Abstract
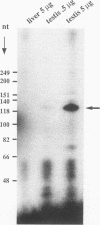
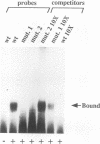
A cDNA encoding a DNA-binding protein has been isolated by screening a mouse testicular expression cDNA library with a concatemer of a 12-bp putative protein-binding element present in the promoter of the testis-specific gene PGK-2. Sequence analysis of the isolated cDNA indicated the presence of an open reading frame that encodes a protein with two conserved DNA-binding motifs known as the high-mobility-group (HMG) boxes. Northern (RNA) blot analysis demonstrated that expression of the gene is restricted to the postpuberal testis. The DNA-binding activity and sequence specificity of the recombinant HMG protein were confirmed by DNA mobility shift assay using the initial concatemer of the PGK-2 promoter element as a probe as well as the wild-type or mutated versions of the 12-bp element within its natural sequence context. Immunocytochemical staining of adult testis sections with polyclonal antisera recognizing this recombinant HMG protein demonstrated that it is located predominantly in the nuclei of elongated spermatids at steps 9 and 10. These results suggest that this novel HMG box protein gene may be involved in the regulation of gene expression of the haploid male genome. The gene from which the cDNA was derived has been termed testis-specific HMG (tsHMG).
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander-Bridges M., Ercolani L., Kong X. F., Nasrin N. Identification of a core motif that is recognized by three members of the HMG class of transcriptional regulators: IRE-ABP, SRY, and TCF-1 alpha. J Cell Biochem. 1992 Feb;48(2):129–135. doi: 10.1002/jcb.240480204. [DOI] [PubMed] [Google Scholar]
- Blanar M. A., Rutter W. J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992 May 15;256(5059):1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- Boer P. H., Adra C. N., Lau Y. F., McBurney M. W. The testis-specific phosphoglycerate kinase gene pgk-2 is a recruited retroposon. Mol Cell Biol. 1987 Sep;7(9):3107–3112. doi: 10.1128/mcb.7.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke P. S., Wolgemuth D. J. Zfp-37, a new murine zinc finger encoding gene, is expressed in a developmentally regulated pattern in the male germ line. Nucleic Acids Res. 1992 Jun 11;20(11):2827–2834. doi: 10.1093/nar/20.11.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cunliffe V., Koopman P., McLaren A., Trowsdale J. A mouse zinc finger gene which is transiently expressed during spermatogenesis. EMBO J. 1990 Jan;9(1):197–205. doi: 10.1002/j.1460-2075.1990.tb08096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunliffe V., Williams S., Trowsdale J. Genomic analysis of a mouse zinc finger gene, Zfp-35, that is up-regulated during spermatogenesis. Genomics. 1990 Oct;8(2):331–339. doi: 10.1016/0888-7543(90)90290-b. [DOI] [PubMed] [Google Scholar]
- Denny P., Ashworth A. A zinc finger protein-encoding gene expressed in the post-meiotic phase of spermatogenesis. Gene. 1991 Oct 15;106(2):221–227. doi: 10.1016/0378-1119(91)90202-m. [DOI] [PubMed] [Google Scholar]
- Denny P., Swift S., Connor F., Ashworth A. An SRY-related gene expressed during spermatogenesis in the mouse encodes a sequence-specific DNA-binding protein. EMBO J. 1992 Oct;11(10):3705–3712. doi: 10.1002/j.1460-2075.1992.tb05455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. P., Lisowsky T., Parisi M. A., Clayton D. A. DNA wrapping and bending by a mitochondrial high mobility group-like transcriptional activator protein. J Biol Chem. 1992 Feb 15;267(5):3358–3367. [PubMed] [Google Scholar]
- Fisher R. P., Topper J. N., Clayton D. A. Promoter selection in human mitochondria involves binding of a transcription factor to orientation-independent upstream regulatory elements. Cell. 1987 Jul 17;50(2):247–258. doi: 10.1016/0092-8674(87)90220-0. [DOI] [PubMed] [Google Scholar]
- Foulkes N. S., Schlotter F., Pévet P., Sassone-Corsi P. Pituitary hormone FSH directs the CREM functional switch during spermatogenesis. Nature. 1993 Mar 18;362(6417):264–267. doi: 10.1038/362264a0. [DOI] [PubMed] [Google Scholar]
- Giese K., Cox J., Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992 Apr 3;69(1):185–195. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- Gold B., Fujimoto H., Kramer J. M., Erickson R. P., Hecht N. B. Haploid accumulation and translational control of phosphoglycerate kinase-2 messenger RNA during mouse spermatogenesis. Dev Biol. 1983 Aug;98(2):392–399. doi: 10.1016/0012-1606(83)90368-8. [DOI] [PubMed] [Google Scholar]
- Goto M., Koji T., Mizuno K., Tamaru M., Koikeda S., Nakane P. K., Mori N., Masamune Y., Nakanishi Y. Transcription switch of two phosphoglycerate kinase genes during spermatogenesis as determined with mouse testis sections in situ. Exp Cell Res. 1990 Feb;186(2):273–278. doi: 10.1016/0014-4827(90)90306-u. [DOI] [PubMed] [Google Scholar]
- Handel M. A. Genetic control of spermatogenesis in mice. Results Probl Cell Differ. 1987;15:1–62. doi: 10.1007/978-3-540-47184-4_1. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Hayashi H., Iwai K. Tetrahymena HMG nonhistone chromosomal protein. Isolation and amino acid sequence lacking the N- and C-terminal domains of vertebrate HMG 1. J Biochem. 1989 Apr;105(4):577–581. doi: 10.1093/oxfordjournals.jbchem.a122707. [DOI] [PubMed] [Google Scholar]
- Hecht N. B. Regulation of 'haploid expressed genes' in male germ cells. J Reprod Fertil. 1990 Mar;88(2):679–693. doi: 10.1530/jrf.0.0880679. [DOI] [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Kelly M., Burke J., Smith M., Klar A., Beach D. Four mating-type genes control sexual differentiation in the fission yeast. EMBO J. 1988 May;7(5):1537–1547. doi: 10.1002/j.1460-2075.1988.tb02973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene K. C. Poly(A) shortening accompanies the activation of translation of five mRNAs during spermiogenesis in the mouse. Development. 1989 Jun;106(2):367–373. doi: 10.1242/dev.106.2.367. [DOI] [PubMed] [Google Scholar]
- Kolodrubetz D., Burgum A. Duplicated NHP6 genes of Saccharomyces cerevisiae encode proteins homologous to bovine high mobility group protein 1. J Biol Chem. 1990 Feb 25;265(6):3234–3239. [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau Y. F., Chan K. M., Sparkes R. Male-enhanced antigen gene is phylogenetically conserved and expressed at late stages of spermatogenesis. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8462–8466. doi: 10.1073/pnas.86.21.8462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey J. R. Nucleotide sequence of the promoter region of a tissue-specific human retroposon: comparison with its housekeeping progenitor. Gene. 1987;61(3):291–298. doi: 10.1016/0378-1119(87)90192-2. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Oyen O., Myklebust F., Scott J. D., Cadd G. G., McKnight G. S., Hansson V., Jahnsen T. Subunits of cyclic adenosine 3',5'-monophosphate-dependent protein kinase show differential and distinct expression patterns during germ cell differentiation: alternative polyadenylation in germ cells gives rise to unique smaller-sized mRNA species. Biol Reprod. 1990 Jul;43(1):46–54. doi: 10.1095/biolreprod43.1.46. [DOI] [PubMed] [Google Scholar]
- Parisi M. A., Clayton D. A. Similarity of human mitochondrial transcription factor 1 to high mobility group proteins. Science. 1991 May 17;252(5008):965–969. doi: 10.1126/science.2035027. [DOI] [PubMed] [Google Scholar]
- Robinson M. O., McCarrey J. R., Simon M. I. Transcriptional regulatory regions of testis-specific PGK2 defined in transgenic mice. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8437–8441. doi: 10.1073/pnas.86.21.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Singh H., Clerc R. G., LeBowitz J. H. Molecular cloning of sequence-specific DNA binding proteins using recognition site probes. Biotechniques. 1989 Mar;7(3):252–261. [PubMed] [Google Scholar]
- Singh J., Rao M. R. Interaction of rat testis protein, TP, with nucleic acids in vitro. Fluorescence quenching, UV absorption, and thermal denaturation studies. J Biol Chem. 1987 Jan 15;262(2):734–740. [PubMed] [Google Scholar]
- Thomas K. H., Wilkie T. M., Tomashefsky P., Bellvé A. R., Simon M. I. Differential gene expression during mouse spermatogenesis. Biol Reprod. 1989 Oct;41(4):729–739. doi: 10.1095/biolreprod41.4.729. [DOI] [PubMed] [Google Scholar]
- Travis A., Amsterdam A., Belanger C., Grosschedl R. LEF-1, a gene encoding a lymphoid-specific protein with an HMG domain, regulates T-cell receptor alpha enhancer function [corrected]. Genes Dev. 1991 May;5(5):880–894. doi: 10.1101/gad.5.5.880. [DOI] [PubMed] [Google Scholar]
- Tsuda K., Kikuchi M., Mori K., Waga S., Yoshida M. Primary structure of non-histone protein HMG1 revealed by the nucleotide sequence. Biochemistry. 1988 Aug 9;27(16):6159–6163. doi: 10.1021/bi00416a050. [DOI] [PubMed] [Google Scholar]
- Wolgemuth D. J., Viviano C. M., Watrin F. Expression of homeobox genes during spermatogenesis. Ann N Y Acad Sci. 1991;637:300–312. doi: 10.1111/j.1749-6632.1991.tb27317.x. [DOI] [PubMed] [Google Scholar]
- van de Wetering M., Clevers H. Sequence-specific interaction of the HMG box proteins TCF-1 and SRY occurs within the minor groove of a Watson-Crick double helix. EMBO J. 1992 Aug;11(8):3039–3044. doi: 10.1002/j.1460-2075.1992.tb05374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]