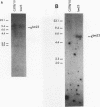
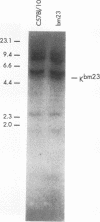
Abstract
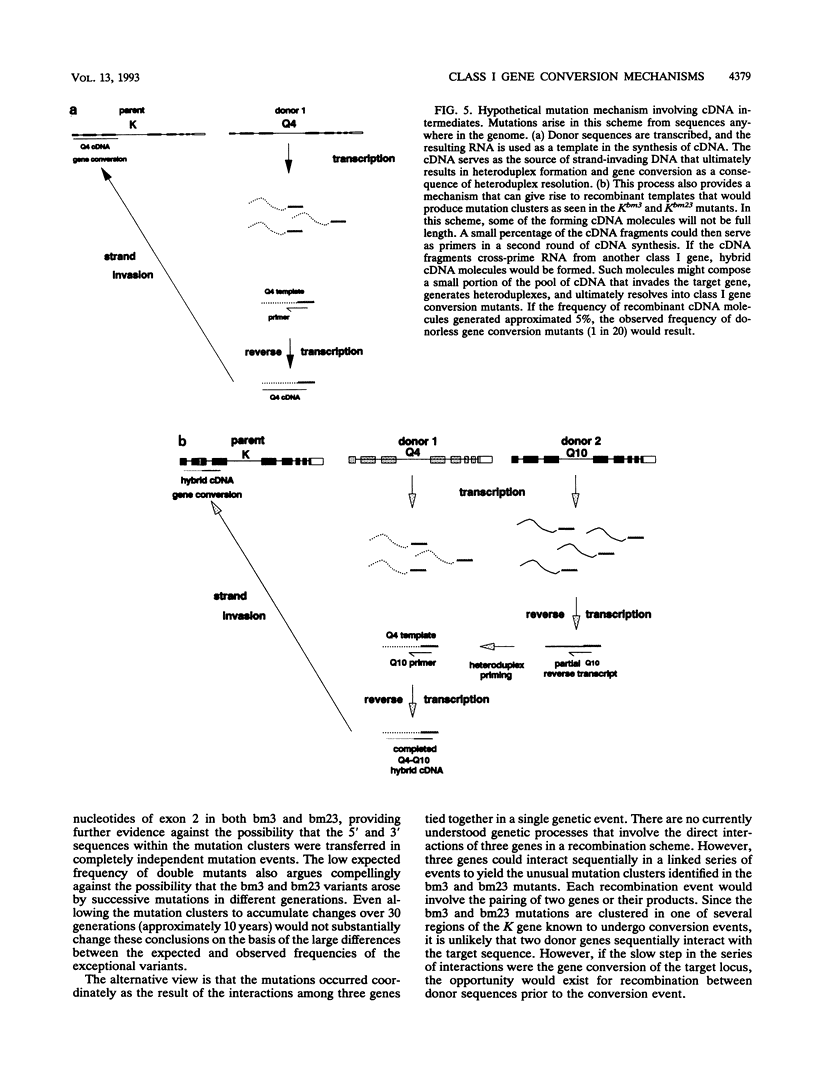
Genetic diversity among the K and D alleles of the mouse major histocompatibility complex is generated by gene conversion among members of the class I multigene family. The majority of known class I mutants contain clusters of nucleotide changes that can be traced to linked family members. However, the details of the gene conversion mechanism are not known. The bm3 and bm23 mutations represent exceptions to the usual pattern and provide insight into intermediates generated during the gene conversion process. Both of these variants contain clusters of five nucleotide substitutions, but they differ from the classic conversion mutants in the important respect that no donor gene for either mutation could be identified in the parental genome. Nevertheless, both mutation clusters are composed of individual mutations that do exist within the parent. Therefore, they are not random and appear to be templated. Significantly, the bm3 and bm23 mutation clusters are divided into overlapping regions that match class I genes which have functioned as donor genes in other characterized gene conversion events. The unusual structure of the mutation clusters indicates an underlying gene conversion mechanism that can generate mutation clusters as a result of the interaction of three genes in a single genetic event. The unusual mutation clusters are consistent with a hypothetical gene conversion model involving extrachromosomal intermediates.
Full text
PDF


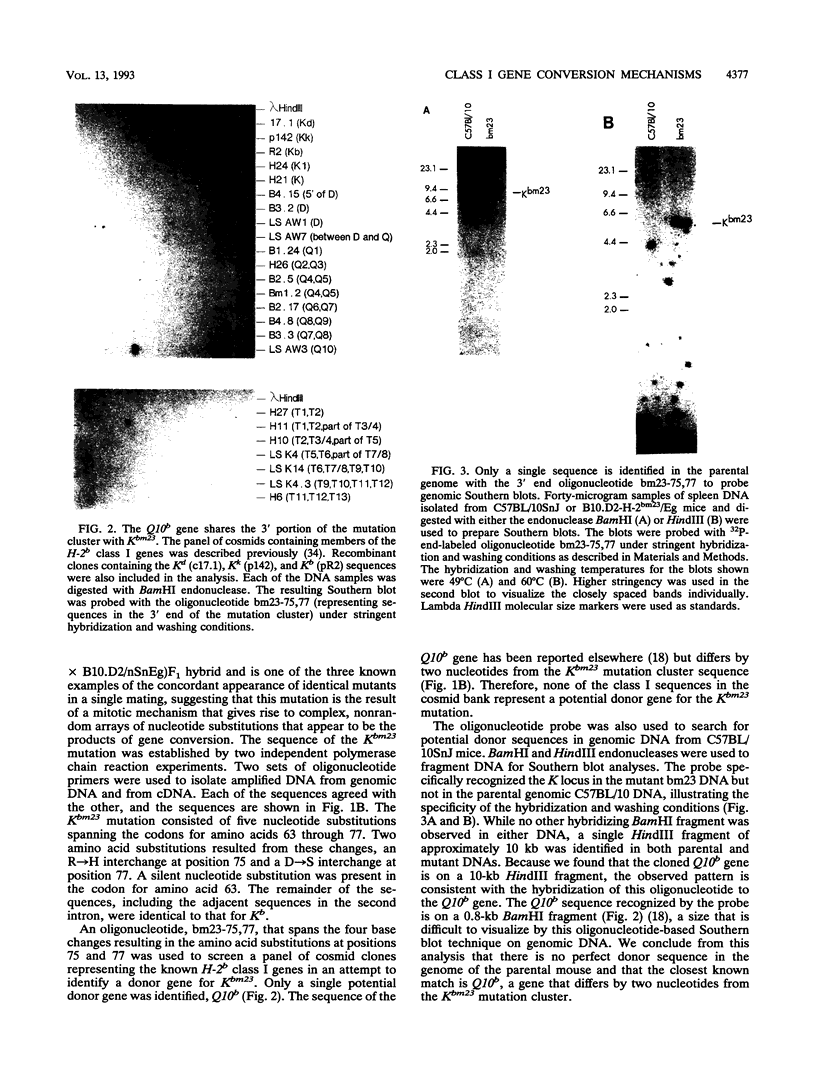
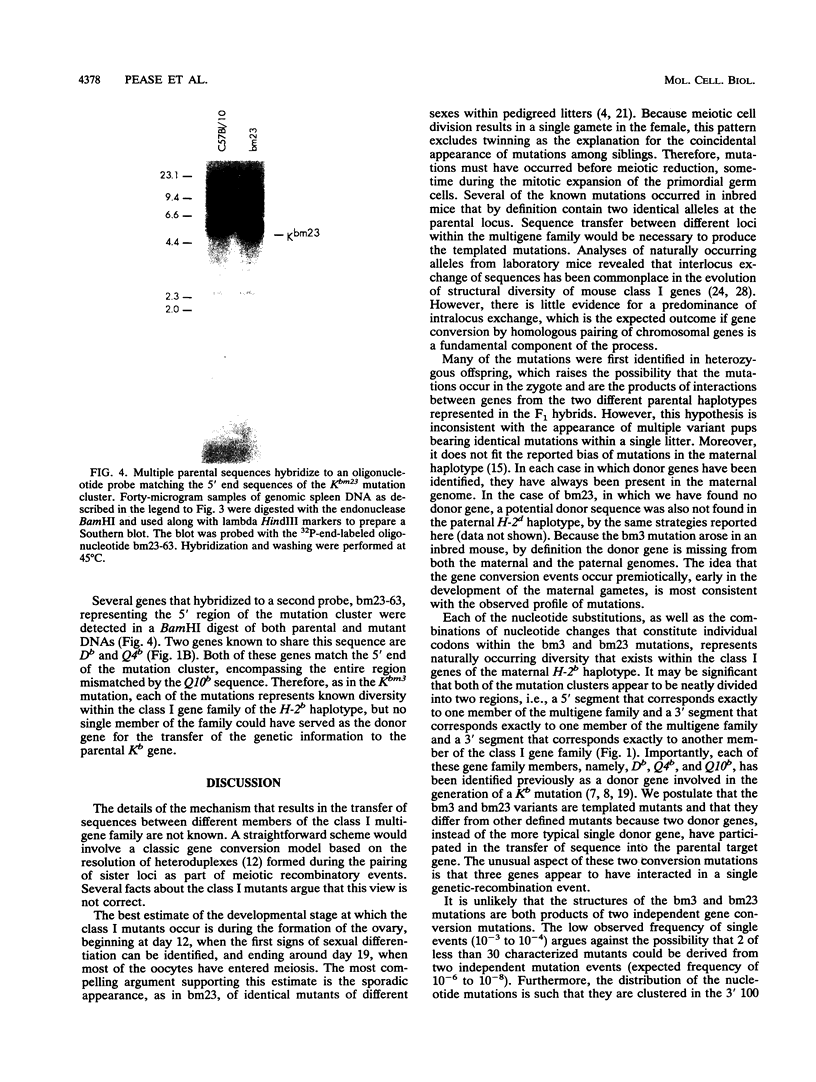


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Derr L. K., Strathern J. N. A role for reverse transcripts in gene conversion. Nature. 1993 Jan 14;361(6408):170–173. doi: 10.1038/361170a0. [DOI] [PubMed] [Google Scholar]
- Duran L. W., Pease L. R. Tracing the evolution of H-2 D region genes using sequences associated with a repetitive element. J Immunol. 1988 Jul 1;141(1):295–301. [PubMed] [Google Scholar]
- Egorov O. S., Egorov I. K. H-2bm23, a new Kb mutant similar to, but not identical with H-2bm3. Immunogenetics. 1984;20(1):83–87. doi: 10.1007/BF00373449. [DOI] [PubMed] [Google Scholar]
- Ewenstein B. M., Uehara H., Nisizawa T., Melvold R. W., Kohn H. I., Nathenson S. G. Biochemical studies on the H-2K antigens of the MHC mutants bm3 and bm11. Immunogenetics. 1980;11(4):383–395. doi: 10.1007/BF01567805. [DOI] [PubMed] [Google Scholar]
- Flaherty L., Elliott E., Tine J. A., Walsh A. C., Waters J. B. Immunogenetics of the Q and TL regions of the mouse. Crit Rev Immunol. 1990;10(2):131–175. [PubMed] [Google Scholar]
- Geliebter J., Nathenson S. G. Microrecombinations generate sequence diversity in the murine major histocompatibility complex: analysis of the Kbm3, Kbm4, Kbm10, and Kbm11 mutants. Mol Cell Biol. 1988 Oct;8(10):4342–4352. doi: 10.1128/mcb.8.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Schulze D. H., Pease L. R., Weiss E. H., Mellor A. L., Flavell R. A., Nathenson S. G. Interaction between Kb and Q4 gene sequences generates the Kbm6 mutation. Mol Cell Biol. 1986 Feb;6(2):645–652. doi: 10.1128/mcb.6.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi S., Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Three spontaneous H-2Db mutants are generated by genetic micro-recombination (gene conversion) events. Impact on the H-2-restricted immune responsiveness. J Exp Med. 1988 Dec 1;168(6):2319–2335. doi: 10.1084/jem.168.6.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand W. H., Horton R. M., Pease L. R., Martinko J. M. Nucleotide sequence analysis of H-2Df and the spontaneous in vivo H-2Dfm2 mutation. Mol Immunol. 1992 Jan;29(1):61–69. doi: 10.1016/0161-5890(92)90157-s. [DOI] [PubMed] [Google Scholar]
- Holliday R. Molecular aspects of genetic exchange and gene conversion. Genetics. 1974 Sep;78(1):273–287. doi: 10.1093/genetics/78.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton R. M., Hildebrand W. H., Martinko J. M., Pease L. R. Structural analysis of H-2Kf and H-2Kfm1 by using H-2K locus-specific sequences. J Immunol. 1990 Sep 15;145(6):1782–1787. [PubMed] [Google Scholar]
- Knight K. L. Restricted VH gene usage and generation of antibody diversity in rabbit. Annu Rev Immunol. 1992;10:593–616. doi: 10.1146/annurev.iy.10.040192.003113. [DOI] [PubMed] [Google Scholar]
- Loh D. Y., Baltimore D. Sexual preference of apparent gene conversion events in MHC genes of mice. Nature. 1984 Jun 14;309(5969):639–640. doi: 10.1038/309639a0. [DOI] [PubMed] [Google Scholar]
- Martinko J. M., Solheim J. C., Geliebter J. The H-2Kkml mutation: a single nucleotide substitution is responsible for multiple functional differences in a class I MHC molecule. Mol Immunol. 1988 Mar;25(3):267–274. doi: 10.1016/0161-5890(88)90018-1. [DOI] [PubMed] [Google Scholar]
- McCormack W. T., Tjoelker L. W., Thompson C. B. Avian B-cell development: generation of an immunoglobulin repertoire by gene conversion. Annu Rev Immunol. 1991;9:219–241. doi: 10.1146/annurev.iy.09.040191.001251. [DOI] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Kress M., Jay G., Flavell R. A. A nonpolymorphic class I gene in the murine major histocompatibility complex. Cell. 1984 Jan;36(1):139–144. doi: 10.1016/0092-8674(84)90082-5. [DOI] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Ramachandran K., Flavell R. A. A potential donor gene for the bm1 gene conversion event in the C57BL mouse. Nature. 1983 Dec 22;306(5945):792–795. doi: 10.1038/306792a0. [DOI] [PubMed] [Google Scholar]
- Melvold R. W., Kohn H. I., Dunn G. R. History and genealogy of the H-2Kb mutants from the C57BL/6Kh colony. Immunogenetics. 1982;15(2):177–185. doi: 10.1007/BF00621950. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Nathenson S. G., Leinwand L. A. Mapping class I gene sequences in the major histocompatibility complex. Nature. 1982 Jul 22;298(5872):382–385. doi: 10.1038/298382a0. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers P. A., Smithies O. Short gene conversions in the human fetal globin gene region: a by-product of chromosome pairing during meiosis? Genetics. 1986 Feb;112(2):343–358. doi: 10.1093/genetics/112.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen J. K., Horton R. M., Cai Z. L., Pease L. R. Structural diversity of the classical H-2 genes: K, D, and L. J Immunol. 1992 Feb 1;148(3):953–967. [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Robinson P. J., Bevec D., Mellor A. L., Weiss E. H. Sequence of the mouse Q4 class I gene and characterization of the gene product. Immunogenetics. 1988;27(2):79–86. doi: 10.1007/BF00351079. [DOI] [PubMed] [Google Scholar]
- Sullivan K. F., Lau J. T., Cleveland D. W. Apparent gene conversion between beta-tubulin genes yields multiple regulatory pathways for a single beta-tubulin polypeptide isotype. Mol Cell Biol. 1985 Sep;5(9):2454–2465. doi: 10.1128/mcb.5.9.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Deininger P. L., Efstratiadis A. Nonviral retroposons: genes, pseudogenes, and transposable elements generated by the reverse flow of genetic information. Annu Rev Biochem. 1986;55:631–661. doi: 10.1146/annurev.bi.55.070186.003215. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Golden L., Fahrner K., Mellor A. L., Devlin J. J., Bullman H., Tiddens H., Bud H., Flavell R. A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984 Aug 23;310(5979):650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki L. J., Gefter M. L. Gene conversion and the generation of antibody diversity. Annu Rev Biochem. 1989;58:509–531. doi: 10.1146/annurev.bi.58.070189.002453. [DOI] [PubMed] [Google Scholar]