Abstract
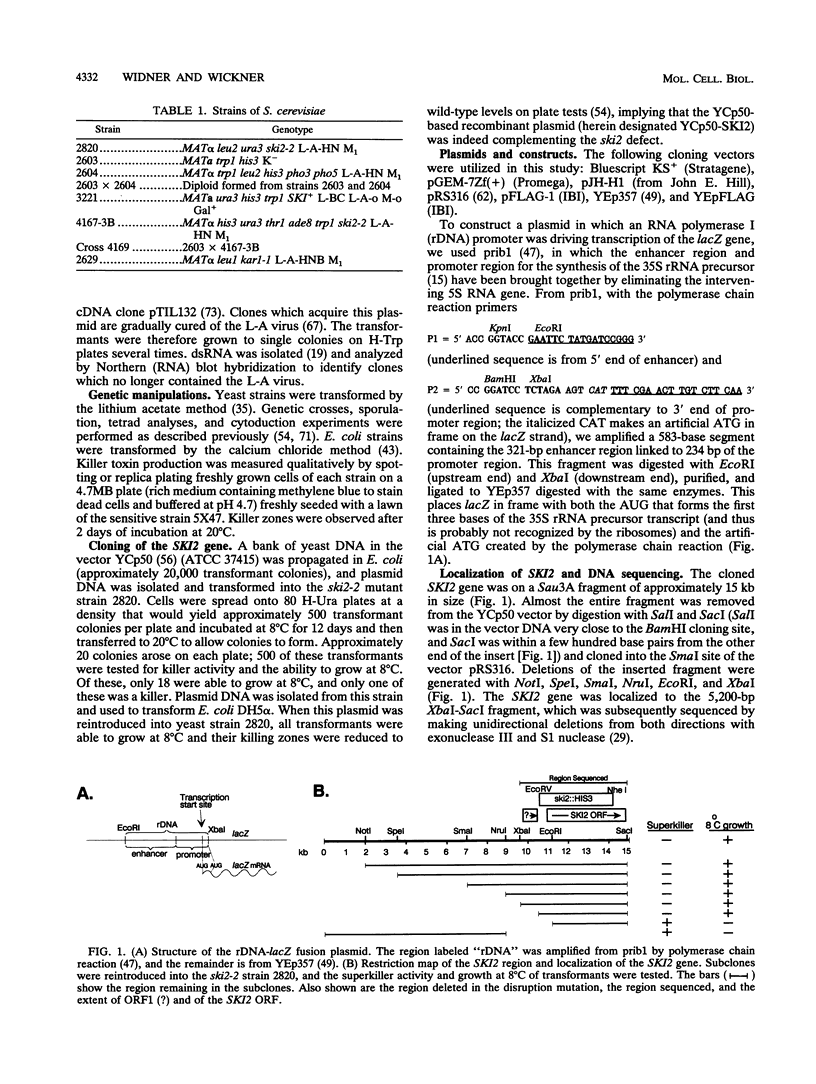
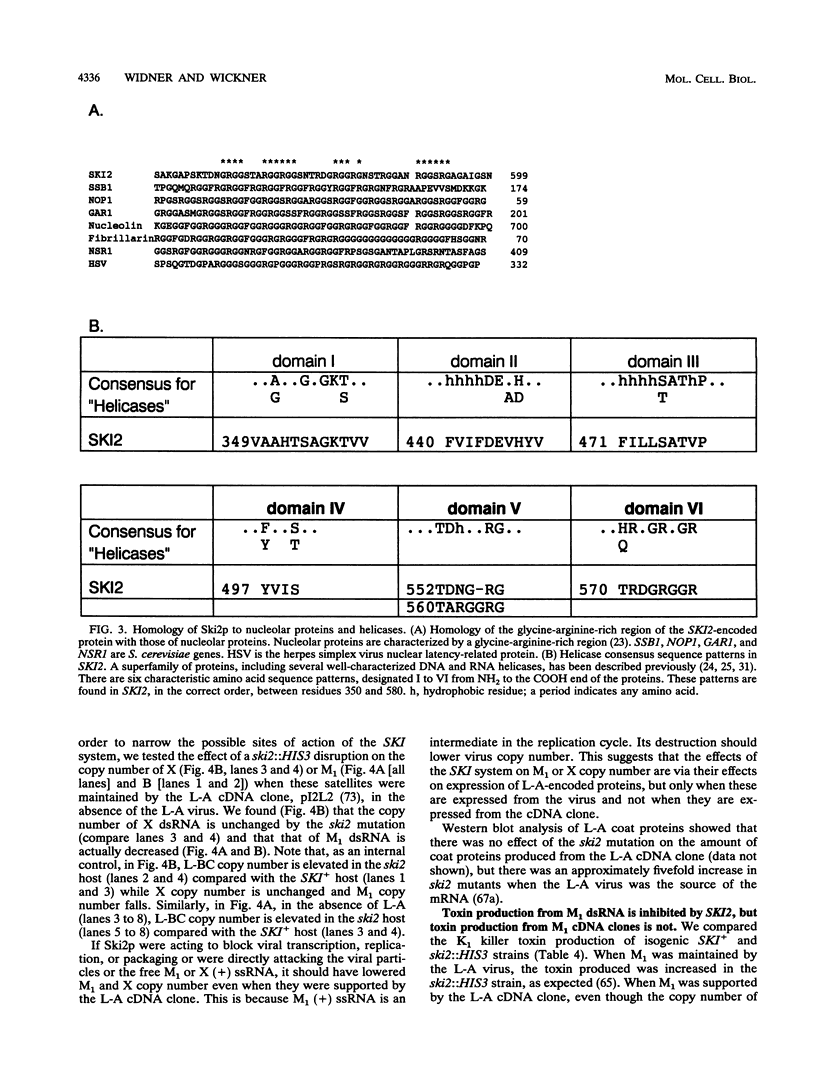
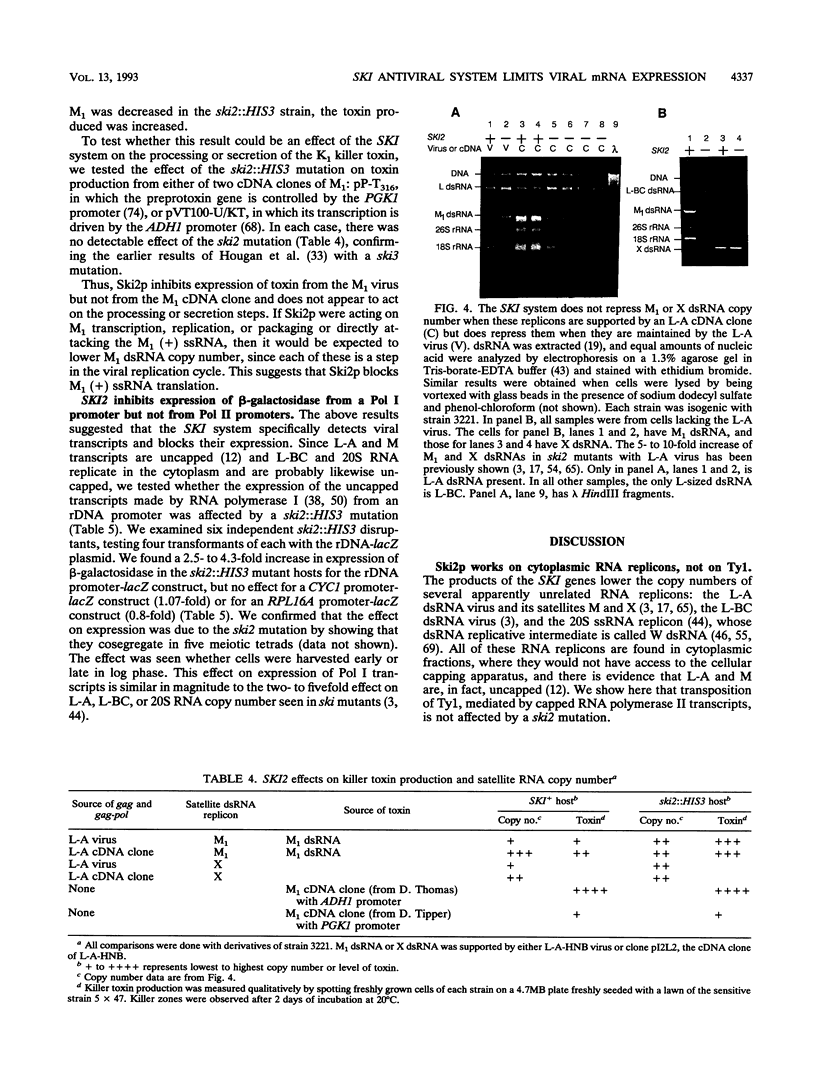
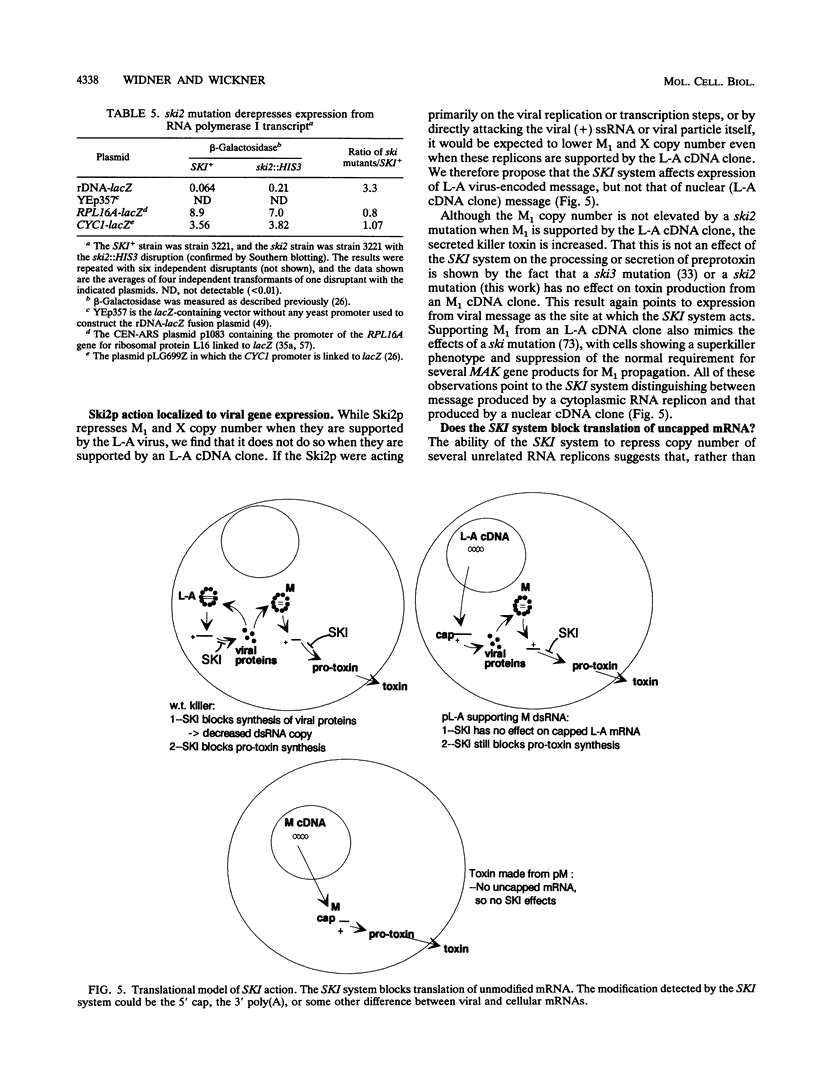
The SKI2 gene is part of a host system that represses the copy number of the L-A double-stranded RNA (dsRNA) virus and its satellites M and X dsRNA, of the L-BC dsRNA virus, and of the single-stranded replicon 20S RNA. We show that SKI2 encodes a 145-kDa protein with motifs characteristic of helicases and nucleolar proteins and is essential only in cells carrying M dsRNA. Unexpectedly, Ski2p does not repress M1 dsRNA copy number when M1 is supported by aN L-A cDNA clone; nonetheless, it did lower the levels of M1 dsRNA-encoded toxin produced. Since toxin secretion from cDNA clones of M1 is unaffected by Ski2p, these data suggest that Ski2p acts by specifically blocking translation of viral mRNAs, perhaps recognizing the absence of cap or poly(A). In support of this idea, we find that Ski2p represses production of beta-galactosidase from RNA polymerase I [no cap and no poly(A)] transcripts but not from RNA polymerase II (capped) transcripts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Ambros V., Pettersson R. F., Baltimore D. An enzymatic activity in uninfected cells that cleaves the linkage between poliovirion RNA and the 5' terminal protein. Cell. 1978 Dec;15(4):1439–1446. doi: 10.1016/0092-8674(78)90067-3. [DOI] [PubMed] [Google Scholar]
- Ball S. G., Tirtiaux C., Wickner R. B. Genetic Control of L-a and L-(Bc) Dsrna Copy Number in Killer Systems of SACCHAROMYCES CEREVISIAE. Genetics. 1984 Jun;107(2):199–217. doi: 10.1093/genetics/107.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S., Hanson I., Kelly A., Pappin D. J., Trowsdale J. A homologue of the Drosophila female sterile homeotic (fsh) gene in the class II region of the human MHC. DNA Seq. 1992;2(4):203–210. doi: 10.3109/10425179209020804. [DOI] [PubMed] [Google Scholar]
- Bernstein H. D., Sonenberg N., Baltimore D. Poliovirus mutant that does not selectively inhibit host cell protein synthesis. Mol Cell Biol. 1985 Nov;5(11):2913–2923. doi: 10.1128/mcb.5.11.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc A., Goyer C., Sonenberg N. The coat protein of the yeast double-stranded RNA virus L-A attaches covalently to the cap structure of eukaryotic mRNA. Mol Cell Biol. 1992 Aug;12(8):3390–3398. doi: 10.1128/mcb.12.8.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Burn V. E., Jayachandran S., Tipper D. J. Yeast killer dsRNA plasmids are transcribed in vivo to produce full and partial-length plus-stranded RNAs. Nucleic Acids Res. 1983 Feb 25;11(4):1077–1097. doi: 10.1093/nar/11.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Sturgeon J. A., Tipper D. J. Encapsidation of yeast killer double-stranded ribonucleic acids: dependence of M on L. J Bacteriol. 1980 Jul;143(1):463–470. doi: 10.1128/jb.143.1.463-470.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbon H. M., Lapeyre B., Amalric F. Structure of the mouse nucleolin gene. The complete sequence reveals that each RNA binding domain is encoded by two independent exons. J Mol Biol. 1988 Apr 20;200(4):627–638. doi: 10.1016/0022-2836(88)90476-7. [DOI] [PubMed] [Google Scholar]
- Bruenn J., Keitz B. The 5' ends of yeast killer factor RNAs are pppGp. Nucleic Acids Res. 1976 Oct;3(10):2427–2436. doi: 10.1093/nar/3.10.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey H., Boone C., Zhu H., Vernet T., Whiteway M., Thomas D. Y. Genetic and molecular approaches to synthesis and action of the yeast killer toxin. Experientia. 1990 Feb 15;46(2):193–200. doi: 10.1007/BF02027313. [DOI] [PubMed] [Google Scholar]
- Dinman J. D., Icho T., Wickner R. B. A -1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):174–178. doi: 10.1073/pnas.88.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion E. A., Warner J. R. The major promoter element of rRNA transcription in yeast lies 2 kb upstream. Cell. 1984 Dec;39(3 Pt 2):663–673. doi: 10.1016/0092-8674(84)90473-2. [DOI] [PubMed] [Google Scholar]
- Esteban R., Wickner R. B. A deletion mutant of L-A double-stranded RNA replicates like M1 double-stranded RNA. J Virol. 1988 Apr;62(4):1278–1285. doi: 10.1128/jvi.62.4.1278-1285.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Wickner R. B. A new non-mendelian genetic element of yeast that increases cytopathology produced by M1 double-stranded RNA in ski strains. Genetics. 1987 Nov;117(3):399–408. doi: 10.1093/genetics/117.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett J. G., Gallie D. R. RNA delivery in Saccharomyces cerevisiae using electroporation. Yeast. 1992 Dec;8(12):1007–1014. doi: 10.1002/yea.320081203. [DOI] [PubMed] [Google Scholar]
- Fried H. M., Fink G. R. Electron microscopic heteroduplex analysis of "killer" double-stranded RNA species from yeast. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4224–4228. doi: 10.1073/pnas.75.9.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura T., Ribas J. C., Makhov A. M., Wickner R. B. Pol of gag-pol fusion protein required for encapsidation of viral RNA of yeast L-A virus. Nature. 1992 Oct 22;359(6397):746–749. doi: 10.1038/359746a0. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Wickner R. B. Gene overlap results in a viral protein having an RNA binding domain and a major coat protein domain. Cell. 1988 Nov 18;55(4):663–671. doi: 10.1016/0092-8674(88)90225-5. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Mastrangelo M. F., Sanders N. J., Shafer B. K., Strathern J. N. Transposon tagging using Ty elements in yeast. Genetics. 1988 Sep;120(1):95–108. doi: 10.1093/genetics/120.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. P., Lehtonen H., Caizergues-Ferrer M., Amalric F., Tollervey D., Lapeyre B. GAR1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO J. 1992 Feb;11(2):673–682. doi: 10.1002/j.1460-2075.1992.tb05099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989 Jun 26;17(12):4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 1983;101:181–191. doi: 10.1016/0076-6879(83)01013-7. [DOI] [PubMed] [Google Scholar]
- Hannig E. M., Thiele D. J., Leibowitz M. J. Saccharomyces cerevisiae killer virus transcripts contain template-coded polyadenylate tracts. Mol Cell Biol. 1984 Jan;4(1):101–109. doi: 10.1128/mcb.4.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S. R., Mozer B. A., Bhatia-Dey N., Dawid I. B. The Drosophila fsh locus, a maternal effect homeotic gene, encodes apparent membrane proteins. Dev Biol. 1989 Jul;134(1):246–257. doi: 10.1016/0012-1606(89)90094-8. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Henríquez R., Blobel G., Aris J. P. Isolation and sequencing of NOP1. A yeast gene encoding a nucleolar protein homologous to a human autoimmune antigen. J Biol Chem. 1990 Feb 5;265(4):2209–2215. [PubMed] [Google Scholar]
- Hodgman T. C. A new superfamily of replicative proteins. Nature. 1988 May 5;333(6168):22–23. doi: 10.1038/333022b0. [DOI] [PubMed] [Google Scholar]
- Hopper J. E., Bostian K. A., Rowe L. B., Tipper D. J. Translation of the L-species dsRNA genome of the killer-associated virus-like particles of Saccharomyces cerevisiae. J Biol Chem. 1977 Dec 25;252(24):9010–9017. [PubMed] [Google Scholar]
- Hougan L., Thomas D. Y., Whiteway M. Cloning and characterization of the SKI3 gene of Saccharomyces cerevisiae demonstrates allelism to SKI5. Curr Genet. 1989 Sep;16(3):139–143. doi: 10.1007/BF00391469. [DOI] [PubMed] [Google Scholar]
- Icho T., Wickner R. B. The double-stranded RNA genome of yeast virus L-A encodes its own putative RNA polymerase by fusing two open reading frames. J Biol Chem. 1989 Apr 25;264(12):6716–6723. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong A. Y., Clark M. W., Gilbert M., Oehm A., Campbell J. L. Saccharomyces cerevisiae SSB1 protein and its relationship to nucleolar RNA-binding proteins. Mol Cell Biol. 1987 Aug;7(8):2947–2955. doi: 10.1128/mcb.7.8.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiledjian M., Dreyfuss G. Primary structure and binding activity of the hnRNP U protein: binding RNA through RGG box. EMBO J. 1992 Jul;11(7):2655–2664. doi: 10.1002/j.1460-2075.1992.tb05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klootwijk J., de Jonge P., Planta R. J. The primary transcript of the ribosomal repeating unit in yeast. Nucleic Acids Res. 1979 Jan;6(1):27–39. doi: 10.1093/nar/6.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Inouye M. Yeast NSR1 protein that has structural similarity to mammalian nucleolin is involved in pre-rRNA processing. J Biol Chem. 1992 Aug 15;267(23):16252–16258. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W. C., Zabetakis D., Mélèse T. NSR1 is required for pre-rRNA processing and for the proper maintenance of steady-state levels of ribosomal subunits. Mol Cell Biol. 1992 Sep;12(9):3865–3871. doi: 10.1128/mcb.12.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd R. E., Toyoda H., Etchison D., Wimmer E., Ehrenfeld E. Cleavage of the cap binding protein complex polypeptide p220 is not effected by the second poliovirus protease 2A. Virology. 1986 Apr 15;150(1):299–303. doi: 10.1016/0042-6822(86)90291-6. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Fishel R., Wickner R. B. Circular single-stranded RNA replicon in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7628–7632. doi: 10.1073/pnas.87.19.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Sarkar G., Sommer S. S., Wickner R. B. A yeast antiviral protein, SKI8, shares a repeated amino acid sequence pattern with beta-subunits of G proteins and several other proteins. Yeast. 1993 Jan;9(1):43–51. doi: 10.1002/yea.320090106. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Wickner R. B. Yeast 20 S RNA replicon. Replication intermediates and encoded putative RNA polymerase. J Biol Chem. 1991 Jul 5;266(19):12779–12783. [PubMed] [Google Scholar]
- Mestel R., Yip M., Holland J. P., Wang E., Kang J., Holland M. J. Sequences within the spacer region of yeast rRNA cistrons that stimulate 35S rRNA synthesis in vivo mediate RNA polymerase I-dependent promoter and terminator activities. Mol Cell Biol. 1989 Mar;9(3):1243–1254. doi: 10.1128/mcb.9.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic mapping in yeast. Methods Cell Biol. 1975;11:221–233. doi: 10.1016/s0091-679x(08)60325-8. [DOI] [PubMed] [Google Scholar]
- Myers A. M., Tzagoloff A., Kinney D. M., Lusty C. J. Yeast shuttle and integrative vectors with multiple cloning sites suitable for construction of lacZ fusions. Gene. 1986;45(3):299–310. doi: 10.1016/0378-1119(86)90028-4. [DOI] [PubMed] [Google Scholar]
- Nikolaev N., Georgiev O. I., Venkov P. V., Hadjiolov A. A. The 37 S precursor to ribosomal RNA is the primary transcript of ribosomal RNA genes in Saccharomyces cerevisiae. J Mol Biol. 1979 Jan 25;127(3):297–308. doi: 10.1016/0022-2836(79)90331-0. [DOI] [PubMed] [Google Scholar]
- Olson M. V., Dutchik J. E., Graham M. Y., Brodeur G. M., Helms C., Frank M., MacCollin M., Scheinman R., Frank T. Random-clone strategy for genomic restriction mapping in yeast. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7826–7830. doi: 10.1073/pnas.83.20.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee S. K., Icho T., Wickner R. B. Structure and nuclear localization signal of the SKI3 antiviral protein of Saccharomyces cerevisiae. Yeast. 1989 May-Jun;5(3):149–158. doi: 10.1002/yea.320050304. [DOI] [PubMed] [Google Scholar]
- Ribas J. C., Wickner R. B. RNA-dependent RNA polymerase consensus sequence of the L-A double-stranded RNA virus: definition of essential domains. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2185–2189. doi: 10.1073/pnas.89.6.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley S. P., Sommer S. S., Wickner R. B. Superkiller mutations in Saccharomyces cerevisiae suppress exclusion of M2 double-stranded RNA by L-A-HN and confer cold sensitivity in the presence of M and L-A-HN. Mol Cell Biol. 1984 Apr;4(4):761–770. doi: 10.1128/mcb.4.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cousiño N., Esteban L. M., Esteban R. Molecular cloning and characterization of W double-stranded RNA, a linear molecule present in Saccharomyces cerevisiae. Identification of its single-stranded RNA form as 20 S RNA. J Biol Chem. 1991 Jul 5;266(19):12772–12778. [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Rotenberg M. O., Woolford J. L., Jr Tripartite upstream promoter element essential for expression of Saccharomyces cerevisiae ribosomal protein genes. Mol Cell Biol. 1986 Feb;6(2):674–687. doi: 10.1128/mcb.6.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. J., Hambidge S. J., Kirkegaard K. Direct introduction and transient expression of capped and non-capped RNA in Saccharomyces cerevisiae. Nucleic Acids Res. 1991 Sep 25;19(18):4949–4953. doi: 10.1093/nar/19.18.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T., Tollervey D., Kern H., Frank R., Hurt E. C. A yeast nucleolar protein related to mammalian fibrillarin is associated with small nucleolar RNA and is essential for viability. EMBO J. 1989 Dec 20;8(13):4015–4024. doi: 10.1002/j.1460-2075.1989.tb08584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer S. S., Wickner R. B. Gene disruption indicates that the only essential function of the SKI8 chromosomal gene is to protect Saccharomyces cerevisiae from viral cytopathology. Virology. 1987 Mar;157(1):252–256. doi: 10.1016/0042-6822(87)90338-2. [DOI] [PubMed] [Google Scholar]
- Thiele D. J., Hannig E. M., Leibowitz M. J. Multiple L double-stranded RNA species of Saccharomyces cerevisiae: evidence for separate encapsidation. Mol Cell Biol. 1984 Jan;4(1):92–100. doi: 10.1128/mcb.4.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Guerry P., Wickner R. B. Chromosomal superkiller mutants of Saccharomyces cerevisiae. J Bacteriol. 1978 Dec;136(3):1002–1007. doi: 10.1128/jb.136.3.1002-1007.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A., Wickner R. B. "Superkiller" mutations suppress chromosomal mutations affecting double-stranded RNA killer plasmid replication in saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Jan;77(1):527–530. doi: 10.1073/pnas.77.1.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle R. P., Wickner R. B. Elimination of L-A double-stranded RNA virus of Saccharomyces cerevisiae by expression of gag and gag-pol from an L-A cDNA clone. J Virol. 1993 May;67(5):2764–2771. doi: 10.1128/jvi.67.5.2764-2771.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet T., Dignard D., Thomas D. Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52(2-3):225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- Wesolowski M., Wickner R. B. Two new double-stranded RNA molecules showing non-mendelian inheritance and heat inducibility in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):181–187. doi: 10.1128/mcb.4.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B., Icho T., Fujimura T., Widner W. R. Expression of yeast L-A double-stranded RNA virus proteins produces derepressed replication: a ski- phenocopy. J Virol. 1991 Jan;65(1):155–161. doi: 10.1128/jvi.65.1.155-161.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Plasmids controlled exclusion of the K2 killer double-stranded RNA plasmid of yeast. Cell. 1980 Aug;21(1):217–226. doi: 10.1016/0092-8674(80)90129-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Kane J., Zhang X. Y., Zhang M., Tipper D. J. Role of the gamma component of preprotoxin in expression of the yeast K1 killer phenotype. Yeast. 1993 Mar;9(3):251–266. doi: 10.1002/yea.320090305. [DOI] [PubMed] [Google Scholar]



