1991 May;11(5):2665–2674. doi: 10.1128/mcb.11.5.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins A. S., Mercer J. A., Jenkins N. A., Copeland N. G. Patterns of Evi-1 expression in embryonic and adult tissues suggest that Evi-1 plays an important regulatory role in mouse development. Development. 1991 Feb;111(2):479–487. doi: 10.1242/dev.111.2.479. [DOI] [PubMed] [Google Scholar]
- Pevny L., Simon M. C., Robertson E., Klein W. H., Tsai S. F., D'Agati V., Orkin S. H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991 Jan 17;349(6306):257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]